Abstract
Background In Legg-Calvé-Perthes disease (LCPD), 4 major patterns (coxa plana, coxa magna, coxa vara, subluxation) of the femoral head are commonly observed. However, direct observation of pathological specimens is rarely possible. An animal model of LCPD may clarify the pathogenesis of femoral head deformity.
Animals and methods In 26 piglets, we interrupted the vascular supply to the capital femoral epiphysis by cutting the ligamentum teres and ligating the femoral neck containing the epiphyseal artery. 6–7 piglets in each experimental group were killed at early (2 and 4 weeks: P2 and P4), intermediate (12 weeks: P12), and late (20 weeks: P20) periods. We examined the extracted femoral heads macroscopically and radiographically.
Results The mean decrease in epiphyseal height was 1.5 mm, 4.1 mm, 5.0 mm, and 7.5 mm in P2, P4, P12 and P20, respectively (rs = 0.76, p = 0.002). The mean increase of diameter was 4.1 mm, 6.9 mm, and 6.8 mm in P4, P12 and P20, respectively. Decrease of the articulotrochanteric distance was mild in P2 and P4, and severe in P12 and P20. Subluxation of the femoral head was observed only in P12 and P20 piglets.
Interpretation The piglet model of LCPD was useful in the early stage of devascularization for investigation of the developmental pattern of femoral head deformity. However, when the piglets had grown to 20 weeks old or more—that is, to full skeletal maturity—the femoral head and acetabulum showed severe deformities that were most likely caused by heavy body weight. ▪
The clinical importance of Legg-Calvé-Perthes disease (LCPD) lies in a deformity of the femoral head which is related to the development of secondary arthritis (Shapiro Citation2001). Since surgery is rarely performed on the diseased femoral head, direct observation of pathological specimens is rarely possible. For the experimental study of LCPD, young growing rabbits and puppies have been widely used (Sanchis et al. Citation1973, Rowe and Park Citation1986), but macroscopic and radiographic findings in these animals cannot explain the pathogenesis of femoral head deformity. Recently, piglets have been shown to be the better LCPD model (Babyn et al. Citation1998, Kim and Su Citation2002).
We used a piglet model of LCPD to determine the pathoanatomical changes of the femoral head in relation to macroscopic and radiographic findings.
Animals and methods
We used 30 crossbred (Yorkshire and Landrace) piglets, 4–5 weeks old and weighing 7–9 kg. In all piglets the left hip was experimented on and the right hip served as control. The interruption of the vascular supply to the capital femoral epiphysis was accomplished by cutting the ligamentum teres and ligating the femoral neck (containing the epiphyseal artery) with a no. 2 Ethibond suture (Babyn et al. Citation1998, Kim and Su Citation2002).
4 of the 30 piglets were excluded because of early death in 2 cases and fractures of the operated femur in the other 2. Of the remaining 26 piglets, 6–7 in each experimental group were killed at early (weeks 2 and 4), intermediate (week 12), and late (week 20) periods (). The groups of piglets were denoted P2, P4, P12 and P20, respectively. The early group of piglets corresponds to the stage of avascular or early fragmentation in human LCPD, and was expected to demonstrate initial deformities. The intermediate group was expected to demonstrate deformities during the active stage of the disease, corresponding to the fragmentation and healing stages in human LCPD. The late group was expected to demonstrate the residual deformities that can be observed after skeletal maturity.
Table 1. Categorization of 26 piglets according to postoperative periods
Both femora and acetabula were extracted from the killed piglets and they were examined macroscopically and radiographically, and stored there-after in 10% formalin for histological confirmation of the vascular infarct and its resulting deformities ().
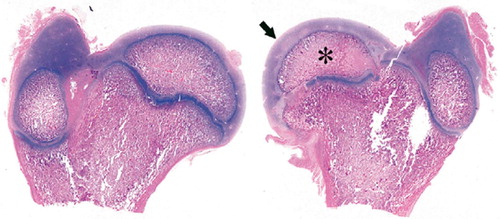
Figure 1. Histological specimen of right (Rt) and left (Lt) femurs extracted from a piglet killed at postoperative week 4.The left femoral head (Lt) showed avascular necrosis of the capital femoral epiphysis.Marked reduction in size of ossific nucleus (*) and considerable hyperplasia of articular cartilage (arrow) were seen in the operated femur. (Hematoxylin and eosin, ×1).
Observations and measurements
At the macroscopic and radiographic examinations, we evaluated 4 major patterns of response which are commonly observed in human LCPD (Shapiro Citation2001): coxa plana, coxa magna, coxa vara, and lateral subluxation of the femoral head. All measurements were performed using a Digimatic Caliper (Mitutoyo Co., Kawasaki, Japan).
Coxa plana was evaluated in 2 ways. Firstly, the decrease in the epiphyseal height in the radiograph was measured. The height of the epiphysis was the largest perpendicular distance between the physeal line and the highest point of the epiphysis. Coxa plana was defined as being when the epiphyseal height of the affected femoral head was 90% or less than the unaffected normal femoral head. Secondly, the flattening of the articular surface, as observed both grossly and radiographically, was divided into 2 groups: localized and generalized.
Coxa magna was defined as being when the widest measured diameter of the affected femoral head was at least 10% larger than that of the unaffected head.
Coxa vara was assessed by the overgrowth of the greater trochanter. This overgrowth was measured by a modified Keret and MacEwen (Citation1991) method to determine the trochanter-head relationship (articulotrochanteric distance, ATD). It was compared quantitatively by measuring the vertical epiphyseal-trochanteric distance on both sides, i.e. from the level of the tip of the greater trochanter to the level of the most proximal point of the epiphysis (Leitch et al. Citation1991). Trochanter overgrowth was considered to be present if the distance was reduced by 20% or more compared to the unaffected side. Trochanter overgrowth was considered to be mild if the level of the affected trochanteric tip was distal to the top of the epiphysis of the same side, but severe if the level of the trochanteric tip was proximal to the epiphysis.
The fourth pattern, lateral subluxation of the femoral head, was evaluated by the acetabulum head index (Heyman and Herndon Citation1950) on radiographs.
The experiment was approved by the Chonnam National University Committee for Animal Experimentation.
Statistics
Correlations were determined by the Spearman's rank correlation test (correlation coefficient rs). We used the SPSS software package (SPSS for Windows release 10.0; SPSS Inc., Chicago, IL). A p-value of less than 0.05 was considered to be statistically significant.
Results ()
4 patterns of femoral head deformity were observed.Coxa plana was first observed in 1 of the 6 P2 piglets, and then in 4 of the 6 P4 piglets, in 4 of the 7 P12 piglets, and in all 7 P20 piglets (rs = 0.55, p = 0.004). The mean decrease in epiphyseal height was 1.5 mm (14%), 4.1 mm (37%), 5.0 mm (33%), and 7.5 mm (43%) in the P2, P4, P12 and P20 groups, respectively (). The mean decrease in the epiphyseal height gradually increased with time (rs = 0.76, p = 0.002).
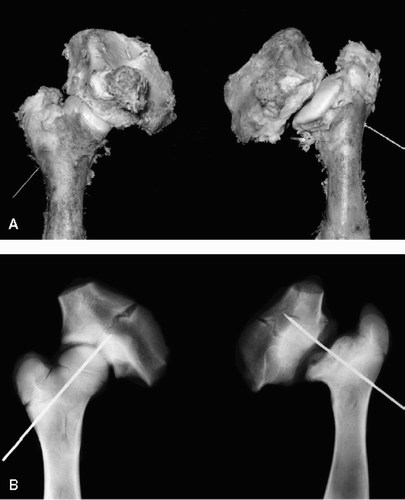
Figure 2. Gross (A) and radiographic (B) findings from extracted femurs from a piglet killed at postoperative week 20 showing (to the right) decreased epiphyseal height (44%), generalized flattening of the articular surface, coxa magna (16%), and severe GT overgrowth.
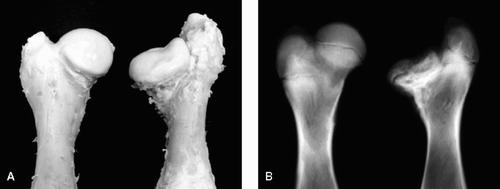
Table 2. Responses of the femoral head in 26 piglets with vascular infarct
Flattening of the articular surface was first observed in 1 of the 6 P2 piglets and became more common with time: 2 in the 6 P4 piglets, 4 in the 7 P12 piglets, and in all 7 P20 piglets (rs = 0.62, p = 0.001). Flattening of the articular surface could be divided into 2 patterns: localized and generalized. Localized flattening was more common in the early group, but in contrast, generalized flattening was more common in the intermediate and late groups.
Coxa magna was observed in 12 of the 26 piglets and was first observed in 1 of the 6 P4 piglets, and then in 5 of the 7 P12 piglets and in 6 of the 7 P20 piglets (rs = 0.70, p < 0.001). The mean increase in diameter in each postoperative group was 4.1 mm (16% of normal femoral head), 6.9 mm (21%), and 6.8 mm (18%) in P4, P12 and P20, respectively (rs = 0.22, p = 0.5). There was no increase of coxa magna from P12 (6.9 mm increase) to P20 (6.8 mm increase). According to both the radiographic and the histological findings, coxa magna at P4 was the result of hyperplasia of the articular cartilage covering the femoral head (). In contrast, coxa magna in the intermediate and late groups (P12 and P20) was the result of remarkable flattening and broadening of the epiphysis.
Decrease of ATD appeared from the early post-operative period and gradually increased to the final follow-up in P20. It was first observed in 4 of the 6 P2 piglets, and then in 4 of the 6 P4 piglets, in 6 of the 7 P12 piglets, and in all 7 P20 piglets (rs = 0.34, p = 0.09). The grade of reduction was mild in all 4 P2 piglets, mild in 2 and severe in 2 in P4, mild in 2 and severe in 4 in P12, and severe in all 7 P20 piglets.
Subluxation of the femoral head was observed only in P12 and P20 piglets (). Subluxation was observed in 5 of the 7 P12 piglets and in all 7 P20 piglets (rs = 0.84, p < 0.001).
Discussion
In our piglet model, coxa plana developed by two mechanisms: decrease of the epiphyseal height and flattening of the articular surface. Devascularization of the femoral head causes necrosis. Subsequently, the necrotized femoral head ceases to grow and becomes fragile. As the piglet continues to bear its body weight, the ossific nucleus may collapse. With continued walking, there can be flattening of the articular surface. In a piglet model, Kim and Su (Citation2002) observed mild femoral head flattening at 4 weeks after the induction of ischemia, and severe flattening at 8 weeks, but they did not describe changes after 8 weeks and until the full growth of the piglet. We found earlier development of coxa plana: both decrease in epiphyseal height and flattening of the articular surface in 1 of the 6 P2 piglets (i.e. 2 weeks after operation). The incidence and severity of coxa plana gradually increased with time. The mean decrease in epiphyseal height at skeletal maturity was 7.5 mm—almost one-half of the normal unaffected epiphyseal height. The flattening of the articular surface in the early period was localized to the superolateral portion of the articular surface, which was pressed by the acetabular margin, and became generalized with time.
Many causes for coxa magna in LCPD have been proposed: hyperplasia of the cartilaginous part of the epiphysis (Abril et al. Citation2000, Yazici et al. Citation2002), increase in the local blood supply (Powers and Bach Citation1977), or a compensating growth process of the femoral neck (Meyer Citation1977). We observed a decrease in epiphyseal height which occurred in the early period after operation and became more pronounced with time. Interestingly, this decrease in the epiphyseal height was closely related to coxa magna in terms of the time of appearance and extent, which suggests that the decrease in the epiphyseal height may in part explain the increase in its diameter. A compression force applied to the plastic epiphysis might cause these changes. Reactive hypertrophy of the cartilaginous part and also mechanical compression play an important role in early onset of coxa magna. A compensating growth process of the femoral neck, in order to maintain normal sphericity despite epiphyseal flattening, is a contributory factor in the late remodeling stage of the disease.
Coxa vara is a frequent residual deformity of the femoral neck in patients with LCPD. Salter and Thompson (Citation1984) stated that pathogenesis of femoral neck deformity is a result of ischemia of the physeal plate. Diminished growth and premature fusion of the physis of the capital femoral epiphysis in association with continued normal growth of the greater trochanteric epiphysis could change the relation between the tip of the trochanter and the most superior portion of the femoral head, such that the trochanter is found to be at the level of, or higher than, the articular cartilage of the head (Shapiro Citation2001). In our study, the decrease in ATD appeared from the early postoperative period and became gradually more severe toward the final week 20. Generally speaking, the decrease was mild in the early period after surgery and became severe in the intermediate and late periods after the surgery.
Subluxation of the femoral head is a frequent abnormal finding observed both in the early and late stages of human LCPD. In the early stage of the disease, subluxation develops because of hypertrophy of the synovium and hyperplasia of the cartilage. In the late stage, subluxation is caused by the disproportion between the femoral head and the acetabulum because of coxa magna (Shapiro Citation2001). In our experimental study, the cause of subluxation was not found to be similar to that in human LCPD. Subluxation of the femoral head was observed only in P12 and P20 piglets. The hips with subluxation showed remarkable deformation in both the acetabulum and the femoral head.
The body weight of the piglets was 7–9 kg when the operation was done, and it increased to 15 kg at P2, 22 kg at P4, 62 kg at P12, and 107 kg at P20. This is a 13-fold increase in body weight over the 20 weeks, whereas the length of the total femur only doubled during this time.
The mean body weight of Korean children is 23 kg at the age of 7 years, which corresponds to the mean age of disease onset, and 64 kg at age 20—only a 3-fold increase in body weight over 13 years (Lee et al. Citation2002). The greater increase in body weight in piglets might cause a total collapse in such a deformed hip, as was observed at P12 and P20.
In conclusion, we were able produce a pathoanatomical condition similar to human LCPD by disrupting the vascular supply to the capital femoral epiphysis in the piglet. The piglet model of human LCPD may be useful for investigation of the developmental pattern of femoral head deformity following devascularization. However, it must be remembered that the macroscopic and radiographic simulation of pathoanatomy was satisfactory only in the early stage of devascularization, when piglets are not so heavy. When the piglets have grown to skeletal maturity, the femoral head and acetabulum become flattened and collapsed.
No competing interests declared.
Author contributions
SMR: study conception and design, data analysis and writing the manuscript. JJL and HYS: experimental surgery and measurements and observations of material and reference review. JYC, ESM, EKS: data anlysis, interpretation and critical review of the manuscript.
- Abril J C, Ferrer A, Castillo F, Ferrer-Torrelles M. An intraarticular hip process with chondrolysis simulating Perthes disease: a report of five cases. J Pediatr Orthop 2000; 20: 729–35
- Babyn P S, Kim H K W, Gahunia H K, Lemaire C, Salter R B, Fornasier V, Pritzker K P. MRI of the cartilaginous epiphysis of the femoral head in the piglet hip after ischemic damage. J Magn Reson Imaging 1998; 8: 717–23
- Heyman C H, Herndon C H. Legg-Perthes disease. J Bone Joint Surg (Am) 1950; 32: 767–78
- Keret D, MacEwen G D. Growth disturbance of the proximal part of the femur after treatment for congenital dislocation of the hip. J Bone joint Surg (Am) 1991; 73: 410–23
- Kim H K W, Su P H. Development of flattening and apparent fragmentation following ischemic necrosis of the capital femoral epiphysis in a piglet model. J Bone Joint Surg (Am) 2002; 84: 1329–34
- Lee D Y, Choi I H, Chung C Y, Cho T J. Synopsis of pediatric orthopaedics, 2nd ed. Newest medical publishing company. Seoul 2002; 561–4
- Leitch J M, Paterson D C, Foster B K. Growth disturbance in Legg-Calvé-Perthes disease and the consequences of surgical treatment. Clin Orthop 1991, 262: 178–84
- Meyer J. Legg-Calvé-Perthes' disease. Radiological results of treatment and their late clinical consequences. Acta Orthop Scand (Suppl 167), 1977: 3–131
- Powers J A, Bach P J. Coxa magna. South Med J 1977; 70: 1297–9
- Rowe S M, Park S K. Necrosis of femoral head in growing rabbit. J Korean Orthop Assoc 1986; 1: 29–34
- Salter R B, Thompson G H. Legg-Calvé-Perthes disease: the prognostic significance of the subchondral fracture and a two-group classification of the femoral head involvement. J Bone Joint Surg (Am) 1984; 66: 479–89
- Sanchis M, Zahir A, Freeman M A R. The experimental simulation of Perthes disease by consecutive interruptions of the blood supply to the capital femoral epiphysis in the puppy. J Bone Joint Surg (Am) 1973; 55: 335–42
- Shapiro F. Legg-Calve-Perthes disease. Pediatric orthopedic deformities, F Shapiro. Academic Press, San Diego 2001; 272–375
- Yazici M, Aydingöz Ü, Aksoy M C, Akgün R C. Bipositional MR imaging vs. arthrography for the evaluation of femoral head sphericity and containment in Legg-Calvé-Perthes disease. Clin Imaging 2002; 26: 342–6