Abstract
Today, aseptic loosening is the most common cause of revision of major arthroplasties. Aseptic loosening accounts for more than two-thirds of hip revisions and almost one-half of knee revisions in Sweden. Several theories on the cause of aseptic loosening have been proposed. Most of these theories, however, are based on empiric observations, experimental animal models or anecdotal cases. In this review, we discuss the most common theories concerning aseptic loosening. It emerges from this review that aseptic loosening has a multifactorial etiology and cannot be explained by a single theory.
Today, total hip replacement (THR) and total knee replacement (TKR) are being performed world-wide with excellent results. Approximately 13,000 THRs and 6,000 TKRs are performed annually in Sweden. Aseptic loosening is the major complication and causes more than 70% of the revisions of hips in Sweden (Herberts and Malchau Citation2000) and 44% of knee revisions (Robertsson et al. Citation2001). Revisions, however, are only performed in 8–9% of hip arthroplasties (Herberts and Malchau Citation2000) and 8% of knee arthroplasties (Robertsson et al. Citation2001). Prophylaxis against infection and thrombosis has reduced the risk of complications that might otherwise endanger the life of the implanted patient. Some major problems remain to be solved. Revision surgery is dangerous for the patient, and is both expensive and time-consuming. In addition, the results are not as good as with primary arthroplasties. Great efforts are needed to reduce the revision rate further, and every small improvement will be an important step forward.
Introduction of new implants must be performed stepwise in order to avoid potential disasters (Malchau Citation1995, Kärrholm Citation2003). New implants must be evaluated before being introduced clinically on a large scale. Implant register studies are a slow and blunt instrument; radiographic findings are more precise at predicting early problems. Radiostereometric analysis (RSA) (Selvik Citation1989), which can predict the outcome of an implant after 2 years (Ryd Citation1992), is even more precise. Histological evaluation based on post-mortem retrieved well functioning implants adds knowledge of the implants before the clinical signs of failure. However, very few of these studies exist in the literature, perhaps because they are difficult to perform in a standardized manner.
Data from the national hip registry of Sweden uses revision as a criterion of failure or endpoint, which implies an overestimation of the true success rates. Hence, some patients with loose implants are not taken into account in such register studies. Söderman et al. (Citation2001) found a 20% failure rate in hip arthroplasties using a disease-specific self-administered questionnaire (WOMAC), indicating that revision as a criterion of failure results in an underestimation of the true failure rate. Kesteris et al. (Citation1998) investigated the outcome of a hip prosthesis by registry studies and found a revision rate of 5.6% over a 10-year period, indicating excellent long-term results. A radiographic investigation of 70 hip arthroplasties from the same cohort as the latter study demonstrated that one-third of the hips fulfilled radiographic criteria for aseptic loosening after 10 years (Iwase et al. Citation2002). Thus, revision rate is a blunt instrument when considering long-term results and should be complemented with radiological, clinical and histological investigations.
Several comprehensive reviews and theses on the topics of bone cement (Morberg Citation1991, Lewis Citation1997), polyethylene (Lewis Citation2001), wear particles (Campbell Citation1995, Bauer Citation2002) and aseptic loosening (Schwarz et al. Citation2000a, Jacobs et al. Citation2001) have been published. The aim of this article is to present an up-to-date review on the topic of aseptic loosening.
Theories of aseptic loosening
Extensive localized bone resorption resulting in loosening without infection was described by Harris et al. (Citation1976) in 4 hip arthroplasties. Osteolysis may be linear, i.e. equally distributed around the implant, or focal, i.e. islands of bone loss in close relation to the implant. Focal osteolysis may occur with no symptoms at all and the implant may remain stable.
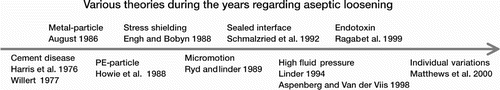
There are several theories about the causes of aseptic loosening (), i.e. osteolysis resulting in loosening of the implant (linear or focal, or a combination of the two). The different theories will be discussed in detail below (). The first and—for several years—dominant theory was the particle disease theory, which could be divided into subgroups (see below). The effect of this theory was the development of uncemented implants, but the outcome was no better than with the early cemented systems—which indicated that the reasons for aseptic loosening are most probably multifactorial.
Figure 2. Drawing of a knee prosthesis with examples of different pathways leading to aseptic loosening.
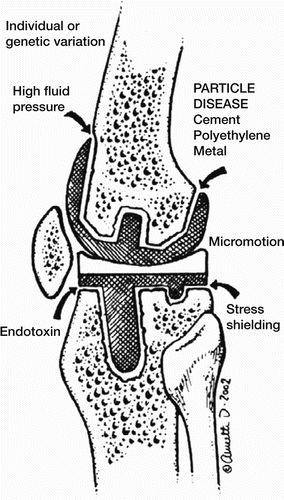
Schmalzried et al. (Citation1992a) described the effective joint space. They hypothesized that wear particles from the polyethylene cup are dispersed into the joint fluid directly or that cement particles are generated at the interface by debonding or cement fractures; this could cause third-body wear and accelerated PE wear at the articulation. Access to the joint fluid for the wear particles is dependent on the contact between implant and bone, or between implant and cement, if present, and bone. Wear debris activates macrophages, which activate osteoclasts or become osteoclasts themselves and initiate bone resorption. The resulting bone loss will enlarge the interface and ease the flow of joint fluid, resulting in higher transportation capacity of the debris and gradual loosening of the implant.
Support for the above theory concerning effective joint space comes from the authors’own study on cemented acetabular components retrieved at autopsy (Schmalzried et al. Citation1992b). Autopsy studies are important, since the well-functioning implant can be studied when no secondary changes suchas osteoporosis and osteolysis have appeared. Results from such studies have indicated that debonding and cement fractures are important factors in the failure of cemented implants of the femur (Collier et al. Citation1992, Schmalzried et al. Citation1992b, Citation1993). It is obvious that loosening starts long before radiological and clinical signs appear. On the acetabular side, the earliest sign of loosening is resorption of the bone immediately adjacent to the cement mantle around the cup (Schmalzried et al. Citation1992b, Citation1993). Schmalzried et al. (Citation1993) proposed that loosening of an acetabular component is a biological event resulting from wear particles, while loosening of the femoral component is mainly mechanical.
A high pressure theory was presented by Aspenberg and Van der Vis (Citation1998a), and it is most likely that high pressure has a role in the pathogenesis of aseptic loosening (see below). According to the theory, the high pressure of the joint fluid moves the wear debris to the effective joint space where bone cells and the macrophages become exposed to it.
Particle disease
Several animal studies have addressed the question of particles alone being responsible for bone resorption around implants. However, as shown in Table, the amounts and sizes of particles have varied between the different studies (Howie et al. Citation1988, Goodman Citation1994, Bobyn et al. Citation1995, Dowd et al. Citation1995, Frökjaer et al. Citation1995, Goodman et al. Citation1995, Citation1999, Kraemer et al. Citation1995, Allen et al. Citation1996, Aspenberg and Herbertsson Citation1996, Shanbhag et al. Citation1997, Van Der Vis et al. Citation1997, Kim et al. Citation1998, Frökjaer et al. Citation1999, Lalor et al. Citation1999, Brooks et al. Citation2000, Rahbek et al. Citation2000, Citation2001, Bechtold et al. Citation2001, Bi et al. Citation2001b, Sundfeldt et al. Citation2002). The time of introduction of the particles and the design of the studies also varied greatly.
Cement
Polymethylmethacrylate (PMMA) or bone cement as a fixation medium for hip prostheses was first described by Haboush (Citation1953), and later developed by Charnley (Citation1960). The long-term results were less satisfactory initially, but are excellent nowadays—at least for the elderly, not very active patient. Cementation is currently the main method for fixation of prosthetic components in both hips and knees in Sweden. Improvements in the handling techniques such as vacuum mixing, compression with a cement gun and precooling of the cement, combined with improvements in surgical technique such as medullary canal plugging and high-pressure lavage of the trabecular bone, are part of the reason for this popularity. Cement failure has been investigated, and small cement fractures are thought to play a significant role in the initiation of failure (Willert et al. Citation1990, Jasty et al. Citation1991). Debonding between cement and implant is also thought to reduce the longevity of the implant fixation (Willert et al. Citation1990, Jasty et al. Citation1991). In hips, the stresses between stem implant and cement are mostly shear, whereas compressive stress forces prevail in the acetabulum. In polished stems, where debonding occurs more easily, the forces transmitted to the cement are different from those in precoated or blasted stems designed to avoid debonding (Lu and McKellop Citation1997, Lennon and Prendergast Citation2001, Nuno and Amabili Citation2002). This may affect the production of cement wear particles, resulting in a different size distribution as well as a different effective joint space for distribution of the particles. In the knee implant, the forces are mainly compression and a knee implant is not designed to allow any subsidence except during the initial bone remodeling (Ryd Citation1986, Ryd and Linder Citation1989) and with polyethylene creep.
Air bubbles in the cement may initiate fracture, and different techniques have been tested to reduce the porosity of the cement (Lewis Citation1997). However, porosity may have a positive function: when a cement fracture reaches the pore it can stop and fade out in the pore (Topoleski et al. Citation1993, Lewis Citation1997). Despite this finding, the current opinion is to reduce the porosity as much as possible (Lewis Citation1997).
The theories about reasons for failure of the implant have been discussed for almost three decades. Wear particles from cement, metal and polyethylene (PE) are claimed to play a major role in aseptic loosening. Willert (Citation1977) discussed the balance between production and elimination and hypothesized that when the equilibrium between production and elimination is disturbed, the accumulation of particles in the joint will cause loosening of the prosthesis. Several authors have found a correlation between loosening of prostheses and the amount of wear products produced from bone cement and PE (Harris et al. Citation1976, Mirra et al. Citation1982, Goldring et al. Citation1986, Howie et al. Citation1993, Mjöberg Citation1994). As cement ages, it becomes more brittle—which could be a source of cement particles. Harris et al. (Citation1976) reported 4 cases with extensive osteolysis in cemented hip artroplasties without any signs of infection. They postulated that the fibrous tissue between the bone and the cement could play a major role in the initiation of the osteolysis. In a retrieval study, Jasty et al. (Citation1986) reported 4 cases with bone resorption in a cemented hip replacement with no evidence of infection. The histomorphometrical analysis revealed polymethylmethacrylate debris in the tissues and the authors proposed that micromotion initiates cement failure, with a subsequent release of cement particles. This will in turn cause a foreign body reaction, initiate osteolysis and lead to loosening. Jones and Hungerford (Citation1987) proposed that cement particles were responsible for the loosening of joint implants, and suggested the uncemented alternative for younger patients with higher levels of activity. Willert et al. (Citation1990) investigated tissue from revised hip arthroplasties and found bone cement particles and PE particles in histiocytes and foreign-body giant cells. They proposed a chronological order or cascade, since they found different layers in the granulomatous tissue containing cement or PE particles. This stratification indicated that the tissue is first introduced into cement and then as an effect of the third-body wear on the polyethylene bearing, PE particles are produced. These authors postulated that the amount of wear particles is probably as important as the material of the particles, and suggested that together, cement particles and PE particles were responsible for the aseptic osteolysis. In their model PE particles promote granuloma formation and bone resorption, leading to aseptic implant loosening.
Table. Selected studies where different materials in different animal models were studied
Jasty et al. (Citation1991) investigated 16 well-fixed implants retrieved at post-mortem. They concluded that the fibrous tissue around a loose implant was secondary to the loosening, and not one of the main reasons for the start of prosthetic loosening—as proposed by Goldring et al. (Citation1983, Citation1986). Goldring et al. found that debonding of the cement was common even early after surgery and increased the longer the implant had been in situ, leading to an open interface. This would expose it to joint fluid, thus increasing the effective joint space. Cement fractures were common, and the incidence increased the longer the implant had been in place. With time, these fractures may result in fragmentation of the bone cement, leading to cement particles in the interface and third-body wear of the PE bearing—resulting in PE particles and aseptic loosening.
Polyethylene
Polyethylene is the most common material for one bearing surface in both total hip replacements (THRs) and total knee replacements (TKRs). The resistance of ultra-high-molecular-weight polyethylene (UHMWPE) to wear and fragmentation depends on many factors including the type of resin, the manufacturing method (ram extrusion, compression molding to sheet or compression molding direct to product shape), the temperature and pressure during processing, methods of sterilization (gamma irradiation in air or in the absence of oxygen, or ethylene oxide), crosslinking by gamma irradiation, and storage after manufacture (shelf life).
PE ages by oxidative degradation, which decreases the molecular weight and increases the density, crystallinity, and elastic modulus. Reducing the oxidative degeneration is important when trying to maintain the optimal properties of polyethylene. The surface of the PE must be as smooth as possible in order to reduce friction and wear against the femoral head or femoral condyles.
Howie et al. (Citation1988) presented a study in rats in which a plug of polymethylmethacrylate was inserted into each femur and then exposed to ultra-high-molecular-weight polyethylene particles 2, 4, 6 and 8 weeks after insertion. The aim was to mimic the bone-cement interface in cemented implants. Bone resorption was found around the implant, and at the interface the bone had been replaced by connective tissue. Based on their findings, these authors postulated that particles alone could cause bone resorption in the absence of motion and infection. These results have not been reproduced, however (Howie et al. Citation1993, Van Der Vis et al. Citation1997).
It is very difficult to make any clear connection between PE particles and bone resorption based on animal studies (Howie et al. Citation1988, Søballe et al. Citation1992, Bobyn et al. Citation1995, Dowd et al. Citation1995, Allen et al. Citation1996, Van Der Vis et al. Citation1997, Aspenberg and Van der Vis Citation1998b, Kim et al. Citation1998, Frökjaer et al. Citation1999, Lalor et al. Citation1999, Brooks et al. Citation2000, Rahbek et al. Citation2000, Sundfeldt et al. Citation2002).
Our group (Sundfeldt et al. Citation2002) investigated the effect of repetitive injections of UHMWPE particles in rabbit knees with a weight-bearing, articulating osseointegrated prosthetic joint. We used endotoxin-free UHMWPE particles from a hip simulator with an average diameter of 0.2 mm. 200 mg of UHMWPE particles was injected into each left knee joint every second week. The right knee served as a control, and was injected with saline only. The follow-up times were 26 and 42 weeks. Despite this high load of particles, no radiographic, histological or biomechanical differences between test and control were detected, and we concluded that additional factors are required to initiate the loosening process. Dowd et al. (Citation2000) proposed that PE wear is linear in hip arthroplasty, and they also found a significant correlation between osteolysis and PE wear in a human study. When investigating Charnley arthroplasties, Sochart (Citation1999) found that wear rates below 0.1 mm per year resulted in more than 90% survival of the implant, whereas annual wear greater than 0.2 mm resulted in no implant survival up to 25 years. These two studies imply that loosening of the implant may be affected by the wear rate, and thus particles appear to be involved in the loosening process. PE particles are phagocytosed by macrophages, which act in two major ways in the bone remodeling process. Firstly, they release different cytokines involved in bone remodeling, such as prostaglandin E2 (PGE2), interleukin 1α(IL1α, Interleukin 1ß (IL1ß, Interleukin 6 (IL 6) and tumor necrosis factor α(TNFα). These cytokines modulate osteoblast and osteoclast activity, which in turn increases the osteolysis (Murray and Rushton Citation1990, Howie et al. Citation1993, Kim et al. Citation1998). Secondly, macrophages may differentiate into osteoclasts as a result of stimulation by PE particles (Quinn et al. Citation1998, Sabokbar et al. Citation1998) affecting the bone tissue directly.
PE particles produced in artificial hip and knee joints vary in size and shape (Landy and Walker Citation1988), but it has been suggested that the effect on the macrophages and bone remodeling is caused by the submicronsized particles (Green et al. Citation1998) that are present in both kinds of joint. Since the knee joint is larger, however, the time to reach the critical dose may be longer than in the hip. Aseptic loosening in knee arthroplasties is not as well studied as in hips. The mode of wear is different (Landy and Walker Citation1988), and modularity seems to play a more important role regarding PE wear than in the hip. In the 1970s and early 1980s, osteolysis in the knee was not recognized; nor did modularity exist at that time. Modularity leads to wear on both the articular side and the back side of the PE tray, thus generating PE particles (Parks et al. Citation1998, Engh et al. Citation2001). The locking mechanism of many tibial trays may be insufficient to prevent motion of the PE tray and back-side wear.
It seems, however, that aseptic loosening is also linked to high fluid pressures, as discussed below (Aspenberg and Van der Vis Citation1998 a, Citationb, Van der Vis et al. Citation1998 a Citationb), and since the knee joint is much larger than the hip joint, high pressure is less likely to occur. PE wear in the artificial knee occurs by abrasion caused by direct contact (second-body wear), back-side wear when using a metal-backed component (acetabular shell or tibial tray), or from third-body wear. Fatigue wear of the PE due to poor PE resistance, manufacturing properties, and high stress on the PE surface can result in delamination.PE wear also depends on aging of the PE (shelf life): oxidative degradation occurs when storing the implants and shelf life is thus a factor to consider when discussing wear resistance and implant longevity, as well as the type of sterilization chosen for the implant.
Wear resistance of the PE can be improved by cross-linking which is achieved either chemically or by radiation. Radiation sterilization of PE results in cross-linking, but recent research has focused on increasing the cross-linking and the wear resistance of PE by use of radiation of higher doses than used for sterilization (Lewis Citation2001). In vitro results have been promising, and a controlled randomized clinical 2-year follow-up study from our department evaluating cross-linked PE has demonstrated less penetration (measured by RSA), indicating less wear than conventional PE (Digas et al. Citation2003). However, whether or not this would lower the frequency of aseptic loosening remains to be investigated.
In many earlier published studies, it is unclear how the amount of particles exposed to the bone tissue and the implant was calculated. Several studies used the calculations from a radiographic study by Livermore et al. (Citation1990), who found a mean wear rate of 84 mm3 per year in hips. Other clinical studies have found wear in hips varying between 34 and 140 mm3 per year (Jasty et al. Citation1997b, Schmalzried et al. Citation2000, Oparaugo et al. Citation2001). More recent studies performed with mathematical models in combination with artificial hip joint simulators found wear rates varying from 6 mm3 (Pietrabissa et al. Citation1998) to 67 mm3 per year (Raimondi et al. Citation2001). PE particles may be one of the causes of aseptic loosening. However, additional factors are probably required to induce it.
Metal
Because of the indications that PE particles are responsible for aseptic loosening, the interest has returned to metal-metal joint arthroplasties. Metal-to-metal implants have less wear than metal-to-polyethylene implants, and earlier results have been optimistic regarding wear and long-term results. However, aseptic loosening still occurs (August et al. Citation1986, Visuri Citation1987, Jantsch et al. Citation1991, Jacobsson et al. Citation1996, Schmalzried et al. Citation1996). Metal-to-metal implants are highly durable against wear, and newer designs may give better long-term results than old ones (McKellop et al. Citation1996, Willert et al. Citation1996, Higuchi et al. Citation1997). With cobalt-chromium alloys, the wear resistance is high but corrosion resistance is low. Modern implants with metal-to-metal articulation have been reported to have good medium-term results (Dorr et al. Citation2000). Metal wear from titanium implants has been suggested to be responsible for aseptic loosening (Agins et al. Citation1988, Lombardi et al. Citation1989, Buly et al. Citation1992, Huo et al. Citation1992). However, today titanium alloys are used with increased resistance to debris production. In an in vitro study by Rader et al. (Citation1999), cytokine release associated with PE particles was more pronounced than that associated with titanium particles. Blaine et al. (Citation1996) reported that cytokines were released from macrophages after exposure to endotoxin-free titanium particles.
Doorn et al. (Citation1998) studied cobalt-chromium particles retrieved from metal-metal total hip arthroplasties, and they also reported less local activity caused by metal particles than caused by PE-particles. These authors discussed different explanations for their findings. Since particles from metal wear are small (10–400 nm), every macrophage can store more particles—which means that fewer macrophages will be required to store the total amount of particles, and thus fewer cells become activated. Alternatively, metal particles can corrode and disappear, and since they are so small they are more easily excreted from the body. Metal wear particles (aluminium, titanium, cobalt and chromium) may have carcinogenic potential, but metal particles may also affect hematopoesis and intellectual capacity (Jacobs et al. Citation1998). Langkamer et al. (Citation1997) reported of a correlation between metal implants and malignant disease, but there is no evidence that metal wear from joint implants can cause such disease (McGregor et al. Citation2000, Signorello et al. Citation2001, Fryzek et al. Citation2002, Visuri et al. Citation2003). Metal particles also cause third-body wear by themselves, resulting in more wear particles. In summary, one can assume that despite the mild foreign body reaction caused by titanium and cobalt-chromium particles, even these particles induce cytokine release from macrophages—which means that metal particles may also be involved in the process of aseptic loosening.
Ceramic
The particles from ceramic implants are insoluble, and the biological response is probably more a result of the particles than the ceramic material itself. The periprosthetic reaction to ceramic particles has been described by Hatton et al. (Citation2002). There have been studies proposing more cytotoxic effects of particles generated when zirconia ceramic articulates against polyethylene than when titanium articulates against polyethylene (Ito et al. Citation1993). On the other hand, there has been one animal study demonstrating less bone resorption and a lower inflammatory respone to ceramic particles compared to polyethylene and titanium particles (Warashina et al. Citation2003). Osteolysis has been described in ceramic-on-ceramic hip artroplasties (Hatton et al. Citation2002). It is most likely that ceramic particles of a critical size will cause a biological response irrespective of the composition of the material (Green et al. Citation1998). In an in vitro study, Hatton et al. (Citation2003) demonstrated that alumina ceramic wear particles are capable of inducing osteolytic cytokine production by human mononuclear phagocytes. However, the doses of particles were very high and this dose would most likely not occur in the clinical situation. Even if the amounts of ceramic particles being generated in a joint arthroplasty are very small, however, they may still be involved in the process of aseptic loosening.
Mechanisms of cell activation in particle disease
One possible method of preventing aseptic loosening of total joint replacements is inhibition of activated macrophages. The activation of different signaling pathways within the cell may alter and reduce the production of cytokines (Blaine et al. Citation1997).
Phagocytosis of particulate matter is associated with the expression of the major histocompatibility complex HLA-DR on macrophages (Hicks et al. Citation1996). HLA-DR is an immunoglobulin-like molecule involved in the presentation of antigens to T-lymphocytes. The expression of HLA-DR is part of the normal physiological response of immunologically activated macrophages after particle-phagocytosis. This is also indicated by the secretion of TNF-αwhich, as in other inflammatory disorders, appears to be important in the response to particles (Blaine et al. Citation1996). The two major signaling pathways include the protein kinase A- and the protein kinase C-mediated pathways. Activation of these pathways results in a cascade of events leading to either enhancement or suppression of gene expression. One of the well-known mediators of TNF-αinduction is the nuclear transcription factor-κB (NF-κB) and activator protein-1 (AP-1). NFκB is found in the cytoplasm and is composed of a heterodimer of p50 and p65. NF-κB is sequestered in the cytoplasm by an inhibitor κBα(IκBα), which binds to NF-κB and prevents its translocation to the nucleus (Chen and Ghosh Citation1999). TNF-αinduction seems to be affected in some partof NF-κB binding to the kB2 site of the TNF-αpromotor (Soloviev et al. Citation2002). An important issue in order to find therapeutic strategies is to determine the cellular response to wear particles with different properties. An in vitro study showed that phagocytosis of titanium particles by macrophages was not necessary for induction of TNF-αor IL-6 release. A ligation of the macrophage CD11b/CD18 complement receptors by specific antibodies against integrin receptors resulted also in incrased cytokine secretion. Particle-induced release of TNF-αand IL-6 did not require phagocytosis but appeared to be dependent on tyrosine kinase and serine/threonine kinase activity, resulting in upregulation of NF-κB expression (Nakashima et al. Citation1999). However, the size and concentration of particles influence macrophage activation and cytokine levels (Thomsen and Gretzer Citation2001). The membrane receptor may be inactivated above or below a certain size and concentration of particles. Activation of the receptor induced activation of the transcription factor NF-κB and nuclear factor IL-6 (NF-IL-6) within 1 h after exposure to titanium particles (Nakashima et al. Citation1999). Different signaling pathways are involved in macrophage activation by either particles or bacterial antigens. An in vivo model using titanium particles implanted in mouse calvarias was used by Schwarz et al. (Citation2000 a, Citationb) and showed activation of NF-κB and increased production of TNF-α.The results also showed that TNF-αdirectly induces fibroblast proliferation and tissue fibrosis, and recruitment and activation of osteoclasts (Schwarz et al. Citation2000 a, Citationb). Anti-TNF-αhas been used clinically for inhibition of periprosthetic osteolysis (Schwarz et al. Citation2000 a, Citationb). NF-κB has been shown to be required for osteoclast development (Franzoso et al. Citation1997). Lipopolysaccharide (LPS) appears to activate pathways other than the ones activated by particles:both agonists cause NF-κB activation, but LPS has an inhibitory effect on IκB (Soloviev et al. Citation2002).
Metal ions also have an effect on macrophage activation, as shown for example in an in vitro study in which cells were exposed to titanium, chromium and cobalt (Wang et al. Citation1996). The results showed different patterns of IL-1ß and TNF-αexpression, but an inhibited release of transforming growth factor-ß1 (TGF-ß1) irrespective of the metal used. Soluble metal ions can penetrate cells and bind to kinase or nuclear factors involved in signal transduction and cytokine gene expression. NF-κB can also be seen as an anti-apoptotic signaling protein, decreasing the inflammatory reaction and cytokine secretion in order to protect against cell death (Ghosh et al. Citation1998).At present, the role of metal ions and metal particles in signal transduction and gene expression is largely unknown. There is growing evidence, however, that an imbalance of bone-resorbing cytokines and bone-forming cytokines plays an important role in osteolysis.
Micromotion
Micromotion, or inducible movements, was defined by Goodman (Citation1994) as: “Small movements between a prosthesis (whether cemented or noncemented) and the surrounding bone, that are not detectable with conventional radiographic methods.” The golden standard method for verification of micromotion in vivo in a human joint implant is radiostereometric analysis (RSA). RSA is a valuable diagnostic and prognostic tool for joint implants (Selvik Citation1989). Once motion actually commences, however, many events have already occurred at the prosthesis-tissue interface. It seems likely that if micromotion is diagnosed by RSA, osseointegration has not occurred in an uncemented implant, no microlocking has occurred in a porous cemented implant, and debonding has occurred in a smooth cemented implant. Ryd and Linder (Citation1989) studied 3 cases of cemented knee arthroplasty by both RSA and histology. They found that initially all implants migrated up to 3 years postoperatively before migration stopped, and that at the endpoint, all tibial implants were firmly attached to the bone by soft tissue. The initial micromotion in the first years (as found by RSA) may be due to several causes: bone deformation caused by bone resorption or soft tissue deformation in the fibrous capsule around the cemented implants. Kärrholm et al. (Citation1994) found that femoral stem subsidence exceeding 1.2 mm was associated with a revision risk of more than 50%. Ryd (Citation1992) proposed that RSA findings could predict implant loosening in both knees and hips. Using a bone chamber, Goodman (Citation1994) reported from experiments on rabbits that a short period with micromotion once a day was enough to prevent osseointegration. In another rabbit study using a bone chamber, Aspenberg et al. (Citation1992) demonstrated that micromotion inhibits bone formation.
The surgical technique is important—and if malalignment occurs, the risk of micromotion increases. Motion in an implant postoperatively may jeopardize the long-term result; thus, the initial stability achieved during surgery is vital. This situation is analogous to fracture healing, where stability is vital for healing and for long-term stability. Without stabilization of a fracture or an implant, the healing process will not occur properly, resulting in callus/fibrous tissue with no mechanical strength. On the other hand, micromotion may even occur in stable osseointegrated implants, as demonstrated by Engh et al. (Citation1992b). These authors described autopsy material of 14 femoral stems and found that the osseointegrated implants had a maximum relative motion between bone and the implant of 40 μm. This micromotion was caused by elastic displacement, however. Using a canine model, Jasty et al. (Citation1997a) demonstrated that 20 μm of oscillating motion allowed osseointegration, but at 40 μm the implants were surrounded by a fibrocartilage or fibrous tissue in some areas. This implies that the limits of osseointegration (in dogs) are between 20 and 40 μm of oscillating motion.
In summary, it seems likely that micromotion affects implant stability and it may open the interface to joint fluid and wear products, leading to harmful effects on the bone bed and subsequent loosening of the implant.
Stress shielding
Insertion of an implant in the knee or hip leads to remodeling of the bone as a result of the new loading conditions imposed by the implant. This can lead to bone loss around the implant in areas not subjected to loading, and is usually referred to as stress shielding (Oh and Harris Citation1978). The balance between loading conditions and bone remodeling is called Wolff’s Law: “Every change in the form and function of a bone or of their function alone is followed by certain changes in their internal architecture, and equally definite secondary alteration in their external conformation, in accordance with mathematical laws” (Wolff Citation1870). This implies that bone remodeling adapts the bone tissue depending on loading conditions i.e. when an implant is inserted. However, bone loss after joint implants is generalized in the whole limb, which can be a confounding factor when analyzing stress shielding (Bryan et al. Citation1996). Stress shielding leads to bone loss, which is not a result of osteolysis but of bone remodeling. The extent of bone remodeling depends on several patient-related and implant-related factors such as age, sex, medication, time of primary surgery, activity level, weight, stem stiffness, stem diameter, porous coating and bone quality.
Stress shielding around an implant can be observed in conventional radiographs but this is a poor method for examining bone content (). Dual energy X-ray absorptiometry (DXA) is also used, and is more precise than plain radiographs. Triple-energy radiograph absorptiometry (Adolphson et al. Citation1994, Neander et al. Citation1997, Swanpalmer et al. Citation1998 a, Citationb) was used by Regner et al. (Citation1999) in a knee study and may be better than DXAsince it can be used to measure bone mineral in the presence of different amounts of homogenous and non-homogenous adipose tissues close to the measured bone. Stress shielding is diagnosed as less bone mineral adjacent to the implant. The topic has been investigated and discussed in several studies (Engh and Bobyn Citation1988, McCarthy et al. Citation1991, Engh et al. Citation1992a, Maloney et al. Citation1996, Bugbee et al. Citation1997, Van Lenthe et al. Citation1997). Stress shielding around a femoral intramedullary implant may depend on the stiffness of the implant (Engh et al. Citation1987, Engh and Bobyn Citation1988, Wan et al. Citation1999); it has been demonstrated in dogs that a small implant without press-fit distally results in less stress shielding than a press-fit implant (Engh et al. Citation1987, Niinimaki et al. Citation2001). Harvey et al. (Citation1999) investigated the effect of bony ingrowth and stress shielding on femoral stem stiffness. They found that a highly flexible stem resulted in less bony ingrowth and more fibrous ingrowth around the implant. However, they could not find any significant differences from femoral stress shielding. Stress shielding may facilitate wear particle-induced loosening (McCarthy et al. Citation1991). Bone loss adjacent to the proximal part of the femoral hip implant may open the interface to wear particles (Engh and Bobyn Citation1988, Maloney et al. Citation1996) and expose the interface to wear and joint fluid. Support for osteoporotic bone would become more osteoporotic after a hip implant was found in autopsy retrieval studies by Maloney et al. (Citation1996) and by Engh et al. (Citation1992a). From their findings, increasing patients’bone mass before joint implant surgery may be important. However, these findings may also be an effect of posttraumatic osteopenia (Adolphson et al. Citation1994, Neander et al. Citation1997), indicating that there is also a need to increase bone density already after surgery—in order to prevent progression of osteoporosis and to keep the patient’s bone mineral level unaffected.
High fluid pressure
Linder (Citation1994) discussed the effect of high pressure as part of the loosening/osteolytic process that happens when an implant becomes loose. In an experimental rabbit study, Aspenberg and Van der Vis (Citation1998 a, Citationb) demonstrated that oscillating fluid pressure induced osteolysis and osteocyte death. It was suggested that this pressure increase might have affected the interstitial fluid and osteoclasts and osteoblasts, leading to bone loss. Robertsson et al. (Citation1997) investigated the pressure in 18 hips before revision for aseptic loosening, and found a higher intracapsular pressure than that measured in 34 unrevised, clinically and radiographically stable hips. Anthony et al. (Citation1990) studied a patient in whom a pressure rise to 198 mm Hg was found in a lytic area at passive flexion of 15 degrees. A pressure rise of this magnitude could endanger the perfusion and the oxygenation of the bone, and may result in death of the osteocytes.
Schmalzried et al. (Citation1992a) also discussed the role of high pressure as a cause of osteolysis. Intracapsular pressure is higher in an osteoarthrotic joint than in a normal joint (Schmalzried et al. Citation1997), and this pressure may affect bone which lacks cartilage protection. In normal joints the bone is not exposed to the joint fluid, but when the cartilage is affected either by disease or by trauma the bone is exposed. Inflammation of the joint occurs, which leads to the production of more fluid, thus increasing the pressure in the joint. This may disturb the circulation to the bone, resulting in cysts or geodes as a result of the bone loss. This bone loss is, however, believed to occur without the activation of macrophages (foreign-body reaction), in contrast to the cysts that develop in the prosthetic joint. Oscillating high fluid pressure (70–150 mm Hg) 2 hours a day for 14 days resulted in bone resorption in a study performed in rabbits by Van der Vis et al. (Citation1998a). The period of 2 h was probably not long enough to induce irreversible ischemic damage to the osteocytes (Kälebo et al. Citation1986). If pressure up to 700 mm Hg (Hendrix et al. Citation1983) is present in the effective joint space, the effect may be more harmful to the osteocytes around the prosthesis— resulting in more resorption and osteolysis. It seems obvious that joint fluid pressure is involved in the loosening process. However, regarding it as a single causative factor in the complex cascade of events surrounding a failed implant may result in an oversimplified explanation of the loosening process.
Endotoxin
Bacterial infection in a joint with an artificial implant is a very serious matter. Mariani et al. (Citation1996) investigated joint fluid from 50 patients with clinical signs of infection using polymerase chain reaction (PCR) and conventional culture. They found positive cultures from 15 patients, but the PCR was positive in 32 samples (including the 15 culture positives). In addition, they found no false positives in a control group of samples from 21 uninfected knee joints. 13 of the PCR positive but culture negative reactions were weak, indicating low titers of infection. These findings may support the theory of endotoxin on particles being responsible for macrophage activation. Remnants of bacterial membrane may still be present on the implant even though the bacteria have been killed by antibiotics or by the immune system. The risk of a bacterial infection in an artificial joint is currently very low, and is estimated to be below 0.5% in Sweden (Herberts and Malchau Citation2000). Data from the Swedish national hip arthroplasty registry have demonstrated that Palacos with gentamicin reduces the risk of aseptic loosening compared to Palacos without gentamicin (Malchau et al. Citation2000).
Even if there are no signs of bacteria in any cultures, there may still be remnants of them. The bacterial cell membrane or cell wall contains different antigens or endotoxins: the classic being lipopolysaccharide (LPS) in gram-negative bacteria (E. coli)and lipoteichoic acid (LTA) in gram-positive bacteria (S. aureus).
Ragab et al. (Citation1999) demonstrated that commercially available titanium particles used in animal experiments had endotoxin on their surfaces. They thus concluded that in all previous animal studies, the endotoxin could have been responsible for the effects on the bone-implant interfacial tissue. From this, the dominant theory that aseptic loosening is caused by particles comes under question. These authors also hypothesized that because of the manufacturers’inability to remove endotoxin from the particles, there was probably endotoxin on the implants inserted. Hitchins and Merritt (Citation1999) found that the detection limits for endotoxin are very close to the stimulatory levels of endotoxin, which may result in underdiagnosis of endotoxin levels on joint implants and also on particles used in experimental studies. Bi et al. (Citation2001a) demonstrated that removal of endotoxins from titanium particles reduced the particle- induced osteolysis by 50–70%, whereas the addition of endotoxin to particles restored the particle-induced osteolysis. Their add-back experiment indicated that endo-toxin-induced cytokine production may be involved in aseptic loosening. Akisue et al. (Citation2002) exposed well-characterized human monocyte/macrophages (THP-1) to titanium wear particles (mostly in the submicron size range) from a failed human hip arthroplasty without endotoxin and concluded that endotoxin-free titanium particles may not by themselves initiate cytokine release from human macrophages. Thus, other factors should be investigated for their role in cytokine activation. Taken together, these studies indicate that endotoxins often seem to be present on particles in vivo and in vitro. Furthermore, endotoxin appears to be as phlogistic and osteolytic as material particles per se. However, in an animal study Skoglund et al. (Citation2002) found that endotoxin may be present for only a short time in vivo. The authors proposed that endotoxin may be a limited cause of aseptic loosening. On the other hand, it may trigger macrophage activation, which may in turn initiate the bone resorption. The study was performed on osseointegrated titanium plates, but osseointegrated implants have more resistance to aseptic loosening (Sundfeldt et al. Citation2003) than recently inserted—not yet osseointegrated—implants. The role of low-virulence bacterial colonization and endotoxin contamination for prosthetic loosening is currently under investigation.
Individual or genetic variations
Individual variation between patients regarding PE wear has been observed clinically: whereas some patients may have severe PE wear and no signs of implant failure, other patients may present a picture of barely noticeable PE wear but rapid osteolysis with implant failure. In some patients, this can be explained by their age, activity level or body weight, but in many patients the explanation is unknown. Jasty et al. (Citation1986) proposed that osteolysis may be related to an adverse cellular response that might predict implant loosening. In an in vitro study, Matthews et al. (Citation2000a) reported that human macrophages from three different donors released different amounts of cytokines when exposed to endotoxin-free PE particles. The difference could be up to 20 times. The same authors compared the reaction of cells from six different human donors to endotoxin-free PE particles of known size and quantity (Matthews et al. Citation2000b): differences in cytokine release of up to 15 times were demonstrated in vitro. The authors speculated on the possibility of using a simple blood test before joint replacement surgery in order to identify patients with strong reactions to PE debris. Some individuals may be more sensitive to wear products than others i.e. “implant looseners”. Whether there is a correlation between such increased sensitivity and an increased risk of prosthesis loosening remains to be established. This may explain why it is so difficult to use animals in these studies. In vitro studies focusing on the activities of macrophages make use of human primary cells, human cell lines and cells of animal origin. In the context of patient hetereogenity, the choice of cells and possible animal model for studies on material-cell interactions is not an obvious one. For example, animal macrophages may react completely differently to wear products than human cells. Furthermore, in vitro studies using human cells may be more appropriate than various in vivo animal models. In the light of the results presented by Matthews et al. (Citation2000 a, Citationb), the task of investigating the role of individual and/or genetic variation in aseptic loosening in much greater depth is overdue. Another indirect conclusion is that animal models for the study of aseptic loosening may have to deal with the fact that the response to particles cannot easily be translated to the corresponding situation in humans.
Sealed interface
Schmalzried et al. (Citation1992a) described the effective joint space and how wear products can affect the interfaces around implants. They proposed that wear particles are dispersed in joint fluid and transported by pressure along the bone-implant interface, thus affecting bone tissue around the entire prosthesis. This would depend on the integrity of bone/implant or bone/cement contact. The authors proposed that periprosthetic bone loss might be prevented if the interface barriers were kept intact. In a study of cementless hips, Tanzer et al. (Citation1992) found osteolysis around the femoral stem which increased with time, implying that wear particles had access to the osteolytic areas by way of the effective joint space. Bobyn et al. (Citation1995) examined the role of a sealed interface in implant survival and, based on a retrieval autopsy study and two different canine studies, they concluded that smooth implant surfaces are more susceptible to the effects of PE particles than porous surfaces. The authors proposed that sealing off the interface with a porous implant effectively stops the PE particles from entering the interface and causing subsequent periprosthetic osteolysis. They also proposed that the ingrowth of fibrous tissue effectively stopped the PE particles. The authors also postulated, however, that sealed interfaces could not withstand high loads of wear particles on a permanent basis and that with time osteolytic areas could form anywhere in the peri-implant region. In two different canine studies, Rahbek et al. (Citation2000, Citation2001) found that PE particles injected into knees with HA-coated implants were unable to migrate into the interface.
In an experiment using a rabbit model, our group demonstrated that an osseointegrated implant with a sealed interface may not be affected by UHMWPE particles or may not progress to aseptic loosening (Sundfeldt et al. Citation2002).
In another study from our group (Sundfeldt et al. Citation2003), 35 knees in 30 patients with Miller-Galante-1 knee replacements were followed clinically and radiographically with a mean follow-up time of 12 years. 20 knees were revised. Patello-femoral problems, especially avulsion of the polyethylene from the metal-backed patella and in some cases severe metallosis, were the main reason for revision. The fixation of the components was excellent, however, with a high degree of osseointegration demonstrated at histological sections of retrieved components. The follow-up time for the revisions was 5 years on average. During revision, the metal-backed patellar prosthesis was removed and replaced with cemented polyethylene prostheses. In addition, the tibia liner was replaced but the tibial plateau and the femoral component were left in situ. Despite very high loads of wear particles, there were no clinical or radiological signs of loosening after 5 years. This may be an effect of the fact that the interfaces were sealed because of osseointegration, and that the implant was stable, preventing the wear particles and joint fluid from reaching the interface.
Many papers have discussed the importance of interface sealing for implant survival (Schmalzried et al. Citation1992a, Citation1993, Kraemer et al. Citation1995, Emerson et al. Citation1999, Lalor et al. Citation1999, Rahbek et al. Citation2000, Citation2001). Sealing off the interface in any cemented or uncemented orthopedic joint implant is probably vital for the outcome of the implant. However, the sealing must be established very early—otherwise increasing amounts of wear particles and joint fluid will be pumped into the interface, and intimate contact between bone and cement or bone and implant will not occur, which could lead to implant failure. Early micromotion may also adversely affect the sealing of the interface, and is probably just as harmful to implant outcome.
Conclusions
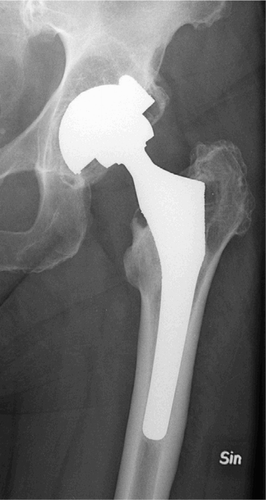
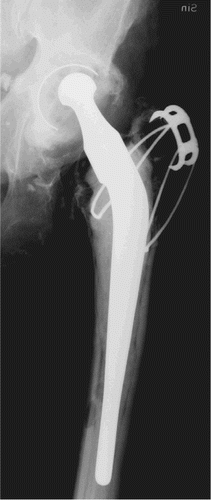
Fixation of the prosthesis is still one of the major issues concerning major arthroplasties. The older patients of today have far greater demands regarding physical activity than 15 years ago. Younger patients suffering from joint disorders also have high demands on returning to normal activity levels after surgery. High activity levels of the patient result in greater wear at the joint. This is a fact, regardless of what materials the implant components are made of. Stress shielding may be high if the design of the implant is inferior, but it will always occur to some extent irrespective of design. High activity levels also increase the load on the components of the implant, resulting in a risk of interface motion and subsequent failure. In addition, progress in medicine has made it possible to perform surgery on patients who are older and more infirm. The expected remaining lifetime for a 65-year-old patient is almost 20 years; thus, implant life must be prolonged or the cost of revision surgery will increase exponentially. Performance of revision surgery in 80-year-old patients because of joint implant failure is a dangerous, difficult and expensive procedure. This will increase, however, since a healthy 80-year-old patient is not interested in ending his or her life disabled, with severe pain because of a failed arthroplasty (). Also, young patients make great demands on their replaced joints. Thus, wear resistance and fixation must be better in order to fulfill these demands.
Figure 4. A hip with Charnley prosthesis.Primary surgery was performed in 1982 and the stem was revised in 1990.Severe polyethylene wear and zones (linear osteolysis) around the cup are present, with focal osteolysis around the distal part of the stem.
Today, aseptic implant loosening is the most common cause of revision worldwide. The underlying mechanism is still unknown, and during the past 15 years a great amount of scientific effort has been focused on the reason for it. Aseptic loosening is a complex and multifactorial event, and finding methods of studying it is vital. For example, both in vitro and in vivo animal models dealing with the problem need to use endotoxin-tested wear particles of adequate material, amount and shape and in addition in vivo studies of implants need to be performed in a joint, where material normally used in clinical practice is present.
Aseptic loosening is probably the result of a combination of several events once established— but the question is how and what initiates it? Particle-related disease has been discussed for several years. Recent discussions have focused on the size of the particles (Green et al. Citation1998) and their biological activity. Cytokines are proteins involved in the immune response, and act as messengers between cells. The cytokine response to particles is the result of a complex activation cascade where, for example, TNF-α, IL-1 and IL-6 appear to be involved in the upregulation and IL-10 appears to be involved in the downregulation of the activation cascade (Gretzer Citation2000). How and why this cascade starts is not well understood, and this is a major area of research.
When investigating the effect of particles on bone in vivo, it may seem appropriate to expose bone tissue to particles directly, as performed by Dowd et al. (Citation1995) and by Shanbhag et al. (Citation1997), but perhaps this will give a false positive reaction since the local concentration of particles will be very high—resulting in a high degree of local macrophage activation.
Recently, many authors have discussed the possibility of endotoxin on the particles being an explanation for macrophage activation and cytokine release (Hitchins and Merritt Citation1999, Ragab et al. Citation1999, Bi et al. Citation2001 a, Citationb, Akisue et al. Citation2002). The endotoxin theory is interesting since macrophages have specific endotoxin receptors (CD14, toll-like receptors) (Mathison et al. Citation1991, Kaisho and Akira Citation2002). The results of the studies above indicate that endotoxins may be involved in the initiation of the peri-implant bone resorption, which could then lead to aseptic loosening. Endotoxins on the surfaces of particles appear to play a major role in inducing cytokine synthesis. However, endotoxin-free particles also seem to affect cytokine release. The reason for this could be that the levels of endotoxin required for macrophage activation are close to the detection limits (Brooks et al. Citation2002).
Some of the implants with aseptic loosening diagnosed radiologically and clinically may have been exposed to low-grade virulent bacteria that have left remnants on the surfaces of the implants (Mariani et al. Citation1996). These findings may be linked to the theory of individual sensitivity to particles, and the presence of endotoxins on particle surfaces may suggest that individual variations in particle-induced macrophage activation arise from different immunological responses to remnants of bacteria (endotoxins).
In vitro studies (Nakashima et al. Citation1999) have demonstrated that macrophages become activated by endotoxin-free particles even if phagocytosis does not occur. It was suggested that particles of a certain size may bind to membrane surface ligands. Our understanding of the mechanism of particle-induced cell activation is improving (). Despite this, there is still no clear evidence that particles by themselves induce aseptic loosening. On the other hand, there are several valid alternative hypotheses.
Figure 5. Figure 5.Schematic diagram of different pathways of communication [OK?] between different cells when affected by particles, cytokines and endotoxin.
![Figure 5. Figure 5.Schematic diagram of different pathways of communication [OK?] between different cells when affected by particles, cytokines and endotoxin.](/cms/asset/eecfe676-a228-462c-a37c-413961e9d249/iort_a_11327144_f0005_b.gif)
Aseptic loosening appears to be a combination of macro-, micro- and nano events. Without micromotion of the implant, aseptic loosening seems unlikely to occur. Animal studies (Aspenberg et al. Citation1992, Goodman Citation1994) and clinical RSA studies (Ryd Citation1986, Citation1992, Kärrholm et al. Citation1994) have demonstrated that micromotion is deleterious to the outcome of an implant.
Particles play a substantial role in the course of events, and it is likely that the size of particles (Green et al. Citation1998, Citation2000, Nakashima et al. Citation1999) is of major importance. The material from which the particles are made may not be as important, however (Murray and Rushton Citation1990, Blaine et al. Citation1996, Rader et al. Citation1999), since different materials induce macrophages to synthesise different amounts of cytokines. Instead, individual differences between patientsdue to genetic factors may play a more significant role than different degrees of particle activation caused by different materials. In addition, endotoxins on the particle surface appear to play a major role in inducing the synthesis of cytokines.
High pressure is unlikely to be the sole cause of aseptic loosening (Aspenberg and Van der Vis Citation1998 a, Citationb, Van der Vis et al. Citation1998 a, Citationb), but it may explain why particles in an opened failed interface have access to the bone; then high pressure would be more of an early symptom than a cause of aseptic loosening.
In summary, aseptic loosening of joint implants is still a major problem and has a multifactorial etiology. For the active or young patient, a more reliable, resistant and permanent interface is required in order to prevent loosening.
Experimental studies investigating basic biological reactions to different materials and techniques are required. It is obvious that animal models can contribute to improved clinical knowledge. Consequently, experimental and clinical researchers in orthopedics should work more closely together to ensure that both approaches are applied to clinical problems.
No competing interests declared.
- Adolphson P, von Sivers K, Dalen N, Jonsson U, Dahlborn M. Decrease in vertebral bone density after hip arthroplasty. A quantitative computed tomography study in 18 arthrosis cases. Acta Orthop Scand 1994; 65(1)12-4
- Agins H H, Alcock N W, Bansal M, Salvati E A, Wilson P D, Jr, Pellicci P M, Bullough P G. Metallic wear in failed titanium-alloy total hip replacements A histological and quantitative analysis. J Bone Joint Surg (Am) 1988; 70(3)347–56
- Akisue T, Bauer T W, Farver C F, Mochida Y. The effect of particle wear debris on NFkappaB activation and proinflammatory cytokine release in differentiated THP-1 cells. J Biomed Mater Res 2002; 59(3)507–15
- Allen M, Brett F, Millett P, Rushton N. The effects of particulate polyethylene at a weight-bearing bone-implant interface A study in rats. J Bone Joint Surg (Br) 1996; 78(1)32–7
- Anthony P P, Gie G A, Howie C R, Ling R S. Localised endosteal bone lysis in relation to the femoral components of cemented total hip arthroplasties. J Bone Joint Surg (Br) 1990; 72(6)971–9
- Aspenberg P, Herbertsson P. Periprosthetic bone resorption Particles versus movement. J Bone Joint Surg (Br) 1996; 78(4)641–6
- Aspenberg P, Van der Vis H. Fluid pressure may cause periprosthetic osteolysis Particles are not the only thing. Acta Orthop Scand 1998a; 69(1)1–4
- Aspenberg P, Van der Vis H. Migration, particles, and fluid pressure A discussion of causes of prosthetic loosening. Clin Orthop 1998b, 352: 75–80
- Aspenberg P, Goodman S, Toksvig-Larsen S, Ryd L, Albrektsson T. Intermittent micromotion inhibits bone ingrowth Titanium implants in rabbits. Acta Orthop Scand 1992; 63(2)141–5
- August A C, Aldam C H, Pynsent P B. The McKee-Farrar hip arthroplasty. A long-term study. J Bone Joint Surg (Br) 1986; 68(4)520–7
- Bauer T W. Particles and periimplant bone resorption. Clin Orthop 2002, 405: 138–43
- Bechtold J E, Kubic V, Soballe K. A controlled experimental model of revision implants: Part I Development. Acta Orthop Scand 2001; 72(6)642–9
- Bi Y, Seabold J M, Kaar S G, Ragab A A, Goldberg V M, Anderson J M, Greenfiel E M. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res 2001a; 16(11)2082–91
- Bi Y, Van De Motter R R, Ragab A A, Goldberg V M, Anderson J M, Greenfield E M. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg (Am) 2001b; 83(4)501–8
- Blaine T A, Rosier R N, Puzas J E, Looney R J, Reynolds P R, Reynolds S D, O'Keefe R J. Increased levels of tumor necrosis factor-alpha and interleukin-6 protein and messenger RNA in human peripheral blood monocytes due to titanium particles. J Bone Joint Surg (Am) 1996; 78(8)1181–92
- Blaine T A, Pollice P F, Rosier R N, Reynolds P R, Puzas J E, O'Keefe R J. Modulation of the production of cytokines in titanium-stimulated human peripheral blood monocytes by pharmacological agents. The role of cAMP-mediated signaling mechanisms. J Bone Joint Surg (Am) 1997; 79(10)1519–28
- Bobyn J D, Jacobs J J, Tanzer M, Urban R M, Aribindi R, Sumner D R, Turner T M, Brooks C E. The susceptibility of smooth implant surfaces to periimplant fibrosis and migration of polyethylene wear debris. Clin Orthop 1995, 311: 21–39
- Brooks R A, Sharpe J R, Wimhurst J A, Myer B J, Dawes E N, Rushton N. The effects of the concentration of high-density polyethylene particles on the bone-implant interface. J Bone Joint Surg (Br) 2000; 82(4)595–600
- Brooks R A, Wimhurst J A, Rushton N. Endotoxin contamination of particles produces misleading inflammatory cytokine responses from macrophages in vitro. J Bone Joint Surg (Br) 2002; 84(2)295–9
- Bryan J M, Sumner D R, Hurwitz D E, Tompkins G S, Andriacchi T P, Galante J O. Altered load history affects periprosthetic bone loss following cementless total hip arthroplasty. J Orthop Res 1996; 14(5)762–8
- Bugbee W D, Culpepper W J, 2nd, Engh C A, Jr, Engh C A, Sr. Long-term clinical consequences of stress-shielding after total hip arthroplasty without cement. J Bone Joint Surg (Am) 1997; 79(7)1007–12
- Buly R L, Huo M H, Salvati E, Brien W, Bansal M. Titanium wear debris in failed cemented total hip arthroplasty. An analysis of 71 cases. J Arthroplasty 1992; 7(3)315–23
- Campbell P. On aseptic loosening of total hip replacements: the role of UHMWPE wear particles. University of Gothenburg, GothenburgSweden 1995, Thesis
- Charnley J. Anchorage of the femoral head prosthesisto the shaft of the femur. J Bone Joint Surg (Br) 1960; 42: 28–30
- Chen F E, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene 1999; 18(49)6845–52
- Collier J P, Bauer T W, Bloebaum R D, Bobyn J D, Cook S D, Galante J O, Harris W H, Head W C, Jasty M J, Mayor M B, (all names). Results of implant retrieval from post-mortem specimens in patients with well-functioning, long-term total hip replacement. Clin Orthop 1992, 274: 97–112
- Digas G, Kärrholm J, Thanner J, Malchau H, Herberts P. Highly cross-linked polyethylene in cemented THR: Randomised study of 61 hips. Clin Orthop 2003, 417: 1–13
- Doorn P F, Campbell P A, Worrall J, Benya P D, McKellop H A, Amstutz H C. Metal wear particle characterization from metal on metal total hip replacements: transmission electron microscopy study of periprosthetic tissues and isolated particles. J Biomed Mater Res 1998; 42(1)103–11
- Dorr L D, Wan Z, Longjohn D B, Dubois B, Murken R. Total hip arthroplasty with use of the Metasul metalon-metal articulation. Four to seven-year results. J Bone Joint Surg (Am) 2000; 82(6)789–98
- Dowd J E, Schwendeman L J, Macaulay W, Doyle J S, Shanbhag A S, Wilson S, Herndon J H, Rubash H E. Aseptic loosening in uncemented total hip arthroplasty in a canine model. Clin Orthop 1995, 319: 106–21
- Dowd J E, Sychterz C J, Young A M, Engh C A. Characterization of long-term femoral-head-penetration rates Association with and prediction of osteolysis. J Bone Joint Surg (Am) 2000; 82(8)1102–7
- Emerson R H, Jr, Sanders S B, Head W C, Higgins L. Effect of circumferential plasma-spray porous coating on the rate of femoral osteolysis after total hip arthroplasty. J Bone Joint Surg (Am) 1999; 81(9)1291–8
- Engh C A, Bobyn J D. The influence of stem size and extent of porous coating on femoral bone resorption after primary cementless hip arthroplasty. Clin Orthop 1988, 231: 7–28
- Engh C A, Bobyn J D, Glassman A H. Porouscoated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg (Br) 1987; 69(1)45–55
- Engh C A, McGovern T F, Bobyn J D, Harris W H. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg (Am) 1992a; 74(7)1009–20
- Engh C A, O'Connor D, Jasty M, McGovern T F, Bobyn J D, Harris W H. Quantification of implant micromotion, strain shielding, and bone resorption with porous-coated anatomic medullary locking femoral prostheses. Clin Orthop 1992b, 285: 13–29
- Engh G A, Lounici S, Rao A R, Collier M B. In vivo deterioration of tibial baseplate locking mechanisms in contemporary modular total knee components. J Bone Joint Surg (Am) 2001; 83(11)1660–5
- Franzoso G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev 1997; 11(24)3482–96
- Fryzek J P, Ye W, Signorello L B, Lipworth L, Blot W J, McLaughlin J K, Nyren O. Incidence of cancer among patients with knee implants in Sweden, 1980–1994. Cancer 2002; 94(11)3057–62
- Frökjaer J, Deleuran B, Lind M, Overgaard S, Søballe K, Bunger C. Polyethylene particles stimulate monocyte chemotactic and activating factor production in synovial mononuclear cells in vivo An immunohistochemical study in rabbits. Acta Orthop Scand 1995; 66(4)303–7
- Frökjaer J, Overgaard S, Lind M, Bünger C, Søballe K. Failure of migration by injected polyethylene particles around press fit implants: an exprimental study in rabbits. Hip International 1999; 9(4)173–7
- Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol 1998; 16: 225–60
- Goldring S R, Schiller A L, Roelke M, Rourke C M, O'Neil D A, Harris W H. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg (Am) 1983; 65(5)575–84
- Goldring S R, Jasty M, Roelke M S, Rourke C M, Bringhurst F R, Harris W H. Formation of a synovial-like membrane at the bone-cement interface Its role in bone resorption and implant loosening after total hip replacement. Arthritis Rheum 1986; 29(7)836–42
- Goodman S B. The effects of micromotion and particulate materials on tissue differentiation. Bone chamber studies in rabbits. Acta Orthop Scand (Suppl 258) 1994; 65: 1–43
- Goodman S, Aspenberg P, Song Y, Knoblich G, Huie P, Regula D, Lidgren L. Tissue ingrowth and differentiation in the bone-harvest chamber in the presence of cobalt-chromium-alloy and high-density-polyethylene particles. J Bone Joint Surg (Am) 1995; 77(7)1025–35
- Goodman S B, Song Y, Chun L, Regula D, Aspenberg P. Effects of TGFbeta on bone ingrowth in the presence of polyethylene particles. J Bone Joint Surg (Br ) 1999; 81(6)1069–75
- Green T R, Fisher J, Stone M, Wroblewski B M, Ingham E. Polyethylene particles of a, critical size‘ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998; 19(24)2297–302
- Green T R, Fisher J, Matthews J B, Stone M H, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res 2000; 53(5)490–7
- Gretzer C. Macrophage-material surface interactions. GothenburgSweden 2000, Thesis
- Haboush E J. A new operation for arthroplasty of the hip based on biomechanics, photoelasticity, fast-setting dental acrylic and other considerations. Bull Hosp Jt Dis 1953; 14: 242–77
- Harris W H, Schiller A L, Scholler J M, Freiberg R A, Scott R. Extensive localized bone resorption in the femur following total hip replacement. J Bone Joint Surg (Am) 1976; 58(5)612–8
- Harvey E J, Bobyn J D, Tanzer M, Stackpool G J, Krygier J J, Hacking S A. Effect of flexibility of the femoral stem on bone-remodeling and fixation of the stem in a canine total hip arthroplasty model without cement. J Bone Joint Surg (Am) 1999; 81(1)93–107
- Hatton A, Nevelos J E, Nevelos A A, Banks R E, Fisher J, Ingham E. Alumina-alumina artificial hip joints Part I: a histological analysis and characterisation of wear debris by laser capture microdissection of tissues retrieved at revision. Biomaterials 2002; 23(16)3429–40
- Hatton A, Nevelos J E, Matthews J B, Fisher J, Ingham E. Effects of clinically relevant alumina ceramic wear particles on TNF-alpha production by human peripheral blood mononuclear phagocytes. Biomaterials 2003; 24(7)1193–204
- Hendrix R W, Wixson R L, Rana N A, Rogers L F. Arthrography after total hip arthroplasty: a modified technique used in the diagnosis of pain. Radiology 1983; 148(3)647–52
- Herberts P, Malchau H. Long-term registration has improved the quality of hip replacement: a review of the Swedish THR Register comparing 160,000 cases. Acta Orthop Scand 2000; 71(2)111–21
- Hicks D G, Judkins A R, Sickel J Z, Rosier R N, Puzas J E, O'Keefe R J. Granular histiocytosis of pelvic lymph nodes following total hip arthroplasty. The presence of wear debris, cytokine production, and immunologically activated macrophages. J Bone Joint Surg (Am) 1996; 78(4)482–96
- Higuchi F, Inoue A, Semlitsch M. Metal-on-metal CoCrMo McKee-Farrar total hip arthroplasty: characteristics from a long-term follow-up study. Arch Orthop Trauma Surg 1997; 116(3)121–4
- Hitchins V M, Merritt K. Decontaminating particles exposed to bacterial endotoxin (LPS). J Biomed Mater Res 1999; 46(3)434–7
- Howie D W, Vernon-Roberts B, Oakeshott R, Manthey B. A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. J Bone Joint Surg (Am) 1988; 70(2)257–63
- Howie D W, Haynes D R, Rogers S D, McGee M A. Pearcy M J The response to particulate debris. Orthop Clin North Am 1993; 24(4)571–81
- Huo M H, Salvati E A, Lieberman J R, Betts F, Bansal M. Metallic debris in femoral endosteolysis in failed cemented total hip arthroplasties. Clin Orthop 1992, 276: 157–68
- Ito A, Tateishi T, Niwa S, Tange S. In-vitro evaluation of the cytocompatibility of wear particles generated by UHMWPE/zirconia friction. Clin Mater 1993; 12(4)203–9
- Iwase T, Wingstrand I, Persson B M, Kesteris U, Hasegawa Y, Wingstrand H. The ScanHip total hip arthroplasty: radiographic assessment of 72 hips after 10 years. Acta Orthop Scand 2002; 73(1)54–9
- Jacobs J J, Skipor A K, Patterson L M, Hallab N J, Paprosky W G, Black J, Galante J O. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg (Am) 1998; 80(10)1447–58
- Jacobs J J, Roebuck K A, Archibeck M, Hallab N J, Glant T T. Osteolysis: basic science. Clin Orthop 2001, 393: 71–7
- Jacobsson S A, Djerf K, Wahlstrom O. Twenty-year results of McKee-Farrar versus Charnley prosthesis. Clin Orthop 1996, 329 Suppl: S60–8
- Jantsch S, Schwagerl W, Zenz P, Semlitsch M, Fertschak W. Long-term results after implantation of McKee-Farrar total hip prostheses. Arch Orthop Trauma Surg 1991; 110(5)230–7
- Jasty M J, Floyd W E, 3rd, Schiller A L, Goldring S R, Harris W H. Localized osteolysis in stable, non-septic total hip replacement. J Bone Joint Surg (Am) 1986; 68(6)912–9
- Jasty M, Maloney W J, Bragdon C R, O'Connor D O, Haire T, Harris W H. The initiation of failure in cemented femoral components of hip arthroplasties. J Bone Joint Surg (Br) 1991; 73(4)551–8
- Jasty M, Bragdon C, Burke D, O'Connor D, Lowenstein J, Harris W H. In vivo skeletal responses to porous-surfaced implants subjected to small induced motions. J Bone Joint Surg (Am) 1997a; 79(5)707–14
- Jasty M, Goetz D D, Bragdon C R, Lee K R, Hanson A E, Elder J R, Harris W H. Wear of polyethylene acetabular components in total hip arthroplasty. An analysis of one hundred and twenty-eight components retrieved at autopsy or revision operations. J Bone Joint Surg (Am) 1997b; 79(3)349–58
- Jones L C, Hungerford D S. Cement disease. Clin Orthop 1987, 225: 192–206
- Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochim Biophys Acta 2002; 1589(1)1–13
- Kesteris U, Robertsson O, Wingstrand H, Önnerfalt R. Cumulative revision rate with the Scan Hip Classic I total hip prosthesis. 1,660 cases followed for 2-12 years. Acta Orthop Scand 1998; 69(2)133–7
- Kim K J, Kobayashi Y, Itoh T. Osteolysis model with continuous infusion of polyethylene particles. Clin Orthop 1998, 352: 46–52
- Kraemer W J, Maistrelli G L, Fornasier V, Binnington A, Zhao J F. Migration of polyethylene wear debris in hip arthroplasties: a canine model. J Appl Biomater 1995; 6(4)225–30
- Kälebo P, Johansson C, Albrektsson T. Temporary bone tissue ischemia in the hind limb of the rabbit A vital microscopic study. Arch Orthop Trauma Surg 1986; 105(6)321–5
- Kärrholm J. Retrospective evaluation of implants—is there a need. Acta Orthop Scand 2003; 74(1)1–3
- Kärrholm J, Borssen B, Lowenhielm G, Snorrason F. Does early micromotion of femoral stem prostheses matter? 4-7-year stereoradiographic follow-up of 84 cemented prostheses. J Bone Joint Surg (Br) 1994; 76(6)912–7
- Lalor P A, Namba R, Mitchell S L, Bearcroff J, Beals N, Sledge C B, Spector M. Migration of polyethylene particles around stable implants in an animal model. J Long Term Eff Med Implants 1999; 9(4)261–72
- Landy M M, Walker P S. Wear of ultra-high-molecular-weight polyethylene components of 90 retrieved knee prostheses. J Arthroplasty (Suppl) 1988; 3: 73–85
- Langkamer V G, Case C P, Collins C, Watt I, Dixon J, Kemp A J, Atkins R M. Tumors around implants. J Arthroplasty 1997; 12(7)812–8
- Lennon A B, Prendergast P J. Evaluation of cement stresses in finite element analyses of cemented orthopaedic implants. J Biomech Eng 2001; 123(6)623–8
- Lewis G. Properties of acrylic bone cement: state of the art review. J Biomed Mater Res 1997; 38(2)155–82
- Lewis G. Properties of crosslinked ultra-high-molecular-weight polyethylene. Biomaterials 2001; 22(4)371–401
- Linder L. Implant stability, histology, RSA and wear--more critical questions are needed A view point. Acta Orthop Scand 1994; 65(6)654–8
- Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg (Am) 1990; 72(4)518–28
- Lombardi A V, Jr, Mallory T H, Vaughn B K, Drouillard P. Aseptic loosening in total hip arthroplasty secondary to osteolysis induced by wear debris from titanium-alloy modular femoral heads. J Bone Joint Surg (Am) 1989; 71(9)1337–42
- Lu Z, McKellop H. Effects of cement creep on stem sub-sidence and stresses in the cement mantle of a total hip replacement. J Biomed Mater Res 1997; 34(2)221–6
- Malchau H. On the importance of stepwise introduction of new hip implant technology : assessment of total hip replacement using clinical evaluation, radiostereometry, digitised radiography and a national hip registry. University of Gothenburg. 1995, Thesis
- Malchau H, Herberts P, Soderman P, Odén A (2000) Prognosis of total hip replacement. Annual meeting of the American Academy of Orthopaedic Surgeons, OrlandoUSA, March, 15–192000, 2000
- Maloney W J, Sychterz C, Bragdon C, McGovern T, Jasty M, Engh C A, Harris W H. The Otto Aufranc Award. Skeletal response to well fixed femoral components inserted with and without cement. Clin Orthop 1996, 333: 15–26
- Mariani B D, Martin D S, Levine M J, Booth R E, Jr, Tuan R S. The Coventry Award. Polymerase chain reaction detection of bacterial infection in total knee arthroplasty. Clin Orthop 1996, 331: 11–22
- Mathison J, Tobias P, Wolfson E, Ulevitch R. Regulatory mechanisms of host responsiveness to endotoxin (lipo-polysaccharide). Pathobiology 1991; 59(3)185–8
- Matthews J B, Besong A A, Green T R, Stone M H, Wroblewski B M, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res 2000a; 52(2)296–307
- Matthews J B, Green T R, Stone M H, Wroblewski B M, Fisher J, Ingham E. Comparison of the response of primary human peripheral blood mononuclear phagocytes from different donors to challenge with model polyethylene particles of known size and dose [In Process Citation]. Biomaterials 2000b; 21(20)2033–44
- McCarthy C K, Steinberg G G, Agren M, Leahey D, Wyman E, Baran D T. Quantifying bone loss from the proximal femur after total hip arthroplasty. J Bone Joint Surg (Br) 1991; 73(5)774–8
- McGregor D B, Baan R A, Partensky C, Rice J M, Wilbourn J D. Evaluation of the carcinogenic risks to humans associated with surgical implants and other foreign bodies. A report of an IARC Monographs Programme Meeting International Agency for Research on Cancer. Eur J Cancer 2000; 36(3)307–13
- McKellop H, Park S H, Chiesa R, Doorn P, Lu B, Normand P, Grigoris P, Amstutz H. In vivo wear of three types of metal on metal hip prostheses during two decades of use. Clin Orthop 1996, 329 Suppl: S128–40
- Mirra J M, Marder R A, Amstutz H C. The pathology of failed total joint arthroplasty. Clin Orthop 1982, 170: 175–83
- Mjöberg B. Theories of wear and loosening in hip prostheses. Wear-induced loosening vs loosening-induced wear-a review. Acta Orthop Scand 1994; 65(3)361–71
- Morberg P H. On bone tissue reactions to acrylic cement. GothenburgSweden 1991, Thesis
- Murray D W. Rushton N Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg (Br) 1990; 72(6)988–92
- Nakashima Y, Sun D H, Trindade M C, Maloney W J, Goodman S B, Schurman D J, Smith R L. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg (Am) 1999; 81(5)603–15
- Neander G, Adolphson P, Hedstrom M, von Sivers K, Dahlborn M, Dalen N. Decrease in bone mineral density and muscle mass after femoral neck fracture. A quantitative computed tomography study in 25 patients. Acta Orthop Scand 1997; 68(5)451–5
- Niinimaki T, Junila J, Jalovaara P. A proximal fixed ana-tomic femoral stem reduces stress shielding. Int Orthop 2001; 25(2)85–8
- Nuno N, Amabili M. Modelling debonded stem-cement interface for hip implants: effect of residual stresses. Clin Biomech (Bristol, Avon) 2002; 17(1)41–8
- Oh I, Harris W H. Proximal strain distribution in the loaded femur. An in vitro comparison of the distributions in the intact femur and after insertion of different hip-replacement femoral components. J Bone Joint Surg (Am) 1978; 60(1)75–85
- Oparaugo P C, Clarke I C, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand 2001; 72(1)22–8
- Parks N L, Engh G A, Topoleski L D, Emperado J. The Coventry Award. Modular tibial insert micromotion. A concern with contemporary knee implants. Clin Orthop 1998, 356: 10–5
- Pietrabissa R, Raimondi M, Di Martino E. Wear of polyethylene cups in total hip arthroplasty: a parametric mathematical model. Med Eng Phys 1998; 20(3)199–210
- Quinn J M, Neale S, Fujikawa Y, McGee J O, Athanasou N A. Human osteoclast formation from blood monocytes, peritoneal macrophages, and bone marrow cells. Calcif Tissue Int 1998; 62(6)527–31
- Rader C P, Sterner T, Jakob F, Schutze N, Eulert J. Cytokine response of human macrophage-like cells after contact with polyethylene and pure titanium particles. J Arthroplasty 1999; 14(7)840–8
- Ragab A A, Van De Motter R, Lavish S A, Goldberg V M, Ninomiya J T, Carlin C R, Greenfield E M. Measurement and removal of adherent endotoxin from titanium particles and implant surfaces. J Orthop Res 1999; 17(6)803–9
- Rahbek O, Overgaard S, Jensen T B, Bendix K, Søballe K. Sealing effect of hydroxyapatite coating: a 12-month study in canines. Acta Orthop Scand 2000; 71(6)563–73
- Rahbek O, Overgaard S, Lind M, Bendix K, Bunger C, Søballe K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles An experimental study in dogs. J Bone Joint Surg (Br) 2001; 83(3)441–7
- Raimondi M T, Santambrogio C, Pietrabissa R, Raffelini F, Molfetta L. Improved mathematical model of the wear of the cup articular surface in hip joint prostheses and comparison with retrieved components. Proc Inst Mech Eng [H] 2001; 215(4)377–91
- Regner L R, Carlsson L V, Kärrholm J N, Hansson T H, Herberts P G, Swanpalmer J. Bone mineral and migratory patterns in uncemented total knee arthroplasties: a ran-domized 5-year follow-up study of 38 knees. Acta Orthop Scand 1999; 70(6)603–8
- Robertsson O, Wingstrand H, Kesteris U, Jonsson K, Önnerfalt R. Intracapsular pressure and loosening of hip prostheses. Preoperative measurements in 18 hips. Acta Orthop Scand 1997; 68(3)231–4
- Robertsson O, Knutson K, Lewold S, Lidgren L. The Swedish Knee Arthroplasty Register 1975-1997: an update with special emphasis on 41,223 knees operated on in 1988-1997. Acta Orthop Scand 2001; 72(5)503–13
- Ryd L. Micromotion in knee arthroplasty. A roentgen stereo-photogrammetric analysis of tibial component fixation. Acta Orthop Scand (Suppl 220) 1986; 1–80
- Ryd L. Roentgen stereophotogrammetric analysis of prosthetic fixation in the hip and knee joint. Clin Orthop 1992, 276: 56–65
- Ryd L, Linder L. On the correlation between micromotion and histology of the bone-cement interface. Report of three cases of knee arthroplasty followed by roentgen stereophotogrammetric analysis. J Arthroplasty 1989; 4(4)303–9
- Sabokbar A, Pandey R, Quinn J M, Athanasou N A. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch Orthop Trauma Surg 1998; 117(3)136–40
- Schmalzried T P, Jasty M, Harris W H. Periprosthetic bone loss in total hip arthroplasty Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg (Am) 1992a; 74(6)849–63
- Schmalzried T P, Kwong L M, Jasty M, Sedlacek R C, Haire T C, O'Connor D O, Bragdon C R, Kabo J M, Malcolm A J, Harris W H. The mechanism of loosening of cemented acetabular components in total hip arthroplasty Analysis of specimens retrieved at autopsy. Clin Orthop 1992b, 274: 60–78
- Schmalzried T P, Maloney W J, Jasty M, Kwong L M, Harris W H. Autopsy studies of the bone-cement interface in well-fixed cemented total hip arthroplasties. J Arthroplasty 1993; 8(2)179–88
- Schmalzried T P, Peters P C, Maurer B T, Bragdon C R, Harris W H. Long-duration metal-on-metal total hip arthroplasties with low wear of the articulating surfaces. J Arthroplasty 1996; 11(3)322–31
- Schmalzried T P, Akizuki K H, Fedenko A N, Mirra J. The role of access of joint fluid to bone in periarticular osteolysis A report of four cases. J Bone Joint Surg (Am) 1997; 79(3)447–52
- Schmalzried T P, Shepherd E F, Dorey F J, Jackson W O, dela Rosa M, Fa'vae F, McKellop H A, McClung C D, Martell J, Moreland J R, Amstutz H C. The John Charnley Award. Wear is a function of use, not time. Clin Orthop 2000, 381: 36–46
- Schwarz E M, Looney R J, O'Keefe R J. Anti-TNF-alpha therapy as a clinical intervention for periprosthetic osteolysis. Arthritis Res 2000a; 2(3)165–8
- Schwarz E M, Lu A P, Goater J J, Benz E B, Kollias G, Rosier R N, Puzas J E, O'Keefe R J. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res 2000b; 18(3)472–80
- Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system [Thesis]. University of Lund, LundSweden 1989; 1–51, Reprint: Acta Orthop Scand (Suppl 232)
- Shanbhag A S, Hasselman C T, Rubash H E. The John Charnley Award Inhibition of wear debris mediated osteolysis in a canine total hip arthroplasty model. Clin Orthop 1997, 344: 33–43
- Signorello L B, Ye W, Fryzek J P, Lipworth L, Fraumeni J F, Jr, Blot W J, McLaughlin J K, Nyren O. Nationwide study of cancer risk among hip replacement patients in Sweden. J Natl Cancer Inst 2001; 93(18)1405–10
- Skoglund B, Larsson L, Aspenberg P A. Bone-resorptive effects of endotoxin-contaminated high-density polyethylene particles spontaneously eliminated in vivo. J Bone Joint Surg (Br) 2002; 84(5)767–73
- Sochart D H. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop 1999, 363: 135–50
- Soloviev A, Schwarz E M, Kuprash D V, Nedospasov S A, Puzas J E, Rosier R N, O'Keefe R J. The role of p105 protein in NFkappaB activation in ANA-1 murine macrophages following stimulation with titanium particles. J Orthop Res 2002; 20(4)714–22
- Sundfeldt M, Widmark M, Johansson C B, Campbell P, Carlsson L V. Effect of submicron polyethylene particles on an osseointegrated implant: an experimental study with a rabbit patello-femoral prosthesis. Acta Orthop Scand 2002; 73(4)416–24
- Sundfeldt M, Johansson C B, Regner L, Albrektsson T, Carlsson L V. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants 2003; 13(4)341–54
- Swanpalmer J, Kullenberg R, Hansson T. The feasibility of triple-energy absorptiometry for the determination of bone mineral, Ca and P in vivo. Physiol Meas 1998a; 19(1)1–15
- Swanpalmer J, Kullenberg R, Hansson T. Measurement of bone mineral using multiple-energy x-ray absorptiometry. Phys Med Biol 1998b; 43(2)379–87
- Søballe K, Hansen E S, Rasmussen H, Jorgensen P H, Bunger C. Tissue ingrowth into titanium and hydroxyapa-tite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10(2)285–99
- Söderman P, Malchau H, Herberts P, Zugner R, Regner H, Garellick G. Outcome after total hip arthroplasty: Part II. Disease-specific follow-up and the Swedish National Total Hip Arthroplasty Register. Acta Orthop Scand 2001; 72(2)113–9
- Tanzer M, Maloney W J, Jasty M, Harris W H. The progression of femoral cortical osteolysis in association with total hip arthroplasty without cement. J Bone Joint Surg (Am) 1992; 74(3)404–10
- Thomsen P, Gretzer C. Macrophage interactions with modified material surfaces. Curr Opin Solid State & Mat Sci 2001; 5(2–3)163–76
- Topoleski L D, Ducheyne P, Cuckler J M. Microstructural pathway of fracture in poly(methyl methacrylate) bone cement. Biomaterials 1993; 14(15)1165–72
- Van Der Vis H M, Marti R K, Tigchelaar W, Schuller H M, Van Noorden C J. Benign cellular responses in rats to dif-ferent wear particles in intra-articular and intramedullary environments. J Bone Joint Surg (Br) 1997; 79(5)837–43
- Van der Vis H, Aspenberg P, De Kleine R, Tigchelaar W, Van Noorden C J. Short periods of oscillating fluid pressure directed at a titanium-bone interface in rabbits lead to bone lysis. Acta Orthop Scand 1998a; 69(1)5–10
- Van der Vis H M, Aspenberg P, Marti R K, Tigchelaar W, Van Noorden C J. Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clin Orthop 1998b, 350: 201–8
- Van Lenthe G H, de Waal Malefijt M C, Huiskes R. Stress shielding after total knee replacement may cause bone resorption in the distal femur. J Bone Joint Surg (Br) 1997; 79(1)117–22
- Wan Z, Dorr L D, Woodsome T, Ranawat A, Song M. Effect of stem stiffness and bone stiffness on bone remodeling in cemented total hip replacement. J Arthroplasty 1999; 14(2)149–58
- Wang J Y, Wicklund B H, Gustilo R B, Tsukayama D T. Titanium, chromium and cobalt ions modulate the release of bone-associated cytokines by human monocytes/macrophages in vitro. Biomaterials 1996; 17(23)2233–40
- Warashina H, Sakano S, Kitamura S, Yamauchi K I, Yamaguchi J, Ishiguro N, Hasegawa Y. Biological reaction to alumina, zirconia, titanium and polyethylene particles implanted onto murine calvaria. Biomaterials 2003; 24(21)3655–61
- Willert H G. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res 1977; 11(2)157–64
- Willert H G, Bertram H, Buchhorn G H. Osteolysis in alloarthroplasty of the hip. The role of bone cement fragmentation. Clin Orthop 1990, 258: 108–21
- Willert H G, Buchhorn G H, Gobel D, Koster G, Schaffner S, Schenk R, Semlitsch M. Wear behavior and histopathology of classic cemented metal on metal hip endoprostheses. Clin Orthop 1996, 329 Suppl: S160–86
- Visuri T. Long-term results and survivorship of the McKee-Farrar total hip prosthesis. Arch Orthop Trauma Surg 1987; 106(6)368–74
- Visuri T, Pukkala E, Pulkkinen P, Paavolainen P. Decreased cancer risk in patients who have been operated on with total hip and knee arthroplasty for primary osteoarthrosis: a meta-analysis of 6 Nordic cohorts with 73,000 patients. Acta Orthop Scand 2003; 74(3)351–60
- Wolff J. Ueber die innere Architectur der Knochen und ihre Bedeutung fur die Frage vom Knochenwachstum. Virchow's Arch 1870; 50: 389–450