Abstract
Background and purpose There have only been a few nationwide studies on the epidemiology and outcome of osteosarcoma. We report the clinical features, treatment, and prognosis of osteosarcoma in Finland for the period 1971–1990.
Methods The study material was derived from popu-lation-based data from the national Finnish Cancer Registry. 302 osteosarcomas were reported during the study period. Histological slides could be retrieved for 199 cases and from histological re-examination, 139 (83%) of these cases were confirmed as osteosarcoma and were included in the analysis. The mean length of follow-up was 8 (0.1–28) years.
Results The overall 5-year survival for the whole study population was 58%, with an improvement in survival during 1981–1990 (65%) compared to the period 1971–1980 (47%) (p=0.01). More chemotherapy was administered in the later time period. For metastasis-free survival, diagnosis in the 1970s as opposed to the 1980s (p=0.01) and large tumor size worsened outcome in univariate analysis. Patients who developed metastatic relapse within 10 months of the diagnosis had worse sarcoma-specific survival than those who developed metastases later. Limb-salvage surgery increased from 12% to 23% for patients with a peripheral tumor, with no increase in local relapses.
Interpretation We recommend aggressive approach to treat recurrent disease, with a view to further improving survival. In a small country such as Finland it is necessary to concentrate treatment to only a few centers, to ensure a high quality of treatment.
Osteosarcoma is a highly malignant primary bone tumor (Huvos Citation1991, Fletcher et al. Citation2002). The prognosis of osteosarcoma has improved significantly due to chemotherapy. Also, surgical treatment has mainly evolved from frequent amputations to limb salvage surgery. The national treatment results for Finland have not been reported previously. Here we report the clinical features and epidemiology of osteosarcoma, and also prognostic factors and separate treatment results, for two consecutive decades (1971–1980 and 1981–1990). The study period corresponds to that in a similar report on chondrosarcoma in Finland published previously by Söderström et al. (Citation2003).
For the present study, data on all patients diagnosed with primary osteosarcoma in the period 1971–1990 in Finland were retrieved from the Finnish Cancer Registry. All osteosarcoma samples were subjected to histological review.
Patients and methods
Patient data
302 patients in Finland had been recorded in the population-based nationwide Cancer Registry as having primary osteosarcoma some time during the years 1971–1990. The Finnish Cancer Registry was started in 1952. It covers the whole of Finland (approximately 5.1 million inhabitants). Since 1961, the reporting of newly diagnosed cancer cases to the Finnish Cancer Registry has been compulsory. Hospitals, pathology laboratories, and general practitioners report cancers using a standardized form. The Central Statistical Office submits information to the Finnish Cancer Registry whenever cancer is mentioned on a death certificate. Thus, the Finnish Cancer Registry includes more than 99% of cancers diagnosed in Finland (Teppo et al. Citation1994).
Detailed clinical data were collected from the patient files and survival data were obtained using national death records from the Population Register Center of Finland. The study was approved by the Joint Ethics Committee of Helsinki University Central Hospital and by the Ministry of Health and Social Affairs.
Histology
Of the 302 osteosarcomas diagnosed in the period 1971–1990, the original hematoxylin and eosin stained histological slides from 199 (66%) patients could be retrieved for re-evaluation (). The 103 patients whose slides could not be re-evalu-ated had been older at diagnosis (mean 40 (SD 25) years as compared to 26 (SD 18) years for those whose slides could be re-evaluated). In addition, the tumors were more often located axially, 30% vs. 18% and less often in the lower limb, 52% vs. 70%. The re-examination was performed by an experienced bone pathologist (TB) without knowledge of either the previous pathological assessment or the clinical outcome. Tumor subtypes were classified according to current WHO criteria (Fletcher et al. Citation2002) and they were graded histologically according to the system described by Huvos (1991). A four-grade malignancy scale was used, in which grades I and II were considered low malignancy and grades III and IV a high degree of malignancy. In the histological re-examination of the 199 tumors that were available, 166 (83%) were confirmed as osteosarcomas. The frequency of wrongly diagnosed osteosarcomas decreased over time, being 22% in the 1970s and 13% in the 1980s. In the re-classification, 108 tumors were subclassified as osteoblastic, 12 as chondroblastic, and 7 as fibroblastic—these constituting the conventional osteosarcoma group. 6 tumors were subclassified as telangiectatic osteosarcomas, 1 as a low-grade central osteosarcoma, 3 as parosteal osteosarcomas, and 2 as periosteal osteosarcomas.
17 patients had metastatic disease at diagnosis, but only patients with localized disease at diagnosis were included in the analyses. The patient files for 6 patients had been destroyed and these patients were excluded from the analysis. 4 patients with extraskeletal osteosarcomas were excluded. Thus, the present series concerns 139 osteosarcoma patients with a mean follow-up of 8.2 (SD 7.2) years.
Table 1. Histological review of the 302 patients by 5-year periods
Chemotherapy and surgery
Chemotherapy was mainly based on Rosen protocols T4–T10 in 1976–1981. In 1982–1989, the treatment was mainly based on the T10 protocol used by the Scandinavian Sarcoma Group (SSG II study) (Saeter et al. Citation1991), and from May 1990 onwards it was based on the SSG VIII study (Smeland et al. Citation2003). In the present study, adequate intensity of chemotherapy was arbitrarily defined as at least methotrexate (MTX) in the preoperative phase combined with postoperative chemotherapy. Amputation was a standard treatment for osteosarcoma prior to the development of radiological and surgical techniques, which have enabled limb-sal-vage surgery (Bielack et al. Citation2002).
Statistics
Metastasis-free survival (MFS) rate, local recur-rence-free survival (LRFS) rate, and overall survival (OS) rate were calculated with Kaplan-Meier analysis. Differences in metastasis-free and overall survival rates of different subgroups were analyzed with the log-rank test for discrete variables and with Cox regression analysis for continuous variables. Continuous variables (age at diagnosis and tumor size) were divided into 2 groups using the median as a cut-off point in order to improve readability in . The level of significance was set at p<0.05. For the analysis of possible changes over the time periods, the chi-square test for linear trends was used. SPSS version 12.0.1 for Windows was used for all analyses.
Table 2. Description of tumor, patient and treatment characteristics of the 139 patients, and the corresponding estimates of 5–year metastases–free (MFS) and overall survival (OS)
Results
Of the 139 patients, only 5 had a low-grade tumor. There was a male predominance of 1.4:1 (81 males and 58 females) with a mean age of 21 (SD 14) years (range 5–71) at diagnosis. The most frequent locations for peripheral tumors (n=122) were femur (n=69, 57%), tibia (n=37, 30%), and humerus (n=11, 9%). 17 tumors (12%) were axial with mandibula, scapula, and pelvic bones being the most frequent sites. The mean value of the largest tumor diameter was 8.4 (SD 3.6) cm, but these values were only reported for 71 tumors.
Surgery
122 patients had osteosarcoma of the limb, and of these 119 patients underwent surgery; 23 patients underwent limb salvage surgery, 78 patients underwent amputation, and 18 patients had an exarticulation. 12% and 23% of patients with a peripheral tumor had limb salvage surgery in the 1970s and 1980s, respectively (p=0.13, chi-square test). All 17 patients with axial tumors underwent surgery.
Chemotherapy
73% of patients received chemotherapy in the 1970s and 86% underwent chemotherapy in the 1980s. 7 of 51 patients (14%) and 65 of 88 patients (74%) received adequate chemotherapy in the 1970s and 1980s, respectively. 32 patients were enrolled in the SSG II study in 1982–1989. In 1990, 4 patients were enrolled in the SSG VIII study.
Survival
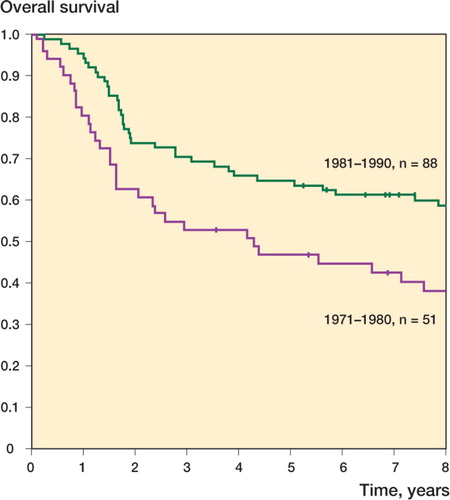
The 5-year overall survival (OS) for the whole study population was 58%. It was 47% during the 1970s, and during the 1980s it was 65% (p=0.01) (). 5 deaths were recorded as complications of therapy: chemotherapy-induced sepsis in 4 patients and cardiomyopathy in 1.
Metastatic disease
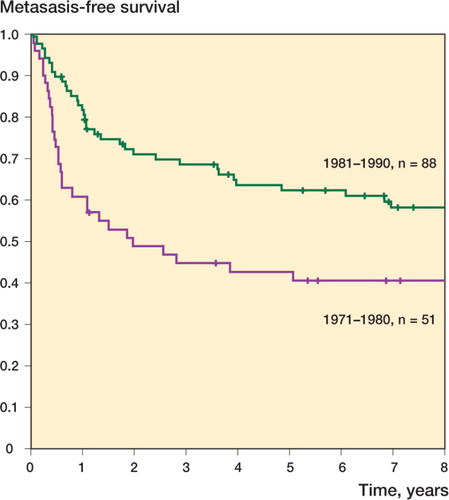
Of the 139 patients, 66 (47%) developed metastatic disease. 5-year metastasis-free survival (MFS) was 43% in the 1970s and 62% in the 1980s (p=0.01) (). 51 patients developed metastatic disease during the 2 first years of follow-up, and 61 during the first 5 years. Isolated lung metastasis was the most common pattern of first metastasis seen in 51 patients, followed by lung combined with at least one other site (8 patients); 6 patients had skeletal metastases only. Patients who developed metastatic relapse within 10 months of diagnosis had worse overall sarcoma-specific survival, with a 2-year survival rate of 18%—as opposed to 73% in those who developed metastases later than 10 months after diagnosis of the primary tumor (p<0.001).
Figure 2. Metastases-free survival plotted as a function of time in 139 patients with a primary localized osteosarcoma at diagnosis.
Of the 51 patients with isolated lung metastases, 25 underwent thoracotomy and metastasectomy, 30 received chemotherapy, and 14 were treated with both chemotherapy and metastasectomy. 23 patients died from first metastasis and 1 patient with first metastasis died from complications of therapy. 4 patients developed no further metastases and have remained disease-free at follow-up (8–14 years). 38 patients developed further metastases. Only 1 patient who had second metastatic relapse (both first and second relapse in the lungs) remains disease-free at follow-up (after 8 years).
Local recurrence
Local recurrence-free survival (LRFS) at 5 years for the whole study population was 82%, and separate 5-year LRFS values for the 2 decades (1971– 1980 and 1981–1990) were 81% and 82%. All 22 local recurrences developed during the first 4 years of follow-up; 19 during the first 2 years. Of the 17 patients with an axial tumor and of the 122 patients with a peripheral tumor, 8 patients and 14 patients (respectively) had local recurrences. Of the 14 local recurrences in peripheral tumors, 8 occurred after limb-sparing surgery (23 patients) and 6 after amputation or exarticulation (96 patients). For patients with a peripheral tumor, the LRFS rates at 5 years were similar in the 1970s and 1980s (85% and 88%, respectively). 15 patients showed both local and distant recurrence.
Univariate analysis on prognostic factors
Four factors (diagnosis in the 1970s as opposed to diagnosis in the 1980s, development of metastatic disease, development of local recurrence, and adequate chemotherapy) were statistically significant with respect to overall survival. For metastasis-free survival, diagnosis in the 1970s as opposed to diagnosis in the 1980s and tumor size (largest diameter) were statistically significant factors in univariate analysis ().
Discussion
To our knowledge, only three nationwide, popu-lation-based studies on clinical presentation and prognosis of osteosarcoma have been published— but without histological reexamination (Larsson and Lorentzon Citation1974, Curry et al. Citation2006, Stiller et al. Citation2006). Stiller et al. (Citation2006) collected information on all patients with osteosarcoma in Britain during 1980–1994 from the National Registry of Childhood Tumours, regional cancer registries in England, the national cancer registries in Scotland and Wales, the UK Children's Cancer Study Group register, special bone tumor centers, and the Birmingham Bone Tumour Service. The 5-year survival rates were 42% for 1980–1984 and 54% for 1985–1989. In multivariate analysis, trial entry and centralization of the treatment to specialist cancer centers were predictive of improved survival. Lars-son and Lorentzon (1974) retrospectively studied all malignant primary bone tumors diagnosed and reported to the National Cancer Registry in Sweden from 1958 through 1968. The study included 242 osteosarcomas, but no survival rates were reported. Curry et al. (Citation2006) retrospectively studied all patients with osteosarcoma in New Zealand 1994– 1999 and compared survival in this cohort to data retrieved from a similar study from 1981 through 1987. There were 96 cases in the 1981–1987 cohort and 84 cases in the 1994–1999 cohort. The 5-year overall survival from osteosarcoma improved from 32% to 44% between the cohorts.
Our study involves only tumors for which original histological specimens were available for review, to obtain a homogenous material. Of the 302 primary osteosarcomas diagnosed in 1971– 1990 and reported to the Finnish Cancer Registry, 199 (66%) were available for review and the diagnosis of osteosarcoma was confirmed in 166 (83%) of these cases. During previous decades, the diagnostic services were not centralized and the local pathologists lacked experience and immunohistochemical methods for diagnosis of bone tumors. Many malignant tumors were classified as osteosarcomas because of their location in bone, even though the tumors did not resemble osteosarcomas histologically.
In our study, formal inclusion in the trial as opposed to no formal inclusion yielded overall survival rates of 69% vs. 55% at 5 years, with borderline statistical significance (p=0.06). This is in agreement with the results published by Stiller (Citation1989). In the present study, chemotherapy was arbitrarily defined as being of adequate intensity if methotrexate was administered at least preoperatively, with any form of chemotherapy after surgery. Adequate chemotherapy had prognostic significance for overall survival in univariate analysis, but it is noticeable that most patients (69 of 76) who had received adequate chemotherapy were treated in the second decade (1981–1990).
In univariate analysis, development of local recurrence had adverse prognostic effect on overall survival. Furthermore, of the 22 patients who had local recurrence 15 also developed distant recurrence. Nearly half of all patients (47%) developed metastatic disease at some point in the course of their disease, with the lungs being the most frequent site of first metastases (77% of first metastatic relapses). Development of metastatic disease was prognostic of poor survival. When analyzed according to decade, however, the overall survival in patients who developed metastatic disease improved significantly, from 8% to 19% at 5 years. This improvement was mainly due to a more aggressive approach to treatment of metastatic disease by chemotherapy and metastasectomy. Patients who developed metastatic disease in a shorter time than the median metastasis-free survival (10 months) had statistically significantly worse sarcoma-specific survival than patients who developed metastatic disease after the median metastasis-free interval. That longer disease-free interval is associated with improved survival has also been reported by Harting et al. (Citation2006). This suggests that there should be more frequent follow-up at the beginning, in order to detect and aggressively treat distant disease.
This is the first nationwide report from Finland on the results of treatment of osteosarcoma. The 5-year overall survival for the whole study population was 58%. This is comparable to the survival rates published elsewhere. In a small country such as Finland (with a population of 5.1 million) where only about 15 primary osteosarcomas are diagnosed annually, it is important to concentrate treatment to only a few centers to ensure high-quality treatment.
MMS was supported by grants from the K. Albin Johansson Foundation and from Finska Läkaresällskapet. The study was also supported by the Helsinki University Central Hospital Research Funds.
No competing interests declared.
MMS and RS examined patient files, reviewed clinical data, did the statistical analysis, and prepared the draft and final versions of the manuscript in collaboration with the other authors. MT, MHT, and AHK contributed ideas and an outline of the study, and prepared the final manuscript together with the other authors. TOB reviewed all histological slides, contributed ideas and an outline of the study, and prepared the final manuscript together with the other authors.
References
- Bielack S S, Kempf-Bielack B, Delling G, Exner G U, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 2002; 20: 776–90
- Curry H, Horne G, Devane P, Tobin H. Osteosarcoma in New Zealand: an outcome study comparing survival rates between 1981-1987 and 1994-1999. N Z Med J 2006; 119: U2234
- Fletcher C D. M, Unri K K, Mertens F. WHO Classification of tumors pathology and genetics tumors of soft tissue and bone. IARC Press, Lyon 2002
- Harting M T, Blakely M L, Jaffe N, Cox C S, Jr., Hayes-Jordan A, Benjamin R S, Raymond A K, Andrassy R J, Lally K P. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatr Surg 2006; 41: 194–9
- Huvos A G, Osteogenic sarcoma. Bone tumors. Diagnosis., treatment and prognosis. W.B. Saunders, Philadelphia 1991; 2: 85–276, Anonymous
- Larsson S E, Lorentzon R. The incidence of malignant primary bone tumors in relation to age., sex and site. A study of osteogenic sarcoma., chondrosarcoma and Ewing's sarcoma diagnosed in Sweden from 1958 tto 1968. J Bone Joint Surg (Br) 1974; 56: 534–40
- Saeter G, Alvegard T A, Elomaa I, Stenwig A E, Holmstrom T, Solheim O P. Treatment of osteosarcoma of the extremities with the T-10 protocol., with emphasis on the effects of preoperative chemotherapy with single-agent high-dose methotrexate: a Scandinavian Sarcoma Group study. J Clin Oncol 1991; 9: 1766–75
- Smeland S, Muller C, Alvegard T A, Wiklund T, Wiebe T, Bjork O, Stenwig A E, Willen H, Holmstrom T, Folleras G, Brosjo O, Kivioja A, Jonsson K, Monge O, Saeter G. Scandinavian Sarcoma Group Osteosarcoma Study SSG VIII: prognostic factors for outcome and the role of replacement salvage chemotherapy for poor histological responders. Eur J Cancer 2003; 39: 488–94
- Soderstrom M, Ekfors T O, Bohling T O, Teppo L H, Vuorio E I, Aro H T. No improvement in the overall survival of 194 patients with chondrosarcoma in Finland in 1971–1990. Acta Orthop Scand 2003; 74: 344–50
- Stiller C A. Survival of patients with cancer. Bmj 1989; 299: 1058–9
- Stiller C A, Passmore S J, Kroll M E, Brownbill P A, Wallis J C, Craft A W. Patterns of care and survival for patients aged under 40 years with bone sarcoma in Britain, 1980–1994. Br J Cancer 2006; 94: 22–9
- Teppo L, Pukkala E, Lehtonen M. Data quality and quality control of a population-based cancer registry. Experience in Finland. Acta Oncol, StockholmSweden 1994; 33: 365–9