Abstract
Using cerium carbonate, lanthanum oxide and manganese nitrate as raw materials, ultrafine particles of Ce–La–Mn mixed oxides were prepared by the sol–gel method combined with supercritical drying technology. The prepared materials were characterised by thermo-gravimetric and differential thermal analysis, X-ray diffraction, Fourier transform infrared spectroscopy and transmission electron microscope. The catalytic properties of ultrafine Ce–La–Mn mixed oxides were tested by the reaction ‘2CO + 2NO = 2CO2 + N2’. The key aim was to examine the effect of supercritical fluid drying technology and Ce-doping on the crystal structure, morphology and catalytic activity of ultrafine Ce–La–Mn mixed oxides. The results show that at 260°C, Ce–La–Mn mixed oxides are brown loose flocculent powders with good dispersibility. The particles are spherical about 10 nm in size. The main crystal components are CeO2, MnO, La5O7NO3 and La(OH)2NO3. After thermal treating at 850°C, Ce–La–Mn mixed oxides were made up of plenty of quasi-global grains smaller than 20 nm; the main crystal components of the Ce–La–Mn mixed oxides are LaMnO3+ λ , La2O3 and CeO2; heat treatment can enhance the crystallinity of the materials. Cerium-doped mixtures just exist as crystal CeO2, which contributes to the crystallisation of Ce–La–Mn mixed oxides in the process of supercritical fluid drying at 260°C, enhancing the catalytic activity of ultrafine Ce–La–Mn mixed oxides which are thermal treated at 850°C.
1. Introduction
Mixed rare earth oxide ultrafine particles have exceptional luminescent, magnetic and electronic properties due to their unfilled 4 f electronic structures. Among them, perovskite-like LaMnO3+ λ is an important three-way catalyst for purifying automobile exhausts Citation1–4. For enhancing the catalytic activity, there are two main methods. One is the ultrafining process. The smaller the diameter of the catalyst, the bigger the specific surface area and the higher the catalytic activity. The other is doping. A fit doping can make defects or change the ions’ valence states in lattice, which lead to better catalytic activity of perovskite Citation5. Ceria is widely used as an oxygen ion conductor in solid oxide fuel cells, oxygen pumps and amperometric oxygen monitors because of its high oxygen conductivity Citation6,Citation7. Ce-doping is aimed to satisfy the catalytic condition and increase the amount of oxygen vacancy, which may enhance the catalytic activity of LaMnO3+ λ Citation8,Citation9.
The methods of preparing mixed oxides include the co-precipitation method and the mechanical mixing method. In these two kinds of preparation processes, the product prepared has characteristics, such as smaller specific surface, lower catalytic activity, higher initiation temperature and shorter service life, which have limited its further use Citation10,Citation11. Compared with these, sol–gel method is a more popular method, because of the advantages, such as well-proportioned grains, big specific surface area, high purity, chemical uniformity, low thermal treating temperature and so on Citation12. Supercritical fluid drying is the method of drying the gel in the solvent's supercritical condition. In this condition, the solvent is in the fluid state, the interface of gas–liquid vanishes, acting force among molecules is reduced and the surface tension is decreased. So, gel skeleton structure would not be changed much after the solvent leaves slowly. Thus, this technology can effectively solve the problem of agglomeration which usually occurs in the process of preparing ultrafine particles Citation13.
In this article, we prepared Ce–La–Mn mixed oxides by sol–gel method combined with supercritical drying technology, discussed the influence of Ce-doping on catalytic property of ultrafine Ce–La–Mn mixed oxides and tried to find a breakthrough to study a new rare earth mixed oxide as catalyst for purifying NO in automobile exhausts.
2. Experimental
2.1. Preparation
Lanthanum nitrate and cerium nitrate solutions with certain concentration were prepared by dissolving lanthanum oxide and cerium carbonate in concentrated nitric acid (AR, 63%). Lanthanum nitrate, cerium nitrate and manganese nitrate (AR, 50%) were mixed according to the molar ratio as 1:1:1, then ammonia solution was added dropwise to prepare Ce–La–Mn hydrosol under high-speed stirring. The hydrosol was separated by high-speed centrifuge and washed several times with absolute ethyl alcohol until that filtrate was neutral, which gave Ce–La–Mn alcogel. La–Mn alcogel was prepared by the same method but the molar ratio of lanthanum nitrate and manganese nitrate is 1:1. La–Mn and Ce–La–Mn alcogels were dried in ethyl alcohol's supercritical condition (260°C, 8 MPa), and then marked as LM260 and CLM260, respectively.
2.2. Thermal treating
LM260 and CLM260 were thermal treated for 2 h under air atmosphere at 850°C, and then marked as LM850 and CLM850, respectively.
2.3. Characterisation
X-ray diffraction (XRD) analysis was performed with a Philips X’ pert diffractometer using Cu-Kα radiation scanning from 10° to 90° with the velocity of 3°/min. The morphology of the particles was observed with a JEM-2000FXII transmission electron microscope (TEM). The thermogravimetric and differential thermal analysis (TG-DTA) was obtained by a WCT-1A microcomputer differential thermal balance. The Fourier transform infrared (FT-IR) spectra were obtained by a NICOLET170SX infrared absorption spectrometer.
2.4. Catalytic activity measurement
The catalytic activity was evaluated in a multiple fixed-bed continuous flow microreactor, and the molar ratio of NO and CO is 0.01:1. The gas space velocity is 1200 h−1. Masses of both the catalysts LM850 and CLM850 are 0.52 g. Concentrations of NO and CO were analysed online by 7890II gas chromatograph (produced by Tianmei Company, Shanghai. Chromatographic column used is Proapak Q column).
3. Results and discussion
3.1. XRD analysis
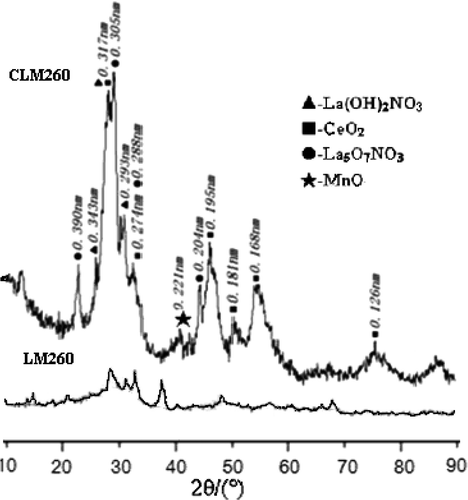
In , it is showed that after supercritical drying at 260°C, crystallinity of Ce–La–Mn mixed oxides is higher than those without cerium. As ceria is easily formed by dehydration, and thereafter crystallisation, the peaks of MnO and La5O7NO3 appeared in CLM260 Citation14. No diffraction peak of perovskite-type LaMnO3+ λ was found in these two patterns, which proved that crystal LaMnO3+ λ cannot be prepared at 260°C. At the same time, it is observed in that only the corresponding basic salt can be got when lanthanum nitrate reacts with alkali. The main corresponding reactions are Equations (Equation1) and (Equation2):
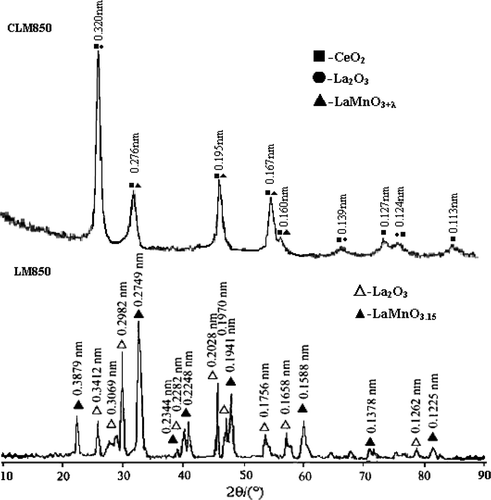
Comparing LM850 with CLM850, the diffraction peaks of Ce-doped LaMnO3+ λ are wider, and it is more amorphous than LaMnO3+ λ , which shows that its crystallinity is lower than pure LaMnO3+ λ . So, the number of oxygen vacancies is enhanced after Ce-doping Citation17.
From , because of active chemical property, rare earth cerium (Ш) was oxidised to give ceria (in which the cerium is Ce(IV)) by oxygen in air at 260°C. Ce(IV) cannot form a perovskite structure with Mn2+, because the formation of perovskite structure is limited by the geometric factor that the tolerance factor t must be limited between 0.75 and 1.00. The tolerance factor t is related with the ionic radii of A, B and O as Equation (Equation4), where r A, r B and r O are the ionic radii of A, B and O in perovskite-type oxides (ABO3), respectively Citation18.
3.2. Thermal analysis
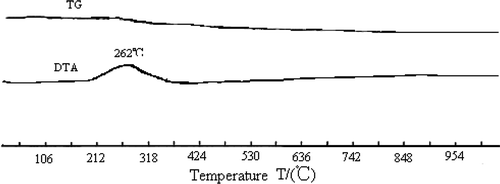
The TG-DTA plot for CLM260 is shown in . There is a distinct exothermic peak at 262°C in the DTA curve corresponding to a mass loss of 3.05% in TG curve. It is more likely to be related to the combustion of alcohol adsorbed on the surface of the sample. In the area between 350°C and 900°C, there were two reactions happening in the system. One is the endothermic process where La2O3 precursors (La5O7NO3 and La(OH)2NO3) are transformed to La2O3 by dehydration. The other is an exothermic one, where simple oxides formed perovskite composite oxides by bulk diffusion and rearrangement. Combining the two reactions, there is a wide slow exothermic process corresponding to the 1.22% mass loss in TG curve.
3.3. TEM analysis
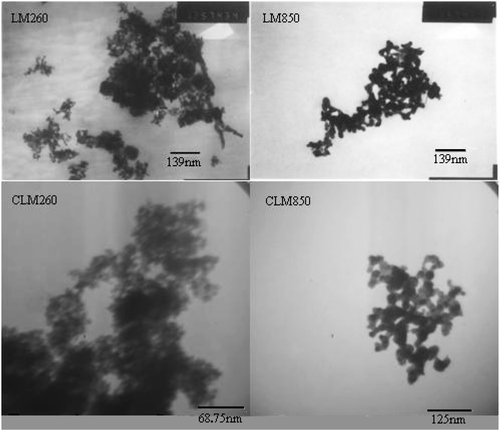
shows the TEM micrographs of different Ce–La–Mn mixed oxides. At 260°C, both LM260 and CLM260 mixed oxides are brown loose flocculent powders with good dispersibility and made up of mass of global grains about 10 nm in size, which indicates that supercritical fluid drying technology solves the problem of agglomeration effectively. After being thermal treated at 850°C, both LM850 and CLM850 mixed oxides are made up of plenty of quasi-global grains about 20 nm in size. Comparing LM260 and CLM260 with LM850 and CLM850, it is inferred that the materials’ diameter increased after thermal treating Citation19, which is due to Ce–La–Mn mixed oxides dehydration. It is showed that the influence of cerium on the size of Ce–La–Mn mixed oxides is little.
3.4. FT-IR analysis
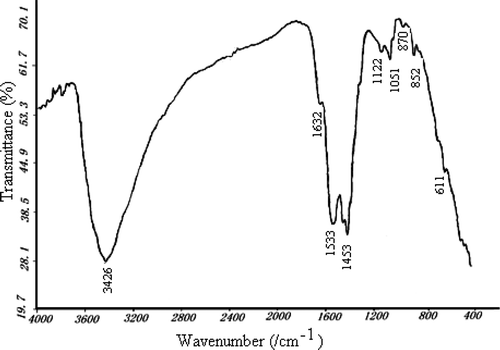
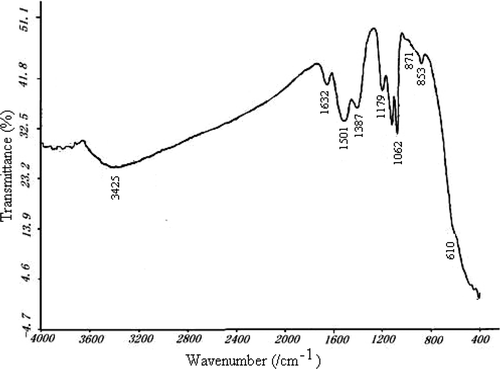
and shows the FT-IR spectra of CLM260 and CLM850, respectively. The corresponding ascriptions with the peaks in the spectra are shown in . The peak whose wave number is about 3426 cm−1 corresponds to stretching vibration of bond O–H. Wave number 1632 cm−1 corresponds to bending vibration of bond C–O. Wave numbers 1533–1387 cm−1 correspond to the stretching vibration of bond N–O. Wave numbers 1179–1051 cm−1 correspond to stretching vibration of bond C–O.
Table 1. Ascription of FT-IR adsorption peaks in and .
Wave numbers 870 cm−1, 852 cm−1 and 611 cm−1 correspond to the stretching vibration of bonds Ce–O, La–O and Mn–O, respectively. and both show La–O, Ce–O and Mn–O groups in FT-IR spectra, which is accordant with XRD analysis.
Comparing and , we see that the peak area and strength of bonds O–H, C–O and N–O decreased observably by thermal treating at 850°C, which shows that the impurity elements like C, H, N in Ce–La–Mn aerogel can be eliminated by thermal treating.
3.5. Catalytic activity
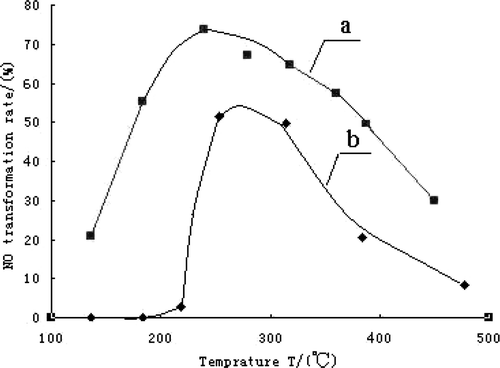
is the temperature profile of NO transformation rate over the CLM850 comparing with LM850. The maximum of NO transformation rate is 54% at 255°C in curve b and 71% at 240°C in curve a. From , it shows that the catalytic activity enhanced obviously after Ce-doping. There are two reasons for the enhancement of catalytic activity after Ce-doping.
First, a number of oxygen vacancies exist in Ce–La–Mn mixed oxides lattice. Oxygen vacancy is helpful for NO catalytic reduction. The N–O bond can easily be broken off when the oxygen atom in NO adsorbed on oxygen vacancy. The oxygen atom adsorbed on oxygen vacancy can produce CO2 by the catalytic oxidation of CO. Catalytic mechanism of oxygen vacancy is given below Citation20:
Second, CeO2 can improve the ambi-condition of redox catalysis, as shown in the two processes below Citation9. In the oxidation process, CeO2 transforms to Ce2O3 and brings free radical oxygen. Then, free radical oxygen combines with CO while CO2 is produced. Free radical oxygen may affect the migration of the perovskite lattice oxygen, which also advances the catalytic activity of ultrafine LaMnO3+ λ .
In , NO transformation rate rises with increasing temperature at first. But at high temperature, NO transformation rate declines with increasing temperature. The reason is that the reaction 2CO + 2NO = 2CO2 + N2 is exothermic. When temperature increases, the balance moves to endothermic reaction which is the reverse. So, it is necessary to choose the right temperature to ensure the high yield and good rate for NO transformation reaction.
4. Conclusions
Ultrafine perovskite-type LaMnO3+ λ can be prepared by the sol–gel method combined with supercritical drying technology, which solves the problem of agglomeration effectively in the process of preparing ultrafine particles. At 260°C, Ce–La–Mn mixed oxides are brown loose flocculent powder with good dispersibility. The particles are spherical, about 10 nm in size. The main crystal components are CeO2, MnO, La5O7NO3 and La(OH)2NO3. After thermal treating at 850°C, Ce–La–Mn mixed oxides were made up plenty of quasi-global grains smaller than 20 nm; the main crystal components of the Ce–La–Mn mixed oxides are LaMnO3+ λ , La2O3 and CeO2; Heat treatment can enhance the crystallinity of the materials. Cerium-doped mixture just exists as crystal CeO2, which contributes to the crystallisation of Ce–La–Mn mixed oxides in the process of supercritical fluid drying at 260°C, enhancing the catalytic activity of ultrafine Ce–La–Mn mixed oxides which are thermal treated at 850°C.
Acknowledgements
The authors gratefully acknowledge the financial support by Key Scientific Research Project of Hubei Province Education Department (2002A01018), Research Project for Excellent Talents in University of Hubei (2002B11004) and Important Research Fund of Wuhan University of Science and Technology (2008XY12).
References
- Davide , F and Lucio , F . 1998 . Methane combustion on some perovskite-like mixed oxides . Appl. Catal. B: Environ. , 16 : 119 – 126 .
- Seiyama , T . 1992 . Total oxidation of hydrocarbons on perovskite oxides . Catal. Rev. Sci. Eng. , 34 : 281 – 300 .
- Yong , F and Yu , Y . 1975 . The oxidation of hydrocarbons and CO over metal oxides IV perovskite-type oxides . J. Catal. , 36 : 266 – 275 .
- Rojas , ML , Fierro , JLG , Tejuca , LG and Bell , AT . 1990 . Preparation and characterization of LaMn1-xCuxO3+λ perovskite oxides . J. Catal. , 124 : 41 – 51 .
- Teiji , N and Makoto , M . 1983 . Reduction-oxidation and catalytic properties of La1-xSrxCoO3 . J. Catal. , 83 : 151 – 153 .
- Arai , H , Hamada , J , Eguchi , K and Seiyama , T . 1986 . Catalytic combustion of methane over various perovskite-type oxides . Appl. Catal. , 26 : 265 – 276 .
- Roy , S , Marimuthu , A , Hegde , MS and Madras , G . 2007 . High rates of NO and N2O reduction by CO, CO and hydrocarbon oxidation by O2 over nano crystalline Ce0.98Pd0.02O2−δ: Catalytic and kinetic studies . Appl. Catal. B: Environ. , 71 : 23 – 31 .
- Nolan , M , Parker , SC and Watson , GW . 2005 . The electronic structure of oxygen vacancy defects at the low index surfaces of ceria . Surf. Sci. , 595 : 223 – 232 .
- Shapovalov , V and Metiu , HJ . 2007 . Catalysis by doped oxides: CO oxidation by AuxCe1−xO2 . J. Catal. , 245 : 205 – 214 .
- Shu , J and Kaliaguine , S . 1998 . Well-dispersed perovskite-type oxidation catalysts . Appl. Catal. B: Environ. , 16 : L303 – L308 .
- Simonot , L , Garin , F and Maire , G . 1997 . A comparative study of LaCoO3, Co3O4 and LaCoO3-Co3O4 I. Preparation, characterisation and catalytic properties for the oxidation of CO . Appl. Catal. B: Environ. , 11 : 167 – 179 .
- Hench , LL and West , JK . 1990 . The sol-gel process . Chem. Rev. , 90 : 33 – 72 .
- Cao , Y , Hu , J , Hong , Z , Deng , J and Fan , K . 2002 . Characterization of high-surface-area zirconia aerogel synthesized from combined alcohothermal and supercritical fluid drying techniques . Catal. Lett. , 81 : 107 – 112 .
- Choi , HJ , Moon , J , Shim , HB , Han , KS , Lee , E and Jung , KD . 2006 . Preparation of nanocrystalline CeO2 by the precipitation method and its improved methane oxidation activity . J. Am. Ceram. Soc. , 89 : 343 – 345 .
- Wang , CX , Dou , BS , Fan , SR , Yu , ZL , Xie , XF and Wu , Y . 1984 . Defects in perovskite-type catalysts CaxLa1-xMnO3+λ and their role in ammonia oxidation . Sci. China Ser. B, , 8 : 12 – 22 .
- Colón , G , Navío , JA , Monaci , R and Ferino , I . 2000 . CeO2–La2O3 catalytic system Part I. Preparation and characterisation of catalysts . Phys. Chem. Chem. Phys. , 2 : 4453 – 4459 .
- Wang , Y , Wang , Y , Meng , YL , Ding , H and Shan , Y . 2008 . A highly efficient visible-light-activated photocatalyst based on Bismuth- and Sulfur-Co doped TiO2 . J. Phys. Chem. C, , 112 : 6620 – 6626 .
- Pena , MA and Fierro , JLG . 2001 . Chemical structures and performance of perovskite oxides . Chem. Rev. , 101 : 1981 – 2017 .
- Markovic , D , Kusigerski , V , Tadic , M , Blanusa , J , Jaglicic , Z , Cvjeticanin , N and Spasojevic , V . 2010 . The influence of the heat treatment on the structural and magnetic properties of nanoparticle La0.7Ca0.3MnO3 prepared by glycine–nitrate method . J. Alloys Compounds , 494 : 52 – 57 .
- Ferri , D , Forni , L , Dekkers , MAP and Nieuwenhuys , BE . 1998 . NO reduction by H2 over perovskite-like mixed oxides . Appl. Catal. B: Environ. , 16 : 339 – 345 .