Abstract
This article reports the synthesis and characterisation of Barium titanate (BaTiO3) nanocomposite and its application as opto-electronic humidity sensor. Titanium tetrachloride and barium hydroxide were mixed in molar ratio 1 : 1 in deionised water under continuous stirring at room temperature. Later, sodium hydroxide solution was added to above solution with continuous stirring. Finally, BaTiO3 gel was obtained. The synthesised nano-composite material was characterised using a scanning electron microscope, X-ray diffraction (XRD) and UV-Visible spectrophotometer. SEM image of the composite film shows that the film is porous having uniform grains. From XRD the minimum crystallite size of BaTiO3 was found to be 8 nm using Debye–Scherer formula. UV-Visible absorption spectroscopy was used for optical characterisation of the film. It was found that the optical band gap of the composite material was 3.50 eV. Barium titanate thin film was deposited on the base of an equilateral prism using sol–gel spin coating process at 4000 rpm. The humidity sensing properties of the film was investigated at different angles of incidence. It was observed that the intensity of reflected light increased with an increase in relative humidity (%RH) in the range 5–95% at a particular angle of incidence. Sensing element has maximum sensitivity ∼6 µW/%RH, which is quite significant for sensor fabrication purposes.
1. Introduction
Humidity sensing is becoming even more important, mainly in control systems for industrial processes and human comfort. A wide variety of ceramics, polymers, metal oxides and their composites have been investigated as humidity sensors [Citation1–7]. These sensors are mainly based on the variations in electrical parameters such as impedance, resistance and capacitance [Citation8–16]. Another category of the sensors are also available, the principle of which is based on the variations in optical parameters such as refractive index (r.i.), intensity modulation, frequency shift and wavelength modulation [Citation17–19]. They are frequently called optical/opto-electronic sensors. Optical sensors have tremendous advantages over their electrical counterpart as they can operate without any interference from nearby electric or magnetic fields. A wide variety of ceramics, polymers, metal oxides and their composites have been already investigated as humidity sensors [Citation20–22]. Most of the humidity sensors are based on modulation in electrical parameters such as resistance, capacitance or conductance of the sensing materials. Few reports of humidity sensors based on optical measurements are also available [Citation20]. Metal oxides such as TiO2, SnO2, BaO, etc. are considered to be the promising materials for the study because of their inherent chemical and physical stability. TiO2 is a wide and direct band gap semiconductor with many important properties, which makes it commonly used in electronic and optoelectronic applications. BaTiO3 is one of the important lead-free ferroelectric materials. In the past decades, a great interest has been devoted to ferroelectric materials due to their attractive surface morphology and sensing behaviour.
2. Thin film deposition technique
The deposition of thin film functional layers on various substrates is an essential step in many fields of modern technology having wide range of application. The most important methods in the field of interest are subdivided into physical and chemical methods. Physical methods may be characterised by an adsorption and condensation process of the atoms or molecules at the substrate surface. Chemical method involves chemical reaction and so-called precursor molecules dissociate at the hot substrate surface and release the atoms of interest.
Among the chemical deposition methods, chemical solution deposition (CSD) is best suited in our case. This method comprises a range of deposition techniques and of chemical routes. The process starts with the preparation of a suitable coating solution from precursors according to the designated film composition and the chemical route to be used. The coating solution is then deposited onto the substrate and the wet film may undergo drying, hydrolysis and condensation reactions depending on the chemical route. The main advantages of CSD are the excellent control of the film composition through the stoichiometry of the coating solution, easy fabrication and relatively low capital investment.
3. Experimental
3.1. Synthesis of BaTiO3
All chemical reagents used for the preparation of BaTiO3 nanocomposite were of AR grade. The BaTiO3 was prepared by sol–gel method. The process involved the mixing of titanium tetrachloride and barium hydroxide in deionised water under continuous stirring at room temperature. The titanium tetrachloride and barium hydroxide was taken in a molar ratio 1 : 1. Further, 4 mL of sodium hydroxide solution was added drop by drop to the above solution under continuous stirring. The dropping rate must be well controlled for the chemical homogeneity and uniform stoichiometries. The concentration of the final solution was ∼0.8 M. The resulting gel was used as the precursor for preparing the film. The prepared film was annealed at 600°C to obtain the BaTiO3. Overall, the chemical reaction is given as below:
3.2. Preparation of BaTiO3 thin film
Sol–gel spin coating technique is one of the best methods of CSD used to deposit uniform thin films [Citation23]. It is advantageous over to other conventional thin film techniques because it requires less equipment and is potentially less expensive. Besides this, the microstructure and uniformity of the deposited film can easily be controlled by regulating the preparation condition namely, solution concentration/fluid density, fluid viscosity, speed of the spinner [Citation24, Citation25]. In our case, we acquired an equilateral prism as substrate. The substrate was cleaned using an ultrasonic cleaner. A thin film of BaTiO3 gel was deposited on the base of an equilateral prism substrate using a photo-resist spinner at a speed of 4000 rpm and dried for 15 min at 60°C.
4. Characterisations
4.1. Scanning electron microscopy
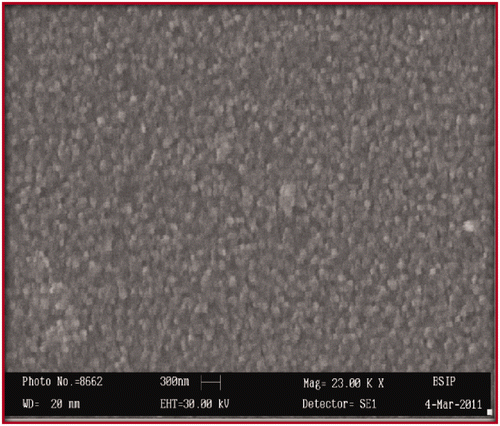
Surface morphology of the sensing material in form of the composite film was investigated using Scanning electron microscope (SEM, LEO-0430, Cambridge). shows a typical SEM image of the composite film at 300 nm scale and 23 KX magnifications. This image exhibits that the film is porous having uniform grains. The average pore size was estimated as 90 nm.
4.2. X-ray diffraction
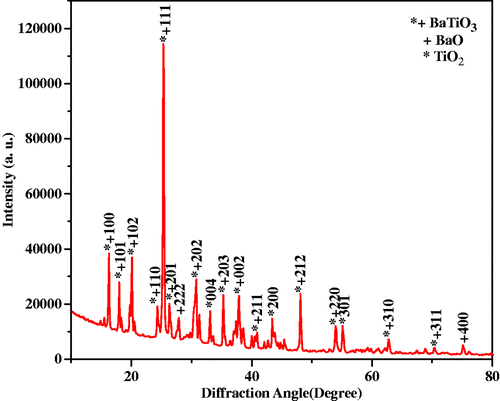
The formation and phase of the film was investigated with X-ray diffractometer recording system X’Pert Pro (PANalytical, The Netherlands) using CuKα (λ = 1.5406 Å) radiation. The instrument was operated using power: 45 kV, 40 mA and scanning parameters: 2θ from 10° to 80°. The X-ray diffraction (XRD) pattern shown in reveals the major formation of BaTiO3, with minor formation of BaO and TiO2. Most of the reflections correspond to BaTiO3. Miller indices [h k l ] for each oxide are indicated on each of the diffraction peaks. The crystal structure of BaTiO3 was hexagonal with lattice parameters a = b = 4.9569 Å, c = 13.9648 Å, α = β = 90°, γ = 120°. Minimum crystallite size was found to be 8 nm using Debye–Scherer formula.
4.3. UV-Visible absorption spectroscopy
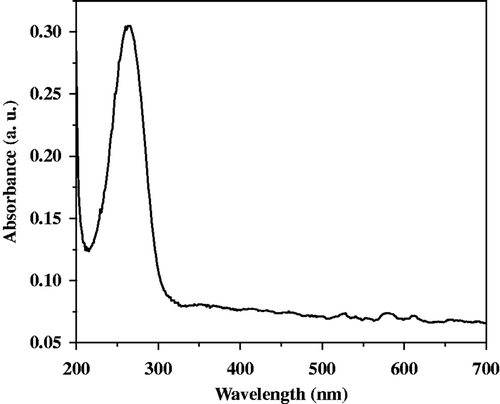
Optical characterisation of the sample was done by using UV-Visible spectrophotometer (Varian, Carry-50 Bio). Variation in optical absorption with wavelength is shown in . Maximum absorption was found at wavelength 260 nm. The optical band gap of the composite material was found to be 3.50 eV.
5. Principle of operation
At the interface between two dielectric media the ratio of reflected light intensity (I r) to incident light intensity (I i) for an unpolarised light beam obtained from Fresnel equations can be expressed as [Citation23]
6. Results and discussion
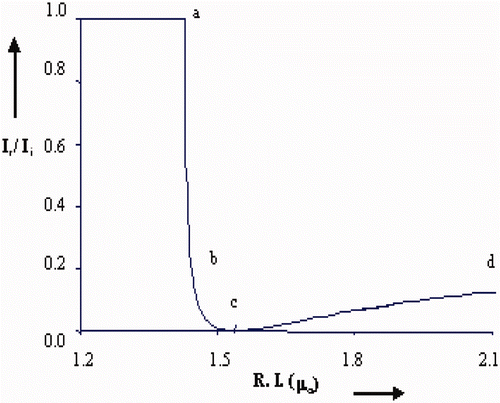
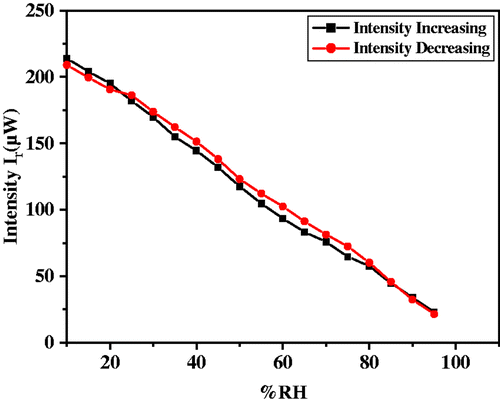
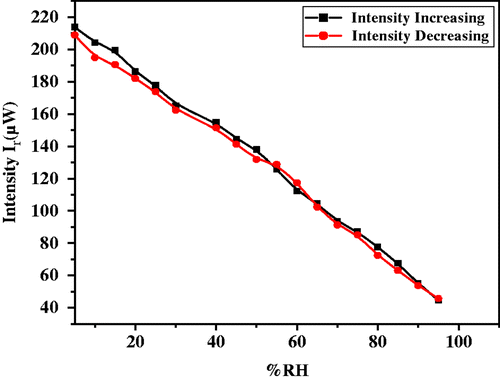
As the capacitive and resistive type humidity sensors have certain limitations, therefore we propose an optical humidity sensor based on modulation in intensity of light due to variation in %RH. For this purpose, the film was exposed to humidity in a controlled humidity chamber. As humidity inside the chamber increases continuously from 10 to 90 %RH, water vapour (humid air) replaces the dry air already present in the pores of the film, which causes continuous increase in r.i. of the film from that of the r.i. of the film in dry air. The increase in humidity increases the rate of adsorption of water vapours and their condensation inside the pores of the film causing an increase in the r.i. of the film. As humidity inside the chamber increases continuously from 10 to 90 %RH, water vapour replaces the dry air which was already present in the pores of the film. The increase in humidity increases the adsorption of water vapours on the surface and their condensation in the pores of the film causing an increase in the r.i. and thickness of the film. The result obtained by experimental investigation is shown in and . These figures show the variation in reflected intensity with %RH for angles of incidence θ i = 52° and 55°, respectively. Figure for θ i = 52° shows slow variation in intensity up to 75 %RH and then rapid increase in intensity. However, curves for θ i = 52° and 55° have identical nature but with shift in intensity. A linear nature can be viewed for the curve with θ i = 55° in entire range of %RH. In case of curve representing variation in intensity for θ i = 55°, there is fast variation in intensity in low humidity region when comparing with other curve in the same region. In the medium and higher humidity region curves show linear nature.
The average sensitivity of the sensor can be defined as
7. Humidity sensing mechanism of BaTiO3
Ceramic sensors are based on the adsorption of water molecules in the pores, and variations of the structure of the adsorbed layer, as a function of humidity. The sensing mechanism of BaTiO3 ceramic humidity sensors at room temperature can be explained using ionic conduction model. The adsorption of water vapours increases with increase in relative humidity. When few water molecules are available at low humidity, they chemisorb on grain surfaces of the ceramic to form hydroxyl groups as surface charge carriers. When initial water molecules are adsorbed, each water molecule is hydrogen-bonded to two hydroxyls, and the dominant surface charge carriers will be H3O+. When more amount of water is absorbed, clustering of the water molecules takes place, forming a film of hydrogen-bonded water molecules, where each water molecule is only single bonded to a hydroxyl group. Since dissociation of H3O+ into H2O and H+ is energetically favourable in liquid water, the dominant charge carrier in high moisture environment is H+.
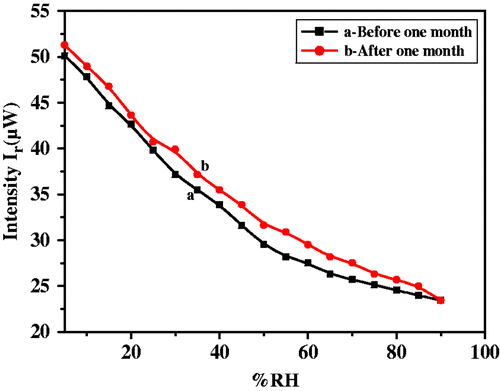
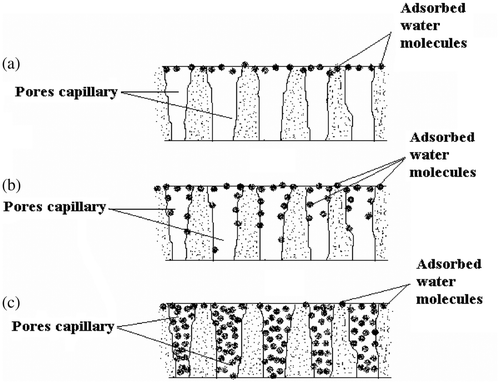
The sensing mechanism, devoted to the surface morphology is based on adsorption of water vapour and capillary condensation. The adsorption mechanism of water vapour with increase in relative humidity is shown in . The range of %RH was divided into three regions namely, dry (below 40 %RH), medium (40–70 %RH) and very humid (above 70 %RH). Initially the BaTiO3 nanocomposite film was free from water molecules, having only dry air in its pores. When exposed to low humidity environment, adsorption of water vapour takes place rapidly on the surface of film. Due to high cationic charge density of metal oxide surface, the electrostatic force is high enough to break one of the O–H bonds of the H2O and form a strong chemical bond between M+ and OH−. Thus, initial layer is chemisorbed. As the %RH increases, adsorption on surface increases. However, in 40–70 %RH range, adsorption takes place on the surface as well as on the walls of capillaries, while in very humid region the condensation of water vapour takes place through the capillaries and forms a meniscus in the capillaries of the film. As the base surface of the prism was coated with a nanocrystalline BaTiO3 nanocomposite, the glass-film interface behaved like a humidity sensor. Moisture detection would depend significantly on the porosity and thickness of the deposited film, as the extent of adsorption of water vapours causes the increase in r.i. of the film.
8. Conclusion
We have successfully fabricated an opto-electronic humidity sensor using a thin film of BaTiO3 nanocomposite on an equilateral borosilicate prism by sol-gel spin coating technique. SEM image shows that the film is porous having uniform grains. From XRD, the minimum crystallite size of BaTiO3 was found to be 8 nm. The sensing element shows maximum sensitivity 6.5 µW/%RH, which is quite significant for sensor fabrication purposes. Being opto-electronic in nature, it can also be employed for remote sensing applications for humidity.
Acknowledgements
Authors are thankful to Department of Science and Technology, India, for financial support in the form of FAST-TRACK project (SR/FTP/PS-21/2009).
References
- Kulwicki , BM . 1991 . Humidity sensors . J. Am. Ceram. Soc. , 74 : 697 – 708 .
- Chen , Z and Lu , C . 2005 . Humidity sensors: A review of materials and mechanism . Sens. Lett. , 3 : 274 – 295 .
- Yamazoe , N and Shimizu , Y . 1986 . Humidity sensors: Principles and applications . Sens. Actuators , 10 : 379 – 398 .
- Traversa , E . 1995 . Ceramic sensors for humidity detection: The state-of-the art and future developments . Sens. Actuators B , 23 : 135 – 156 .
- Morimoto , T , Nagao , M and Tokuda , F . 1969 . The relation between the amounts of chemisorbed and physisorbed water on metal oxides . J. Phys. Chem. , 73 : 243 – 248 .
- Shimizu , Y , Arai , H and Seiyama , T . 1985 . Theoretical studies on the impedance-humidity characteristics of ceramic humidity sensors . Sens. Actuators , 7 : 11 – 22 .
- Yeh , YC , Tseng , TY and Chang , DA . 1990 . Electrical properties of TiO2–K2Ti6O13 porous ceramic humidity sensor . J. Am. Ceram. Soc. , 73 : 1992 – 1998 .
- Grange , H , Bieth , C , Boucher , H and Delapiere , G . 1987 . A capacitive humidity sensor with very fast response time and very low hysteresis . Sens. Actuators , 12 : 291 – 296 .
- Nahar , RK , Khanna , VK and Khokle , WS . 1984 . On the origin of the humidity-sensitive electrical properties of porous aluminium oxide (sensor application) . J. Phys. D Appl. Phys. , 17 : 2087 – 2095 .
- Basu , S , Chatterjee , S , Saha , M , Bandyopadhay , S , Mistry , KK and Sengupta , K . 2001 . Study of electrical characteristics of porous alumina sensors for detection of low moisture in gases . Sens. Actuators B , 79 : 182 – 186 .
- Thoma , P , Colla , J and Stewart , R . 1979 . A capacitance humidity-sensing transducer . IEEE Trans. Compon. Hybrids Manufact. Technol. , 2 : 321 – 323 .
- Yadav , BC , Srivastava , R , Dwivedi , CD and Pramanik , P . 2008 . Moisture sensor based on ZnO nanomaterial synthesized through oxalate route . Sens. Actuators B , 131 : 216 – 222 .
- Yadav , BC , Srivastava , R and Dwivedi , CD . 2008 . Synthesis and characterization of ZnO–TiO2 nanocomposite and its application as humidity sensor . Phil. Mag. , 88 : 1113 – 1124 .
- Srivastava , R , Yadav , BC , Dwivedi , CD and Kumar , R . 2007 . Comparative study of moisture sensing properties of ZnO nanomaterials through hydroxide route by mixing dropwise and sudden . Sens. Trans. J. , 80 : 1295 – 1301 .
- Yadav , BC , Srivastava , R and Dwivedi , CD . 2007 . Synthesis and characterization of ZnO nanorods by the hydroxide route and their application as humidity sensors . Synth. React. Inorg. Metal-Org. Nano-Metal Chem. , 37 : 417 – 423 .
- Yadav , BC , Srivastava , AK and Sharma , P . 2007 . Resistance based humidity sensing properties of TiO2 . Sens. Trans. J. , 81 : 1348 – 1353 .
- Brook , TE , Taib , MN and Narayanaswamy , R . 1997 . Extending the range of fiber-optic relative-humidity sensor . Sens. Actuators B , 39 : 272 – 276 .
- Kharaz , A and Jones , BE . 1995 . A distributed optical-fiber sensing system for multi-point humidity measurement . Sens. Actuators A , 47 : 491 – 493 .
- Walbran , S and Kornyshev , AA . 2001 . The proton transport in polarizable water . J. Chem. Phys. , 114 : 10039 – 10048 .
- Yadav , BC , Yadav , RC and Dwivedi , PK . 2010 . Sol–gel processed (Mg–Zn–Ti) oxide nano-composite film deposited on prism base as an opto-electronic humidity sensor . Sens. Actuators B , 148 : 413 – 419 .
- Yeh , YC , Tseng , TY and Chang , DA . 1990 . Electrical properties of TiO2–K2Ti6O13 porous ceramic humidity sensor . J. Am. Ceram. Soc. , 73 : 1992 – 1998 .
- Grange , H , Bieth , C , Boucher , H and Delapierre , G . 1987 . A capacitive humidity sensor with very fast response time and very low hysteresis . Sens. Actuators , 12 : 291 – 296 .
- Yadav , BC , Pandey , NK , Srivastava , AK and Sharma , P . 2007 . Optical humidity sensors based on titania films fabricated by sol–gel and thermal evaporation methods . Meas. Sci. Technol. , 18 : 260 – 264 .
- Meyerhofer , D . 1978 . Characteristics of resist films produced by spinning . J. Appl. Phys. , 49 : 3993 – 3997 .
- Carcano , G , Ceriani , M and Soglio , F . 1993 . Spin coating with high viscosity photo resist on square substrates-application in the thin hybrid microwave integrated circuit field . Microelectron. Int. , 10 : 12 – 20 .