Abstract
This paper reports on the synthesis of spherical silver nanoparticles and the consequent formation of self-assembled humic-silver supramolecules. The method described in this article is eco-friendly and the supramolecules formed were stable for several months. The silver nanoparticles themselves were synthesised from humic acid extracted from mudbank sediments (from Aleppey, south west coast of India) and characterised by UV-Vis spectra and HRTEM. Tuning of dipole and quadrouple oscillation of spherical nanoparticles was achieved. Variable self-assembled distribution mechanism of humic-silver nanoparticles in the solution responsible for quadrouple intensification was investigated.
1. Introduction
Natural humic materials have been increasingly used in the synthesis of anisotropic nanoparticles. They have also been used successfully in developing nanosensors Citation1–5. Although the supramolecular nature and the reducing potential of humic acid have been the subject of independent investigations, their applications in medical/technological/industrial areas have received scant attention Citation6–12.
The reducing property and polyelectrolyte nature of the humic acid is due to the presence of polar functional groups such as phenols, hydroxyls, carbohydrate subunit and the presence of carboxylic acids Citation9,Citation10. The presence of aromatic and aliphatic moieties in the humic acid molecule renders it amphiphilic and influences its interaction with metals/organics leading to the formation of self-assembled structures Citation13. The reduction potential and self-assembling nature of humic acid can be made use of in the synthesis of chemically engineered metal nanostructures, especially of silver and gold which, because of their plasmonic properties, find application in optical sensing and biomedicine Citation14–16.
Amphiphiles, comprising of polarisable head groups, are used in generating second harmonic signals for nonlinear optical response. Marine humic acid amphiphiles with supramolecular electron (donor–acceptor network) bridged by condensed aromatic rings and conjugated double bonds are cheap source for the green synthesis of molecular units, capable of nonlinear optical response which will find application in optoelectronics. This article reports on the use of humic acid in a single-step synthesis of silver nano particles in aqueous media. The factors that regulate the distribution of silver nano particles are also investigated.
2. Experimental
2.1. Standardisation of humic acid
Humic acid used in the present study was extracted and purified Citation17 from mudbank sediment (refered to as ‘Chakara’ in local language) collected from Punnapra, near Aleppey on the Kerala coast. Mudbanks are unique annually occurring phenomena, confined to the southwest coast of India during the southwest monsoon season (June–September) Citation18–20. They are known as ‘Zones of Bio-rhythm’ as they shelter to abundant aquatic life. Humic acids extracted from the mudbank sediments were standardised by CPMAS 13C solid state NMR (Bruker AVANCE 300) Citation21 and FTIR (Thermo nicolet, Avathar 370). NMR spectral data were analysed using Bruker topspin 1.3 software.
2.2. Synthesis of silver nanoparticles
Stock solutions of humic acid and silver nitrate were prepared. Varying concentrations of silver nitrate (ranging between) 0.0005 to 2.0 mol l−1 were transferred to separate boiling tubes containing 10 ml aliquots of 10 ppm solutions of humic acid (prepared by appropriate dilution of humic acid stock solution using Milli-Q water). Several sets of trial experiments were conducted to optimise the reaction times and conditions. The boiling tubes containing the reactant mixtures were heated in an air oven at 85–90°C while maintaining a pH of about 11.5. A whitish gray precipitate formed immediately upon the addition of silver nitrate solution initially turned colourless and thereafter to pale yellow and golden yellow depending on the concentration of the silver nitrate, indicating the formation of the silver nanoparticles. The time taken for synthesis of silver nanoparticles varied from 15 min to 1 h depending on the concentration of the silver nitrate. This solution was found to be stable for several months.
2.3. Characterisation of silver nanoparticles
The resultant solution of silver nanoparticles was further analysed using UV-Vis spectroscopy (Genesis 10 UV) and HRTEM (JOEL3010). UV-Vis spectral analysis enabled the optical characterisation of silver nanoparticles, while TEM analysis provided information on distribution and shape of these particles. The samples for electron microscopy were prepared by drop casting. Drops of aqueous solution of humic acid protected silver nanoparticles were added to carbon coated copper grids which were dried overnight at room temperature and thereafter used for further analysis.
3. Results and discussion
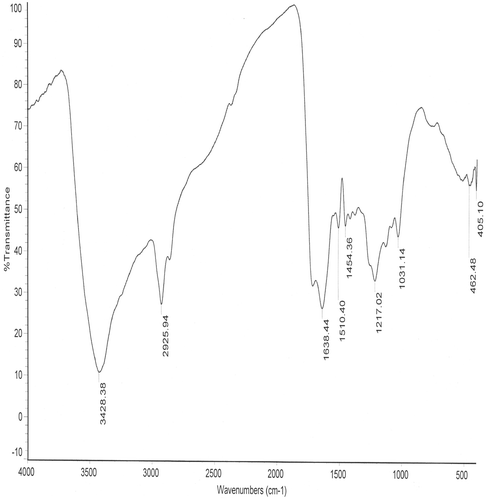
NMR studies and gave evidence of the polyeletrolytic and the amphiphilic nature of humic acid and revealed the presence of oxygenated functional groups in it Citation21, Citation22. FTIR analysis and confirmed the presence of polar functional groups, which facilitated the reduction of alkaline silver nitrate to silver nanoparticles and . The silver nano particles formed were stabilised by the polyeletrolytic nature of humic acid.
Figure 2. CPMAS C-13 solid state NMR spectra of humic acid molecule extracted from mudbank sediments, south west coast of India.
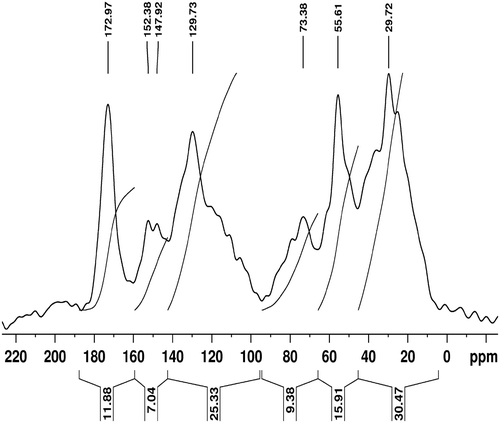
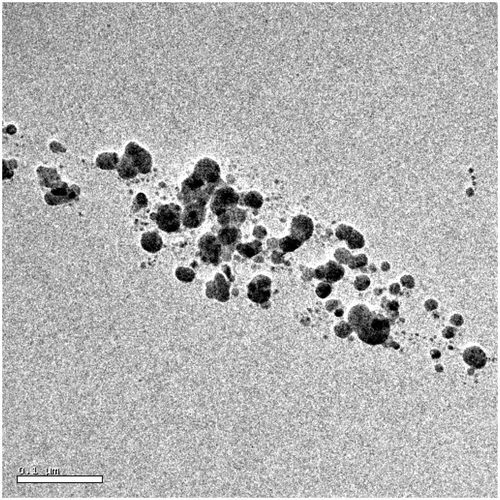
Figure 3. HRTEM images representing the spherical silver nanoparticles synthesised at 0.002 mol l−1 concentration of silver nitrate.
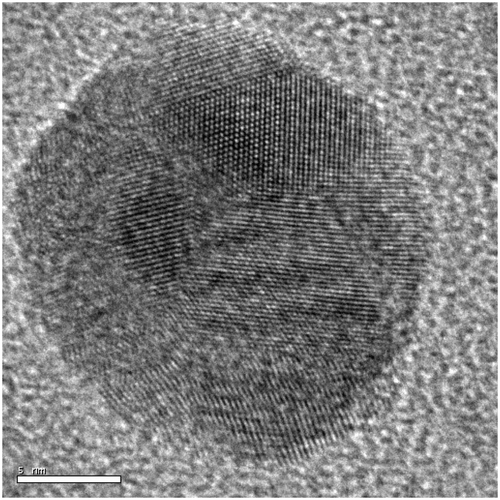
Figure 4. HRTEM image of spherical nanoparticle synthesised at 0.008 mol l−1 concentration of silver nitrate.
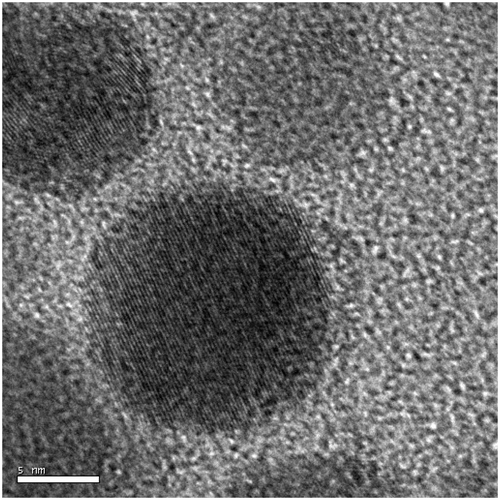
Table 1. Assignment of IR peaks of humic acid molecule extracted from the mudbank sediments.
Table 2. Assignment of solid state C-13 CPMAS spectral data of humic acids extracted from mudbank sediments.
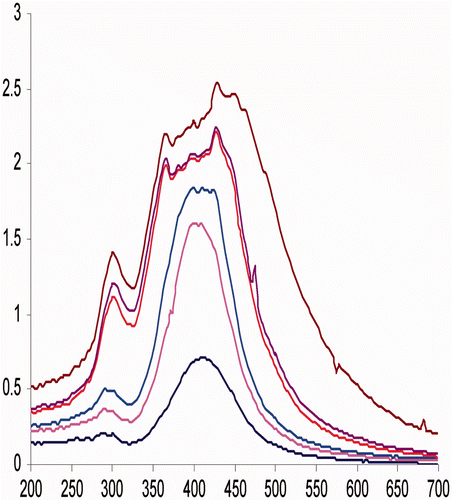
, the trough at 320 nm, the shoulder at 370 nm and the peak at 412 nm have together enabled the identification of silver nanoparticles formation. The 320 nm trough has been the result of an intraband transition, while the shoulder and the peak have been the result of plasmonic absorbances characteristic of spherical silver nanoparticles Citation23. The synthesis of particles at higher concentration of silver nitrate led to the intensification of 370 nm shoulder and to a red shift of 412 nm peak.
Figure 5. HRTEM images of humic protected silver nanoparticles synthesised at 0.002 mol l−1 of silver nitrate concentration. In which silver nanoparticles are poly-dispersed. Dark spots correspond to silver nanoparticles.
3.1. Self-assemblage (clustering) of silver nanoparticles
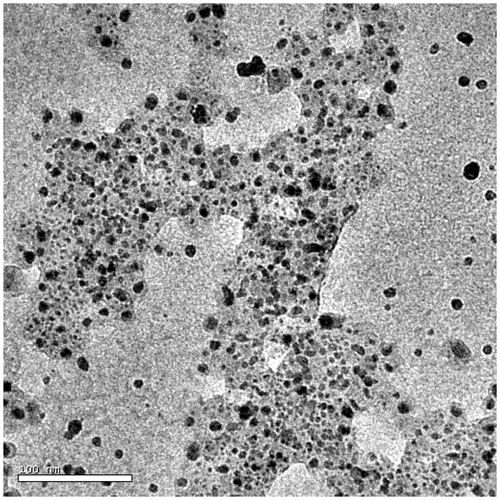
HRTEM images revealed the existence of silver nanoparticles in the solution phase, with variable distribution. This variable distribution is a direct consequence of the amphiphilic nature and of the concentration of silver nanoparticles. Particles synthesised at lower concentrations were found to be polydispersed (), while those at higher concentration were found to be clustered ().
3.2. Consequence of self-assembled supramolecules
The rearrangement of a higher population of nanoparticles to self-assembled humic silver nano-supramolecules directly influenced the plasmonic properties of the solution (). The humic-silver nano-supramolecule solution shows an intensified quadruple peak and the highest peak underwent a red shift by ∼20 nm. The shape and size of the nanoparticles, distribution of the charge, density and effective mass of the electron along with the dielectric property of the media and near field coupling etc., directly influence the plasmonic oscillation Citation24–32. Even though particles synthesised are spherical in nature, intensification of quadruple resonance at 370 nm and red shifting of dipole resonance by ∼20 nm may be considered as a direct consequence of near field coupling.
4. Conclusion
The green synthesis of humic-silver nanoparticles with intensified quadruple peak was attempted. Green synthesis utilised the reducing and stabilisation potential of humic acid molecule. Unique property of humic acid amphiphile to self-assemble, and to bring particles in close proximity was studied to understand the mechanism of quadruple (multipole) intensification in humic-silver nanoparticles. The importance of exploring marine resources for the generation of eco-friendly nanomaterials was also emphasised.
Acknowledgements
The authors are grateful to the financial assistance by University Grant Commission, India and Central NMR facility in National Chemical Laboratory, Pune for Solid state 13C-NMR and Indian Institute of Technology, Madras for HRTEM analysis.
References
- Airoldi , FPS , Silva , WTL , Crespilho , FN and Rezende , MOO . 2007 . Evaluation of the electrochemical behavior of pentachlorophenol by cyclic voltammetry on carbon paste electrode modified by humic acids . Water. Environ. Res. , 79 : 63 – 67 .
- Alvarez-Puebla , RA , dos Santos , DS Jr and Aroca , RF . 2007 . SERS detection of environmental pollutants in humic acid–gold nanoparticle . Composite Materials Analyst , 132 : 1210 – 1214 .
- Baigorri , R , García-Mina , JM , Aroca , RF and Alvarez-Puebla , RA . 2008 . Optical enhancing properties of anisotropic gold nanoplates prepared with different fractions of a natural humic substance . Chem. Mater. , 20 : 1516 – 1521 .
- Crespilho , FN , Zucolotto , V , Siqueria , JR Jr , Constantino , CJ , Nart , FC and Oliveira , ON Jr . 2005 . Immobilization of humic acid in nanostructured layer-by-layer films or sensing applications . Environ. Sci. Technol. , 39 : 5385 – 5389 .
- Stephan , DT and Vimolvan , P . 2008 . Humic acid assisted synthesis of silver nanoparticles and its application to herbicide detection . Mater. Lett. , 62 : 2661 – 2663 .
- Baalousha , M , Motelica-Heino , M , Galaup , S and Le Coustumer , P . 2005 . Supramolecular structure of humic acids by tem with improved sample preparation and staining . Microsc. Res. Tech. , 66 : 299 – 306 .
- Baalousha , M , Motelica-Heino , M and Le Coustumer , P . 2006 . Conformation and size of humic substances: Effects of major cation concentration and type, pH, salinity, and residence time . Colloids and Surfaces A: Physicochem. Eng. Aspects , 272 : 48 – 55 .
- Osterberg , R and Shirshova , L . 1997 . Oscillating, nonequilibrium redox properties of humic acids . Geochim. Cosmochim. Acta , 61 : 4599 – 4604 .
- Bauer , M , Heitmann , T , Macalady , DL and Blodau , C . 2007 . Electron transfer capacities and reaction kinetics of peat dissolved organic matter . Environ. Sci. Technol. , 41 : 139 – 145 .
- Schwarzenbach , R , Stierli , R , Lanz , K and Zeyer , J . 1990 . Quinone and iron porphyrin mediated reduction of nitroaromatic compounds in homogeneous aqueous solution . J. Environ. Sci. Technol. , 24 : 1566 – 1574 .
- Struyk , Z and Sposito , G . 2001 . Redox properties of standard humic acids . Geoderma , 102 : 329 – 346 .
- Changlun , C , Xiangke , W , Hui , J and Wenping , H . 2007 . Direct observation of macromolecular structures of humic acid by AFM and SEM . Colloids and Surfaces A: Physicochem. Eng. Aspects , 302 : 121 – 125 .
- Wandruszka , RV and Engebretson , R . 1997 . The role of selected cations in the formation of pseudomicelles in aqueous humic acid . Talanta , 44 : 805 – 809 .
- Lu , W and Lieber , CM . 2007 . Nanoelectronics from the bottom up . Nat. Mater. , 6 : 841 – 850 .
- Maier , SA . 2006 . Metal nanostructures for subwavelength photonic devices . IEEE J. Sel. Top. Quantum. Electron. , 12 : 1214 – 1220 .
- Willets , KA and Van Duyne , RP . 2007 . Localized surface plasmon resonance spectroscopy and sensing . Annu. Rev. Phys. Chem. , 58 : 267 – 297 .
- Swift , RS . 1985 . “ Fractionation of soil humic substances ” . In Fractionation of Soil Humic Substances in Soil, Sediments, and Water , Edited by: Aiken , GR , McKnight , DM , Wershaw , RL and McCarthy , P . 387 – 408 . Chichester : John Wiley & Sons .
- Nair , SM , Balchand , AN and Nambisan , PNK . 1993 . Phosphate fractionation in mudbank sediments from the south west coast of India . Hydrobiol. , 252 : 61 – 69 .
- Nair , SM and Balchand , AN . 1993 . Phosphate-phosphorus (ad-) sorption chracteristics of sediements from a very high productive coastal zone . Toxicol. Environ. Chem. , 39 : 81 – 95 .
- Tatavarti , R and Narayana , AC . 2006 . Hydrodynamics in a Mud Bank Regime during nonmonsoon and monsoon seasons . J. Coastal Res. , 22 : 1463 – 1473 .
- Conte , P , Spaccini , R and Piccolo , A . 2006 . Advanced CPMAS-13C NMR techniques for molecular characterization of size-separated fractions from a soil humic acid . Anal. Bioanal. Chem. , 386 : 382 – 390 .
- Brunsveld , L , Folmer , BJB , Meijer , EW and Sijbesma , RP . 2001 . Supramolecular polymers . Chem. Rev. , 101 : 4071 – 4098 .
- Kreibig , U and Vollmer , M . 1995 . Optical Properties of Metal Clusters, Springer Series in Materials Science , New York : Springer-Verlag .
- Mock , JJ , Barbic , M , Smith , DR , Schultz , DA and Schultz , S . 2002 . Shape effects in plasmon resonance of individual colloidal silver nanoparticles . J. Chem. Phys. , 116 : 6755 – 6759 .
- Kelly , KL , Coronado , E , Zhao , LL and Schatz , GC . 2003 . The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment . J. Phys. Chem. B. , 107 : 668 – 677 .
- Mock , JJ , Smith , DR and Schultz , S . 2003 . Local refractive index dependence of plasmon resonance spectra from individual nanoparticles . Nano. Lett. , 3 ( 4 ) : 485 – 491 .
- Amendola , V , Bakr , OM and Stellacci , F . 2010 . A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly . Plasmonics , 5 ( 1 ) : 85 – 97 .
- Wiley , BJ , Im , SH , Li , ZY , Mclellan , J , Siekkinen , A and Xia , Y . 2006 . Manuvering the surface plasmon resonance of silver nanostructures through shape – Controlled synthesis . J. Phys. Chem. B. , 110 ( 32 ) : 15666 – 15675 .
- Su , KH , Wei , QH , Zhang , X , Mock , JJ , Smith , DR and Schultz , S . 2003 . Interparticle coupling effects on plasmon resonances of nanogold particles . Nano. Lett. , 3 ( 8 ) : 1087 – 1090 .
- Rechberger , W , Hohenau , A , Leitner , A , Krenn , JR , Lamprecht , B and Aussenegg , FR . 2003 . Optical properties of two interacting gold nanoparticles . Opt. Commun. , 220 : 137 – 141 .
- Kottmann , JP and Martin , OJF . 2001 . Retardation-induced plasmon resonances in coupled nanoparticles . Opt. Lett. , 26 ( 14 ) : 1096 – 1098 .
- David Evanoff , D Jr and George , C . 2005 . Synthesis and optical properties of silver nanoparticles and arrays . Chem. Phys. Chem. , 6 : 1221 – 1231 .