Abstract
Monoclinic tungsten oxide (WO3) nanorods were grown using the hydrothermal method on a seeded W foil. The seed layer was formed by thermal oxidation of W foil at 400°C for 30 min. Cetyltrimethylammonium bromide (CTAB) or hexamethylamine (HMT) was used in the reactive hydrothermal bath, along with sodium tungstate dihydrate (Na2WO4.2H2O) and hydrochloric acid (HCl). The concentration of CTAB was varied from 0.01 M to 0.07 M and the concentration of HMT was varied from 0.01 M and 0.05 M. The result showed that CTAB-assisted hydrothermal reaction produced WO3 nanorods with 4–7 nm diameter, and provided that CTAB concentration was less than 0.07 M. WO3 nanorods could not be obtained when CTAB concentration was 0.07 M. Columnar structured WO3 was produced with the presence of HMT in the hydrothermal bath. This was due to decomposition of HMT to form hydroxyl ions (OH−) that inhibited the growth of nanorods. Cyclic voltammetry (CV) analysis showed better electrochromic property of WO3 nanorods compared to columnar structured WO3.
1. Introduction
Tungsten oxide (WO3) has attracted great attention since it has potential applications as electrochromic, photocatalytic and sensors [Citation1]. Out of these applications, electrochromic property is the most interesting as WO3 film has the ability to undergo optical colouration when voltage is applied. The colouration of WO3 film is useful in the formation of various optical devices. WO3 can change colour from yellowish-transparent to opaque-blue when ions like Li+, Na+ or H+ are inserted or intercalated in its structure. The electrochromism of WO3 has generated interest in the use of WO3 in energy-saving smart/dynamic windows, antiglare mirrors, high contrast displays and active camouflage [Citation2]. WO3 nanostructures have been prepared typically by common methods such as sol-gel, electrodeposition, chemical vapour deposition and sputtering. Hydrothermal reaction is a promising method to prepare various WO3 nanostructures due to its simple manner, low cost and large scale production.
Cetyltrimethylammonium bromide (CTAB) is a cationic surfactant and limited hydrothermal works have been done by using CTAB to produce WO3 [Citation3–9]. All these research studied on the formation of WO3 powder instead of directly grow WO3 film on a substrate. It is believed that by directly growing WO3 on a substrate, better contact between WO3 nanostructures and substrate could be obtained. Hexamethylamine (HMT) is a non-ionic surfactant that could be useful in the hydrothermal growth of WO3. To the authors’ knowledge, no HMT-prepared WO3 has been reported to date. To allow intercalation of ions, a large surface area of WO3 is desired. Huge surface area of WO3 can be achieved by the formation of thin WO3 with nanostructured grains. In this work, WO3, in the form of nanorods, was prepared using the seeded growth hydrothermal reaction on W foils. The foil was thermally oxidised to form a native oxide layer that was used as a seed layer. This seed layer acted as a template for the formation of WO3 nanorods by the hydrothermal reaction in a reactive solution.
2. Experimental details
W foil (1 × 1 cm2) was cleaned ultrasonically with ethanol for 15 min and subjected to thermal oxidation at 400°C for 30 min in air to form WO3 seeds. The seeded W foil was then placed in a screw capped bottle filled with the hydrothermal bath solution. This solution was prepared by dissolving 0.3 M sodium tungstate dihydrate (Na2WO4.2H2O) and surfactants of cetyltrimethylammonium bromide (C19H42BrN; CTAB) or hexamethylamine (C6H12N4; HMT) in deionised water. The amount of surfactants was varied between 0.01–0.07 M in order to investigate the effect of concentration of surfactant on the growth of WO3 nanorods. Hydrochloric acid (HCl) was added into hydrothermal bath solution with continuous stirring to obtain pH 2. The hydrothermal process was conducted at 80°C for 8 h. The morphology of samples was observed using field emission scanning electron microscope (FESEM, Zeiss SUPRA 35) and transmission electron microscope (TEM, Philips CM 12). The phases present in the samples were analysed using an X-ray diffractometer (XRD, Bruker AXS D8 ADVANCE), equipped with Cu Kα radiation. The electrochromic properties of samples were measured by cyclic voltammetry (CV) in 0.1 M sulphuric acid (H2SO4) electrolyte using Autolab (μAutolab Type III). The working electrode, counter electrode and reference electrode were the WO3 electrode, platinum electrode and Ag/AgCl electrode, respectively.
3. Results and discussion
WO3 nanorods were grown for 8 h in the hydrothermal reactive bath containing either CTAB or HMT. The morphologies of the samples are compared. Figure (a) shows the seed layer on W foil, which comprises of compact grains. After the hydrothermal reaction for 8 h with CTAB, WO3 nanorods were observed as in Figure (c)–(g). The mechanism of nanorods formation under the influence of CTAB can be explained with Equations (1) and (2)(1)
(2)
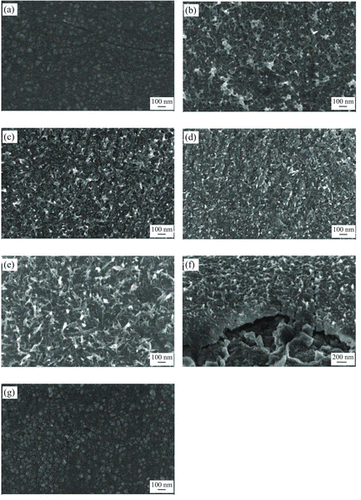
Figure 1. FESEM micrographs of: (a) thermal oxidised W foil, (b) WO3 prepared without surfactant and WO3 prepared with: (c) 0.01 M CTAB, (d) 0.03 M CTAB, (e) 0.05 M CTAB, (f) 0.05 M CTAB (cross sectional view) and (g) 0.07 M CTAB.
CTAB is a positively charged cationic surfactant with a long hydrophobic tail. Na2WO4.2H2O dissolved in deionised water and reacted with HCl to form H2WO4.nH2O. During the hydrothermal reaction at 80°C, H2WO4.nH2O decomposed on WO3 seed layer to form WO3. WO3 comprised of [WO6]6− octahedrons and CTA+ were then attracted to these [WO6]6− octahedrons and allowed one dimensional growth of WO3 nanorods.
For the sample prepared without surfactant, WO3 nanorods could still be obtained as shown in Figure (b). This was due to the presence of NaCl (from Equation (1)) that acted as a capping agent and adsorbed on the crystal planes parallel to the c-axes of WO3 nucleus [Citation10]. When a small amount of CTAB (0.01 M or 0.03 M) was used in the hydrothermal reaction solution, the WO3 nanorods formed were short as shown in Figure (c) and (d). This was due to the insufficient amount of CTAB, and the capping roles of NaCl became more dominant. With the addition of 0.05 M CTAB as in Figure (e) and (f), longer nanorods could be obtained. However, with further increase of CTAB to 0.07 M (Figure (g)), nanorod was not formed, but the film had flat and compact surface. This was due to the presence of excess CTAB in the hydrothermal reaction solution that covered the whole seeds surface. As a result, diffusion of ions could not take place and the formation of WO3 nanorods was inhibited. Figure shows the morphology of the samples prepared with 0.05 M CTAB for 10 h hydrothermal process. By comparing to the sample after hydrothermal reaction for 8 h (Figure (e) and (f)), 10 h sample showed more nanorods formation with better uniformity. This suggested that the hydrothermal reaction time is crucial for the nanorods formation as longer time allows more diffusion process to take place and forms more and longer nanorods.
Figure 2. Morphologies of WO3 prepared with 0.05 M CTAB for 10 h: (a) surface morphology and (b) cross sectional view.

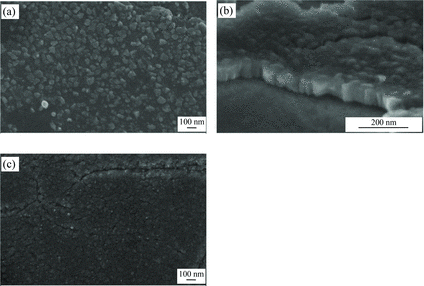
Figure shows the WO3 microstructures prepared using HMT. These HMT-prepared WO3 comprised of columnar structure as shown in Figure (b). It was further determined that HMT experienced thermal degradation during hydrothermal process and released hydroxyl ions (OH−). These OH− ions were then reacted with hydrogen ions (H+) from the acid and form water (H2O). The presence of water neutralised the hydrothermal reaction solution and inhibited the growth of WO3 nanorods. Since OH− reacted with H+ from the HCl, plenty of Cl− ions were left in the solution. Therefore, more Cl− ions were reacted with Na+ from the Na2WO4.2H2O and resulted in the formation of more NaCl. As NaCl would act as the capping agent, it then capped the WO3 seed layer and caused a slow diffusion process of WO42− into the seed layer. This slower process resulted in the formation of columnar structure WO3.
Figure 3. Morphologies of WO3 prepared with: (a) 0.01 M HMT, (b) 0.01 M HMT (cross sectional view) and (c) 0.05 M HMT.
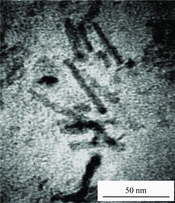
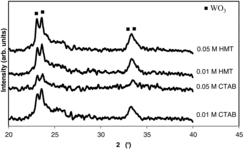
To investigate if indeed nanorods have formed on the surface of WO3, TEM analysis was performed. Figure shows the TEM image of the sample prepared with 0.05 M CTAB. It can be observed that WO3 nanorods have a diameter of 4–7 nm. The XRD patterns of the samples prepared with CTAB and HMT are shown in Figure . The spectra showed that both CTAB and HMT-prepared WO3 had a monoclinic phase that corresponded to ICDD number of 43–1035.
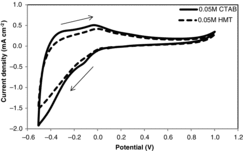
To evaluate the electrochromic properties of WO3 nanorods, cyclic voltammetry (CV) measurement was performed on the samples. The CV measurement was taken between −0.5 and 1.0 V with a scan rate of 100 mVs−1 in 0.1 M H2SO4 electrolyte. Figure shows the cyclic voltammogram of WO3 prepared with 0.05 M CTAB and 0.05 M HMT. With decreasing voltage, current moves negatively, corresponding to intercalation of H+ into WO3 and resulting in colouration of the film. With increasing voltage, current moves positively, which corresponds to de-intercalation of H+ from HxWO3 and resulting in bleaching of the film. This electrochromic process can be represented as:(3)
For the WO3 prepared with 0.05 M CTAB, a cathodic current peak could be observed at −0.10 V, which revealed that an electrochemical process took place at that voltage. By increasing the voltage, two anodic current peaks appeared at −0.30 V and −0.03 V, which corresponded to the shallow trap H+ sites and deep trap H+ sites, respectively [Citation11]. The integrated cathodic-current density is equal to the amount of H+ intercalated to form HxWO3 [Citation2]. By comparing cathodic charge quantities of WO3 prepared with 0.05 M CTAB (nanorods) and 0.05 M HMT (columnar), WO3 nanorods showed a higher charge-insertion density over the same period. This better electrochromic property of WO3 nanorods was due to the higher surface area of WO3 for faster intercalation and de-intercalation process.
4. Conclusions
WO3 nanorods were successfully prepared with CTAB surfactant (< 0.07 M) using the hydrothermal reaction at 8 h. Among these samples, sample with 0.05 M CTAB produced the longest nanorods. Moreover, the hydrothermal reaction using 0.05 M CTAB for 10 h produced more uniform and longer WO3 nanorods. On the other hand, columnar WO3 was obtained in the HMT-prepared samples as HMT thermally degraded in the hydrothermal solution that neutralised the solution and inhibited the growth of nanorods. CTAB-prepared WO3 nanorods showed excellent electrochromic property compared to HMT-prepared WO3 columnar.
Acknowledgements
The authors express their sincere appreciation to the technical support of the School of Materials and Mineral Resources Engineering, Universiti Sains Malaysia. This research was supported by MyBrain15, Ministry of Higher Education, Malaysia, Research University grant 1001/PBAHAN/814086 and PRGS 1001/PBAHAN/8044020.
References
- Wang J, Khoo E, Lee PS, Ma J. Controlled synthesis of WO3 nanorods and their electrochromic properties in H2SO4 electrolyte. J Phys Chem C. 2009;113:9655–9658.
- Lee SH, Deshpande R, Parilla PA, Jones KM, To B, Mahan AH, Dillon AC. Crystalline WO3 nanoparticles for highly improved electrochromic applications. Adv Mater. 2006;18:763–766.
- Li XL, Liu JF, Li YD. Large-scaled synthesis of tungsten oxide nanowires with high aspect ratio. Inorg Chem. 2003;42:921–924.
- Song X, Zhao Y, Zheng Y. Hydrothermal synthesis of tungsten oxide nanobelts. Mater Lett. 2006;60:3405–3408.
- Hong SJ, Jun H, Borse PH, Lee JS. Size effects of WO3 nanocrystals for photooxidation of water in particulate suspension and photoelectrochemical film systems. Int J Hydrogen Energy. 2009;34:3234–3242.
- Yayapao O, Thongtem T, Phuruangrat A, Thongtem S. CTAB-assisted hydrothermal synthesis of tungsten oxide microflowers. J Alloys Compd. 2011;509:820–826.
- Huang Q, Wang L, Wang M, Nan J. Preparation, characterization and the electrogenerated chemiluminescence behaviour of WO3 nanocrystals. J Alloys Compd. 2011;509:9901–9905.
- Liu S, Zhang F, Li H, Chen T, Wang Y. Acetone detection properties of single crystalline tungsten oxide plates synthesized by hydrothermal method using cetyltrimethyl ammonium bromide supermolecular template. Sens Actuators B. 2012;162:259–268.
- You L, Yang F, He X, Sun Y, Lu G. Highly sensitive NO2 sensor based on monodispersed WO3 nanoparticles. 14th International Meeting on Chemical Sensors; 2012 May 20–23; Nuremberg, Germany.
- Wang J, Khoo E, Lee PS, Ma J. Synthesis, assembly, and electrochromic properties of uniform crystalline WO3 nanorods. J Phys Chem C. 2008;112:14306–13412.
- Kim DJ, Pyun SI. Hydrogen transport through rf-magnetron sputtered amorphous WO3 film with three kinds of hydrogen injection sites. Solid State Ion. 1997;99:185–192.