Abstract
The paper reports a green chemistry approach for the synthesis of silver nanoparticles (AgNPs) using hypericin-rich shoot cultures of Hypericum hookerianum as reducing agent. Normal green shoot cultures deficient in hypericin and red-pigmented shoot cultures rich in hypericin (3.01% DW) were raised in Murashige and Skoog nutrient medium containing 1.0 mg/L kinetin (KIN) and 0.2 mg/L naphthaleneacetic acid (NAA), respectively. Dried powder extracts of whole shoots were used for AgNPs formation. The effect of temperature on the formation of AgNPs is investigated. The nanoparticles obtained were characterised using UV–Vis spectroscopy, field emission scanning electron microscopy (FESEM), energy-dispersive X-ray (EDX) and X-ray diffraction (XRD) analyses. The UV–Vis spectra of AgNPs gave surface plasmon resonance (SPR) at 440 nm. The synthesised AgNPs were effective against different multidrug-resistant human pathogens such as Bacillus subtillis (Gram positive) and Pseudomonas aeruginosa (Gram negative) species. Further, the effect of hypericin concentration on anti-bacterial activity was investigated and was found to increase with increase in concentration.
Keywords:
1. Introduction
The versatility of nanotechnology encourages researchers to develop modified syntheses derived from nature, which encompasses alternative approaches towards developing nanomaterials. Green synthesis being safe and ecologically sound for the nanoparticle synthesis is emerging into an important branch of nanotechnology.[Citation1] Nanoparticles derived from green process being versatile enough can be used in many types of technological applications, from delicate electronics to revolutionary medical procedures.[Citation2–8]
In advanced biomedical applications, nanoparticles play a key role to deliver pharmaceutics and can be used in novel diagnostic and therapeutic approaches.[Citation9] Further, functionalised, biocompatible and inert nanomaterials have potential applications in cancer diagnosis and therapy.[Citation10–13] Most commonly studied metal nanoparticles include gold, silver, titanium oxide and iron nanoparticles.[Citation14] Among metal nanoparticles, Ag has received considerable attention in the field of biology and medicine due to its effective cytotoxicity against cancer cells and it also exhibits effective anti-bacterial, anti-fungal, anti-viral, anti-inflammatory and anti-angiogenesis properties.[Citation15,16] Biologically assisted synthesis of metal nanoparticles using green chemistry approach is a key issue in nanoscience research.
In very recent times, nanotechnology is making inroads into plant medicine as well. The advantage of using medicinal plants is that they are easily available in a biodiversity-rich country like India, safe to human and animal health and possess a broad availability of metabolites that may aid in reduction of metal ions. The biomimetic approach of reducing Ag ions to Ag nanoparticles by using medicinal plant extracts is a simple and eco-friendly process against chemical synthesis and is relatively free from elaborate maintenance of microbial cultures required for microbial extracellular synthesis of nanoparticles. Rapid biomolecular hosting of biodegradation, non-toxic and green synthesis of Ag nanoparticles using therapeutic herbal extracts potentially useful in such areas as cosmetics, food and medicine have been already demonstrated in such field-derived species using Chenopodium album,[Citation17] Azadirachta indica,[Citation18] Sorbus aucuparia [Citation19] and Piper betle.[Citation20] However, uniform medicinal plant materials raised through tissue culture have so far not been reported to yield bioactive nanoparticles through such an approach. This would greatly help to develop future plant-based nanomedicine free from bacterial and fungal contaminants, pollutants and variations inherent in natural or cultivated plants.
Plants of the genus Hypericum (Hypericaceae) are herbs and shrubs traditionally used as medicinal plants to cure a range of symptoms and diseases in different parts of the world. Many of them are not yet scrutinised for bioactive metabolites. Hypericum hookerianum Wight & Arn., a perennial round-topped shrub distributed in Palni and Nilgiri hills of the Western Ghats in southern India, has folklore use as an anti-microbial in traditional practices and is pharmacologically confirmed to possess wound healing,[Citation21] anti-bacterial,[Citation21] cytotoxic [Citation22] and anti-tumour [Citation23] properties. In the present paper, we report for the first-time synthesis of silver nanoparticles (AgNPs) using hypericin-rich shoot cultures of H. hookerianum as reducing agent and their concentration-dependent anti-bacterial activity. The study opens up new possibility of synthesising nanoparticles using in vitro cultures.
2. Experimental
The auxin-induced, hypericin-rich shoot cultures of H. hookerianum were raised and maintained in Murashige and Skoog (1962) medium [Citation24] supplemented with 1.0 mg/L kinetin (KIN) and 0.2 mg/L naphthalene acetic acid (NAA) following the procedures described by Padmesh et al. [Citation25] Normal green shoots were grown in the same medium containing 1.0 mg/L KIN. These shoot cultures were used for the experiments. All the chemicals were obtained from Sigma-Aldrich and experiments done in triplicates. Milli-Q water was used throughout the experiments.
The hypericin-rich shoot cultures of H. hookerianum obtained were thoroughly washed and cut into small pieces. The finely cut pieces were shade dried at room temperature for a period of 48 hrs. The dried mass was ball milled (SPEX 8000D, UKRAINE) to obtain a fine powder. Ten grams of the fine powder was weighed and transferred to a beaker with 100 mL water and boiled for 10 min. The resulting extract was allowed to cool to room temperature and was filtered using Whatman number 1 filter paper. The filtrate was collected in a 250-mL Erlenmeyer flask and stored at 4 °C for further use.
2.1. Synthesis of AgNPs
The filtered extract was added to vigorously stirred 20 mL of 2 × 10−3 M aqueous AgNO3 solution at 300 K. The reduction of Ag+ ions to Ago was evident from the slow colour change and the reaction was completed within 1 hr with a stable light yellowish brown colour of the solution, which indicated the formation of colloid (S1).
Similarly, three (S2, S3 and S4) more sets of samples were prepared at 325 K, 350 K and 375 K, respectively. A slight colour change (dark complexion) was noticed in colloids S2 and S3. Colloid S4 was brown in colour. There was noticeable increase in the rate of reduction from S1 to S4.
2.2. Characterisation
The bioreduction of Ag+ ion in solution was monitored using UV–Vis absorption spectrophotometer (JASCO V-530). The metallic nature of the particles was analysed using X-ray diffractometer (INEL) with Co-Kα radiation. The morphology of the AgNPs was examined using field emission scanning electron microscope (FESEM - SUPRA 55 - CARL ZEISS, Germany) and the composition was estimated using energy-dispersive X-ray spectroscopy (EDX).
2.3. Anti-bacterial activity
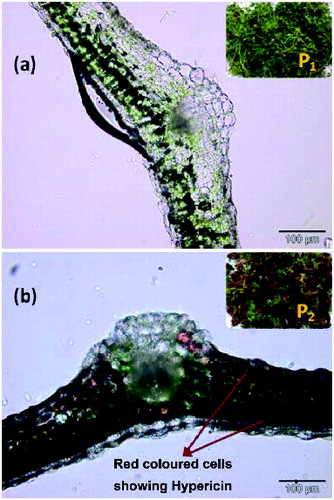
The effect of hypericin concentration on the anti-bacterial activity was studied. For this purpose, normal chlorophyllous shoot cultures deficient in hypericin (P1) raised in MS medium containing 1.0 mg/L KIN and hypericin-induced, red-coloured shoot cultures (P2) raised in medium fortified with 1.0 mg/L and 0.2 mg/L KIN were used. Thin leaf sections of P1 and P2 were examined under Olympus BX51 Upright microscope to observe the distribution of hypericin. The leaf sections of P1 showed normal anatomy with characteristic palisade and spongy parenchyma of the mesophyll, while P2 showed red-coloured cells scattered across different parts of the mesophyll, indicating the presence of hypericin (). AgNPs were synthesised using both P1 and P2 as reducing agents as described in Section 2.1 at 375 K.
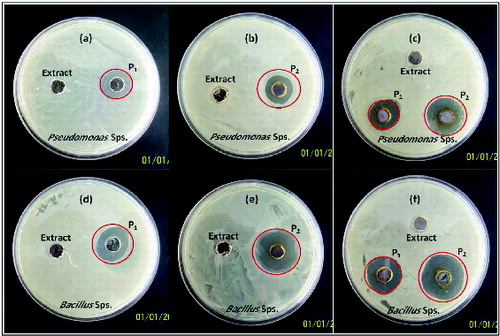
The anti-bacterial activity of the synthesised AgNPs was tested by using Bacillus subtillis (Gram positive) and Pseudomonas aeruginosa (Gram negative) species. The pure cultures of these bacteria were subcultured on nutrient agar medium in petri dishes and wells of 5 mm diameter were made on nutrient agar plates using gel puncture method. A solution of 30μL containing AgNPs was poured into one well to observe the zone of inhibition and in the other well 30 μL shoot extract was added as reference. The petri dishes were incubated at 37 °C for 24 hrs and the different levels of zone of inhibition were observed.
3. Results and discussions
3.1. UV–Vis spectral studies
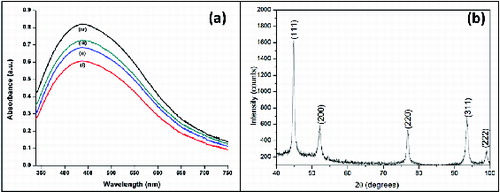
UV–Vis spectroscopy is an important technique to ascertain the formation and stability of metal nanoparticles in aqueous solution. (a) shows the UV–Vis spectra of the Ag colloids S1, S2, S3 and S4. Colour of Ag colloid is attributed to surface plasmon resonance (SPR) arising due to the collective oscillation of free conduction electrons induced by an interacting electromagnetic field.[Citation26] The SPR bands of the colloids are centred at 440 nm. The bands are broad and the intensity increases from S1 to S4 indicating the increase in production of nanoparticles with increase in temperature.
3.2. XRD analysis
The crystalline nature of Ag nanoparticles was confirmed by X-ray diffraction (XRD) analysis. (b) shows the XRD pattern of Ag nanoparticles obtained by using hypericin-rich shoot cultures of H. hookerianum as reducing agent. The prepared solution with AgNPs was drop coated onto a glass slide and was studied with INEL X-ray diffractometer. The diffraction pattern was recorded by Co-Kα1 radiation with λ of 1.78 Å in the region of 2θ ranging within 40o–100o at 0.02°/min and the time constant was 2 s.
The diffraction peaks at 44.80°, 52.28°, 76.78°, 93.48° and 98.96° correspond to the (111), (200), (220), (311) and (222) facets of the face-centred cubic lattice of Ag (JCPDS No: 03-0921). The (200), (220), (311) and (222) Bragg reflections are weak and broadened relative to the intense (111) reflection. This feature [Citation1] indicates that the nanocrystals are highly anisotropic and are (111) oriented. In addition, yet some unassigned peaks were also observed suggesting the crystallisation of bio-organic phase occurring on the surface of AgNPs.[Citation27]
3.3. FESEM studies
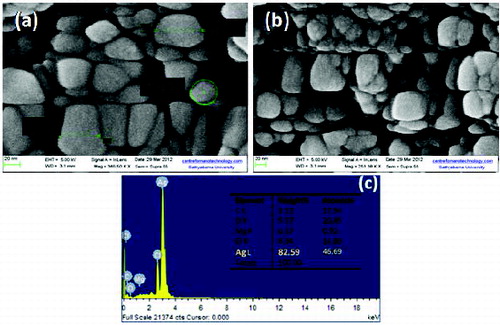
FESEM images of colloids S1 and S4 are shown in (a) and (b). The morphology of synthesised AgNPs with distorted spherical shape and size ranging within 20–65 nm was observed in S1. In colloids S4, the particle size ranging within 10–50 nm was observed. This decreased particle size and increase in production of nanoparticles with increasing temperature is a well-known phenomenon.[Citation28] Thus, the results obtained were in correlation with UV–Vis spectral studies.
3.4. EDX studies
The energy-dispersive spectrum ((c)) revealed the clear identification of the elemental composition profile of the synthesised nanoparticles, which suggests the presence of Ag as the ingredient element. The qualitative analysis showed that the weight percent of Ag is 82.59. Metallic AgNPs typically show an optical absorption peak at 3 keV due to the SPR.[Citation29] However, other elemental signals along with AgNPs were also recorded, which were not dominant in comparison with Ag and hence ignored.
3.5. Anti-bacterial study
Recently, Klabunde et al. [Citation30] demonstrated that reactive metal oxide nanoparticles show excellent bactericidal effect. It has been known for a long time that Ag ions and Ag compounds are highly toxic to most bacterial strains.[Citation31]
The present study focussed on the effect of concentration of hypericin on anti-bacterial activity. The synthesised AgNPs exhibited excellent anti-bacterial activity against pathogens of Gram-positive and Gram-negative bacteria. The result suggests that the synthesised AgNPs undergo interaction with bacterial cell and thereby exhibiting strong action against B. subtillis (Gram positive) and P. aeruginosa (Gram negative) species. The formation of clear zone with restricted bacterial growth around the cavity as shown in is an indication of anti-bacterial activity. The result showed that the anti-bacterial activity exhibited by P2 with both Bacillus and Pseudomonas species is far higher than P1. Further, both P1 and P2 exhibited better anti-bacterial activity with Bacillus species, which can be evidenced from the increase in the diameter of zone of inhibition.
4. Conclusions
Ag nanoparticles were synthesised using shoot cultures of H. hookerianum as reducing agent. The spectroscopic characterisation from UV–Vis, FESEM, EDX and XRD analyses supports the formation, composition and stability of the biosynthesised nanoparticles. The synthesised AgNPs were found to have more anti-bacterial activity with Gram-positive than Gram-negative species. As AgNPs prepared from hypericin-rich P2 extracts showed significantly higher anti-bacterial activities than those of P1, the enhanced activity of the former may be attributed to hypericin abundantly present in them.
Acknowledgements
The authors acknowledge the support from Sathyabama University, Chennai for funding this project. The authors are grateful to Dr B. Deva Prasad Raju, Sri Venkateswara University, Tirupati, and Dr N. John Sushma, Sri Padmavati Mahila University, Tirupati, for technical guidance.
References
- Ankamwar B, Chaudhary M, Sastry M. Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorg Met-Org Nano-Met Chem. 2005;35:19–26.
- Mohanpuria P, NK Rana, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517.
- Jain PK, Huang X, EI-Sayed IH, El-Sayed MA. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–1586.
- Alanazi FK, Radwan AA, Alsarra IA. Biopharmaceutical applications of nanogold. Saudi Pharm J. 2010;18:179–193.
- Pradeep T, Anshup. Noble metal nanoparticles for water purification: a critical review. Thin Solid Films. 2009;517:6441–6478.
- Philip D, Gopchandran KG, Unni C, Nissamudeen KM. Synthesis, characterization and SERS activity of Au-Ag nanorods. Spectrochim Acta A. 2008;70:780–784.
- Zheng B, Qian L, Yuan H, Xiao D, Yang X, Paau MC, Choi MM. Preparation of gold nanoparticles on eggshell membrane and their biosensing application. Talanta. 2010;82:177–183.
- Hu J., Wang Z, Li J. Gold nanoparticles with special shapes: controlled synthesis, surface-enhanced Raman scattering. Sensors. 2007;7:3299–3311.
- Caruthers SD, Wickline SA, Lanza GM. Nanotechnological applications in medicine. Curr Opin Biotechnol. 2007;18:26–30.
- Sengupta S, Evarone D, Capila I, Zhao G, Watson N, Kiziltepe T, Sasisekharan R.Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature. 2005;436:568–572.
- Alivisatos P. The use of nanocrystals in biological detection. Nat Biotechnol. 2004;22:47–52.
- Gao XH, Cui YY, Levenson RM, Chung LW, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–976.
- Singh M, Singh S, Prasad S, Gambhir IS. Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Dig J Nanomat Biostruct. 2008;3:115–122.
- El-Ansary A, Al-Daihan S. On the toxicity of therapeutically used nanoparticles: an overview, J Toxicol. 2009;2009:754810:1–754810:9
- Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS. Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B. 2009;73:332–338.
- Kalishwaralal K, Deepak V, Pandian SRK, Kottaisamy M, Barathmani Kanth S, Kartikeyan B, Gurunathan S. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B. 2010;77:257–262.
- Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A. 2010;369:27–33.
- Shiv Shankar S, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Inter Sci. 2004;275:496–502.
- Dubey SP, Lahtinen M, Sarkka H, Sillanpaa M. Bioprospective of Sorbus aucuparia leaf extract in development of silver and gold nanocolloids. Colloid Surf B. 2010;80:26–33.
- Mallikarjuna K, Dillip GR, Narasimha G, John Sushma N, Deva Prasad Raju B. Phytofabrication and characterization of silver nanoparticles from Piper betle broth. Res J Nanosci Nanotechnol. 2012;2:17–23.
- Mukherjee PK, Saritha GS, Suresh B. Antibacterial spectrum of Hypericum hookerianum. Fitoterapia. 2001;72:558–560.
- Vijayan P, Vinod KS, Badami S, Mukherjee PK, Dhanaraj SA, Suresh B. Selective in vitro cytotoxicity of Hypericum hookerianum towards cancer cell lines. Int J Orient Pharm Exp Med. 2003;3:141–146.
- Dongre SH, Badami S, Godavarthi A. Antitumor activity of Hypericum hookerianum against DLA induced tumor in mice and its possible mechanism of action. Phytother Res. 2008;22:23–29.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–479.
- Padmesh P, Seeni S, Reji JV, Nair GM. Tropical Botanic Garden and Research Institute, assignee. A novel process for the production of hypericin from shoot and callus cultures of Hypericum hookerianum. Indian Patent. 2008;239540.
- Mulvaney P. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 1996;12: 788–800.
- Sathyavathi R, Krishna MB, Rao SV, Saritha S, Rao DN. Biosynthesis of silver nanoparticles using coriandrum sativum leaf extract and their application in nonlinear optics. Adv Sci Lett. 2010;3:138–143.
- Park J, Joo J, Kwon GS, Jang Y, Hyeon T. Synthesis of monodisperse spherical nanocrystals. Angew Chem Int Ed. 2007;46:4630–4660.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasan K. Biosynthesis of sliver nano-particles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 2011;79:594–598.
- Stoimenov PK, Klinger RL, Marchin GL, Kenneth JK. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–6686.
- Slawson RM, Van Dyke MI, Lee H, Trevors JT. Germanium and silver resistance, accumulation, and toxicity in microorganisms. Plasmid. 1992;27:72–79.