Abstract
Naturally occurring forms of materials have complex morphology and excellent performance, which provides new strategies for synthesising materials. In this paper, strontium oxalate crystals with different morphologies can be obtained simultaneously on either side of the membrane of mung bean sprouts (MBS). The as-obtained products were characterised by scanning electron microscopy (SEM) and X-ray diffraction (XRD). The effect of ethylene diamine tetraacetic acid (EDTA) on the morphologies was investigated and discussed. The results showed that EDTA played a more important role in the formation of the products inside the MBS membrane. A presumable mechanism was proposed. This research may provide insights into the cooperation between bionic research and living vegetal cells.
1. Introduction
The complex morphology and excellent performance in many fields of naturally occurring forms has stimulated people's interest in copying nature and in adapting ideas from nature to synthesise materials.[Citation1] During the past decade, many efforts have been made to explore bio-inspired synthesis strategies to obtain complex materials with specific shape, size, orientation and hierarchical organisation.[Citation2–7]
Templates of organism structures are attracting more and more interest due to their inherent hierarchical ordered superstructures,[Citation8] such as tobacco mosaic virus,[Citation9] silica algae,[Citation10] etc. However, in most cases, bio-templates used to synthesise well-defined nanostructures or superstructures [Citation11] are performed under harsh conditions within relatively high temperature, extreme pH value, complicated procedures or other modifiers. Few ordered hierarchical structures have been produced in a heterogeneous environment at ambient temperature and pressure and pH values close to neutral with no additive.
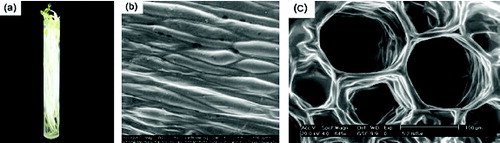
Based on the above considerations, a new method was proposed to control the crystallisation by using living biological template. As was observed in our previous work,[Citation12] mung bean sprout (MBS, the sprout form of mung bean) was a good bio-template for simultaneous synthesis of crystals with two kinds of morphologies. This should contribute to the particular structure of MBS. shows the pictures of a living MBS (1a), the outside surface (1b) and inside stem patterns (1c) of MBS. It can be seen that the epidermis is uneven with well-distributed grooves of widths about 80∼120 μm and diameters around 10∼20 μm (b). The cross section of the inner stems of MBS is composed of many canaliculi with a uniform size of about 100 μm (c). In addition, there are a lot of pleats on the inner stem wall. This template remains stable at temperature between 0°C to 35°C and pH values from 4 to 10. The synthesis process using MBS as template is under mild conditions at room temperature and atmosphere pressure.
Figure 1. (a) Photograph of living MBS; (b) SEM images of the cross section of the interior stem SEM images of the epidermal surface of MBS (outside); (c) the cross section of the interior stem of MBS (inside).
The purpose of this work is to report the growth of strontium oxalate using MBS as a template and study the influence of the surfactant on the growth mechanism.
2. Experimental section
2.1. Synthesis method
All chemical reagents used were of analytical grade and purchased from Sinopharm Chemical Reagent Co. Ltd. Mung bean sprouts were purchased from a supermarket. Before experiment, the plants were washed by distilled water several times to ensure no impurities were attached to them. The typical experimental procedures are described as follows: the MBS were immersed in 0.05 mol/L SrCl2 solution for 20 h; MBS were taken out of SrCl2 solution and washed by distilled water before being divided into two parts; one was immersed in 0.05 mol/L Na2C2O4 for 20 h, and the other was immersed in 0.05 mol/L Na2C2O4 with 0.01 mol/L EDTA solution for 20 h; after being washed by distilled water and absolute ethanol repeatedly, the products accreted inside and outside of MBS were collected separately; before the precipitates were characterised, they were all carefully washed with distilled water and absolute ethanol, followed by centrifugation.
2.2. Characterisation
Powder X-ray diffraction (XRD) patterns were recorded on a Philips PW1700 X-ray diffractometer (XRD) with Cu Kα radiation (λ = 0.15418 nm) (the Netherlands). Scanning electron microscopy (SEM) measurements were performed with a field-emission environmental scanning electron microscope FEI/Phillps XL30 ESEM-FEG (SEM, the Netherlands). Infrared spectra (4000∼500 cm−1) were recorded by an FTIR spectrometer (Nicolet 5DX FTIR). All measurements were carried out at room temperature.
3. Results and discussions
3.1. Morphologies and structures
The morphologies of the products obtained through MBS membrane without any crystal modifiers are shown in . (a) indicates that the product outside the membrane are aggregates composed of some short sheets. The length of sheets is about 5 μm, and the thickness is nearly 500 nm. The inside products, as shown in (b), looks like a dumbbell bouquet. A close observation clearly reveals that all the bouquet-like productions are assembled by substructures like nanorods.
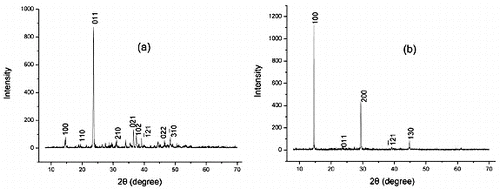
In order to find out whether the phase of the product was changed by MBS, X-ray diffraction (XRD) was used to characterise the products obtained through direct reaction and MBS, respectively. shows the X-ray diffraction (XRD) patterns of the synthesised products by direct reaction of SrCl2 and Na2C2O4 (a) and under the template of MBS (b). All the diffraction patterns can be indexed according to the body-centred tetragonal structure of SrC2O4•H2O with lattice parameters a = 6.47 Å, b = 6.52 Å and c = 6.493 Å (JCPDS, No. 20–1203). But there are very big differences in the crystal face growth direction. a indicates that the (011) face of the product in direct reaction is in a predominant growth position, but the (100) face of the product prepared by MBS is predominant. It can be concluded that MBS didn't change the phase of the product but played an important role in the crystal growth procedure.
Figure 3. XRD patterns of as-prepared strontium oxalate sample: (a) direct reaction; (b) react through MBS.
Former experimental studies of our group on the polymorphic formation of calcium carbonate showed that the crystal modifier played an important role in controlling the formation of the crystalline phase and morphology.[Citation13] In this work, EDTA was used to study the effect of the surfactant on the growth process. The morphologies of the strontium oxalate prepared by MBS in the presence of EDTA as crystal modifiers were observed using SEM. Results are shown in . Products obtained on the outside surface are uniform rods with 2 μm length and 600 nm width (as shown in a), and the inside products are octahedrons (as shown in b).
Figure 4. SEM images of as-prepared strontium oxalate after EDTA is added: (a) outside sample; (b) inside sample.
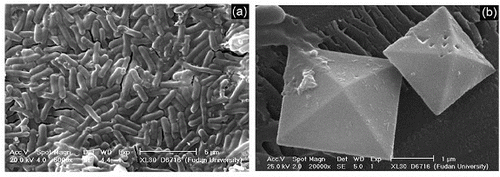
It can be concluded from and that EDTA played important roles in the morphology of the crystals both outside and inside products. The shapes of outside products changed from short sheets composed aggregates to rods, and the inside crystals transformed from dumbbell bouquet into octahedrons. These may attribute to the strong coordination ability of EDTA.
3.2. FT-IR spectroscopy
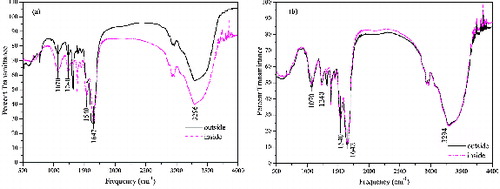
shows the FTIR spectra of SrC2O4 synthesised in this work. The strong broad band (3000–3700 cm−1) centred at 3296 cm−1 can be assigned to hydrogen bonded O–H stretching vibration arising from crystal water. A very strong and sharp band at 1647 cm−1 can be attributed to partial double bond character of carbonyl group stretching vibration.[Citation14] This may be due to one >C = O group and other >C = O group with coordinated bond from carbonyl oxygen to strontium. There was no obvious difference between the inside and outside products.
3.3. Formation mechanism exploration
The above experimental results reveal that MBS themselves play as a template in the formation of two differently shaped strontium oxalate crystallisations. After EDTA was added, the morphologies of both inside and outside products were changed.
Biologically, MBS provided a living bio-template for the formation of crystals. Its special spatial structure made it possible for two different shapes of products obtained simultaneously inside and outside the MBS membrane. Meanwhile, various organic groups, such as hydroxyl, primary amide, carboxylate and some phospholipid groups exist in MBS. According to the theory of soft–hard acid–base, Sr2+ will preferentially combine with hard alkaline groups through electrostatic attraction, such as oxygen in the hydroxyl and carboxylate groups. Consequently, it affected the nucleation process of strontium oxalate. After the C2O42− ion entered MBS membrane, it reacted with combined Sr2+ and then formed strontium oxalate on the interface. Then, induced by the bio-template, the substructure was assembled to the last morphology. This step is also supported by our earlier findings that the superstructures do not really grow from a supersaturated ion solution but by transformation of the primary clusters formed.[Citation15]
While EDTA was added, the process was also under the control of the crystal modifiers. In the process of nucleation, the MBS template was dominant in the nucleation positions through special confinement. In a further growth process, EDTA played a more important role than the organic groups attached on the MBS membrane for its strong coordination. Thus, the growth process was changed and the morphologies of the crystals obtained were different. A detailed mechanism needs to be further investigated in future work.
4. Conclusion
In this work, a living bio-template, MBS was used to control the simultaneous synthesis of differently shaped strontium oxalate crystals. Two kinds of crystals in different shapes were simultaneously grown on the outer surface and the inner stem wall of MBS, respectively. EDTA was used as a modifier and had an effect on the crystal growth process. The formation mechanism is considered to be very complicated and therefore further investigation is necessary. This work may be helpful to study biomineralisation through a living plant template.
Acknowledgements
The authors are grateful for the financial support of the National Natural Science Foundation (Nos. 21101118, 51072134, 91122025, 21103127) of China, the State Major Research Plan (973) of China (No. 2011CB932404), the Nano-Foundation of Shanghai in China (No. 11nm0501300), Fundamental Research Funds for the Central Universities and the Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials (No. 2012MCIMKF03).
References
- Xu AW, Ma YR, Cölfen H. Biomimetic mineralization. J Mater Chem. 2007;17:415–449.
- Liang P, Shen Q, Zhao Y, Wei H, Lieberwirth I, Huang YP, Wang DJ, Xu DF. Petunia-shaped superstructures of CaCO3 aggregates modulated by modified chitosan. Langmuir. 2004;20:10444–10448.
- Imai H, Oaki Y, Kotachi A. A biomimetic approach for hierarchically structured inorganic crystals through self-organization. Bull Chem Soc Jap. 2006;79:1834–1851.
- Yao F, Yang QQ, Yin C, Zhu SM, Zhang D, Moon WJ, Kim YS. Biomimetic Bi2WO6 with hierarchical structures from butterfly wings for visible light absorption. Mater Lett. 2012;77:21–24.
- Nuraje N, Dang XN, Qi JF, Allen MA, Lei Y, Belcher AM. Biotemplated synthesis of perovskite nanomaterials for solar energy conversion. Adv Mater. 2012;24:2885–2889.
- Soumya S, Sreerekha PR, Menon D, Nair SV, Chennazhi KP. Generation of a biomimetic 3D microporous nano-fibrous scaffold on titanium surfaces for better osteointegration of orthopaedic implants. J Mater Chem. 2012;22:1904–1915.
- Nouroozi F, Farzaneh F. Synthesis and characterization of brush-like ZnO nanorods using albumen as biotemplate. J Brazil Chem Soc. 2011;22:484–488.
- Shang TM, Sun JH, Zhou QF, Guan MY. Controlled synthesis of various morphologies of nanostructured zinc oxide: flower, nanoplates, and urchin. Cryst Res Technol. 2007;42:1002–1006.
- Shenton W, Douglas T, Young M, Stubbs G, Mann S. Inorganic-organic nanotube composites from template mineralization of tobacco mosaic virus. Adv Mater. 1999;11:253–256.
- Wang YJ, Tang Y, Dong AG, Wang XD, Ren N, Gao Z. Zeolitization of diatomite to prepare hierarchical porous zeolite materials through a vapour-phase transport process. J Mater Chem. 2002;12:1812–1818.
- Sandhage KH, Dickerson MB, Huseman PM, Caranna MA, Clifton JD, Bull TA, Heibel TJ, Overton WR, Schoenwaelder MEA. Novel, bioclastic route to self-assembled, 3D, chemically tailored meso/nanostructures: shape-preserving reactive conversion of biosilica (diatom) microshells. Adv Mater. 2002;14:429–433.
- Yan Y, Wu QS, Li L, Ding YP. Simultaneous synthesis of dendritic superstructural and fractal crystals of BaCrO4 by vegetal bi-templates. Cryst Growth Des. 2006;6:769–773.
- Zhu DZ, Sun DM, Wu QS. Assembly of nano-superstructural aragonite CaCO3 by living bio-membrane. J Exp Nanosci. 2011;6:622–630.
- Dalal PV, Saraf KB. Growth of strontium oxalate crystals in agar-agar gel. Bull Mater Sci. 2011;34:377–381.
- Shenton W, Davis SA, Mann S. Directed self-assembly of nanoparticles into macroscopic materials using antibody-antigen recognition. Adv Mater. 1999;11:449–452.