Abstract
The present study aims to evaluate the inhibition effectiveness of titanium dioxide nanoparticles in combination with cell wall active antibiotics – ceftazidime and cefotaxime against the multi-drug resistant Pseudomonas aeroginosa isolated from pus, sputum, endo-tracheal tract and broncho-alveolar lavage. Commercial Degussa-P25 TiO2 nanoparticle, antibiotics ceftazidime and cefotaxime were used in this study against multi-drug-resistant nosocomial pathogen. The nanoparticle shows antimicrobial effect on the pathogen at concentrations more than 350 μg/mL, when exposed to ultraviolet (UV) light for an hour. Minimum inhibitory concentration values obtained for the antibiotic cefotaxime were sixfolds higher than the antibiotic ceftazidime. When these antibiotics were used in combination with UV-irradiated metal nanoparticle, ceftazidime resulted in enhanced antimicrobial activity whereas cefotaxime does not show any change.
1. Introduction
Infections caused by multi-drug resistant (MDR) strains Pseudomonas aeruginosa are becoming extremely difficult to treat with conventional antibiotics, leading to a sharp rise in clinical complications.[Citation1] Worldwide, they have been identified as a major nosocomial pathogen.[Citation2,3] Along with the increase in the prevalence of resistant strains due to the abuse of antibacterial agents, there is an urgent need for a new class of antibacterial agents with an entirely different structure and mode of action.[Citation4] During the last decade, various resistant mechanisms have increased worldwide in bacterial pathogens which lead to failure treatment in human and animal diseases.[Citation5] These bacteria are able to adapt rapidly to new environmental conditions such as in the presence of antimicrobial molecules and as a consequence, resistance increases with the antimicrobial use.[Citation6]
In recent years, various nanoparticles have been reported to display good biological activities.[Citation7] Amongst them, TiO2 nanoparticles have attracted the attention of researchers due to its oxidative and hydrolysis properties. As a photocatalyst, it can improve the efficiency of electrolytically splitting water into hydrogen and oxygen which can produce electricity in nanoparticles form. When these nanoparticles are exposed to ultraviolet (UV) light, they become increasingly hydrophilic.[Citation8] In the case of antibacterial coatings, maximum density of nonmaterial on surface results in the generation of reactive oxygen species (ROS) and thus increases the antibacterial activity of TiO2.[Citation9] TiO2 photocatalysts have also been utilised to disinfect a broad spectrum of microorganisms.[Citation10] Recently the metal oxide nanoparticles played a vital role in the novel drug delivery systems. The biosynthesised and chemically synthesised silver nanoparticles showed various biological activities.[Citation11] Recent studies have demonstrated that specially formulated metal oxide nanoparticles have good antibacterial activity[Citation12] and antimicrobial formulations comprising nanoparticles could be effective bactericidal materials.[Citation13,14]
In the present study, the efficacy of TiO2 nanoparticles as an antibacterial agent against MDR P. aeruginosa was explored. Also, the synergistic effect of these nanoparticles with the third generation cephalosporins, viz. cefotaxime (CEF) and ceftazidime (CEZ), was studied to see the enhancement in potency of these drugs.
2. Experimental
2.1. Strains and media
Four clinical isolates of P. aeruginosa were obtained from Sahyadri Hospital and Research Lab Pvt Ltd, Karve Road, Pune, Maharashtra, viz: Isolate 1 – Pus, Isolate 2 – Sputum, Isolate 3 – endo-tracheal tract (ETT), Isolate 4 – broncho-alveolar lavage (BAL). Control strain (NCIM-5029) of P. aeruginosa was obtained from National Chemical Laboratory, Pune. All solutions and reagents were prepared with distilled and deionised water and analytical grade chemicals were used without further purification (Sigma-Aldrich Co., MO, USA). Nutrient agar (NA) was used for the maintenance of clinical isolates of the bacterial strains.
2.2. Characterisation of nanoparticle
The optical property of TiO2 nanoparticles was studied by ultraviolet-visible (UV-vis) spectroscopy. The crystal phase and crystallite size of TiO2 nanoparticles were recorded by X-ray diffraction technique. In this CuKα radiation (λ = 1.54 Å) was used in the 2θ scan range of 10°–80°. The full width at half maximum at the plane (101) was used to estimate the average crystallite size of TiO2.
2.3. Preparation of photocatalytic product
For testing antimicrobial activity in the standard TiO2 nanoparticle film, 1 gram of commercial Degussa P25 TiO2 powder was kept in vigorously shaking conditions to form aqueous solution. Then prepared solution was exposed to UV light for 0, 30 and 60 minutes.
2.4. Antibiotic susceptibility test
Antibiotic susceptibility test was carried out by ‘Disk Diffusion method’.[Citation15,16] Mueller Hinton (MH) agar media was poured in 90 mm Petri dishes and incubated at 37 °C overnight to check sterility. The bacterial suspension was adjusted to an optical density (OD545) according to McFarlands standard (106 CFU/mL). Bacterial suspension was inoculated on each plate by spread plate method and then antibiotic sensitivity disks were placed on the surface of the medium. The plates were then incubated at 37 °C for 18 hours and sensitivity of each antibiotic was determined by checking the zones of inhibition.
2.5. Broth dilution assay
Sterile nutrient broth was poured in dilution tubes. Bacterial suspension was then added along with varying concentrations of TiO2 nanoparticles (100–500 μg/mL) from the two stocks: (1) exposed to UV light for one hour and (2) no UV exposure. These samples are incubated for 24 hours at 37 °C and then minimum inhibitory concentration (MIC) value of TiO2 nanoparticles was established. Here nanoparticles were used as a negative control while the respective strains of P. aeroginosa culture were taken as positive control.
2.6. Antibacterial assay
The antibacterial activity of the TiO2 nanoparticles was checked by using well-diffusion method. Sterile MH agar was poured into petriplates and was swabbed with the overnight culture (106 cells/mL) of bacteria. Then solid medium was gently punctured with the help of cork borer to make a well. After that, TiO2 nanoparticle samples (25–1000 μg/mL) were added from the above-mentioned two stocks into each well and incubated for 24 hours at 37 °C and the antibacterial sensitivity was measured as the zone of inhibition in mm diameter. The same technique was used to establish the MIC values for the antibiotics CEF, CEZ and their combinations with TiO2 nanoparticles.
2.7. Effect of UV irradiation on antimicrobial potential of TiO2 nanoparticles
To check the effect of UV light exposure on antimicrobial potential of TiO2 nanoparticles, three separate stocks were prepared using 50 μg TiO2 nanoparticles were dissolved in distilled water and exposed to UV light (UV-C light – wavelength 254 nm) for 0, 30 and 60 minutes and used for antibacterial assay.[Citation10,Citation17]
3. Results and discussion
In the present study, the antibiotic sensitivity disk consisted of a total of 12 antibiotics viz: Amikasin, Ofloxacin, Gentamycin, Norfloxacin, Cefaclor, Ciprofloxacin, Nitrofuantoin, Cefoperazone, Nalidixic acid, Cefuroxime, Cefadroxil and Netlinocin were used against the selected four different isolates (Isolate 1 from Pus, Isolate 2 from Sputum, Isolate 3 from ETT and Isolate 4 from BAL) along with the control strain of P. aeruginosa. The antimicrobial susceptibility testing showed resistance in 5 out of 12 antibiotics for control and similarly 9, 8, 8 and 8 antibiotics for isolates 1, 2, 3 and 4, respectively, as shown in . This confirms P. aeruginosa as a MDR pathogen.
Table 1. Results of antibiotic susceptibility test against P. aeruginosa.
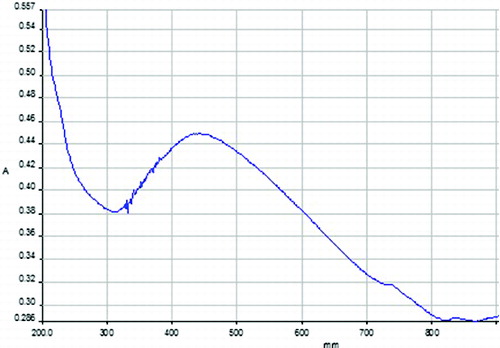
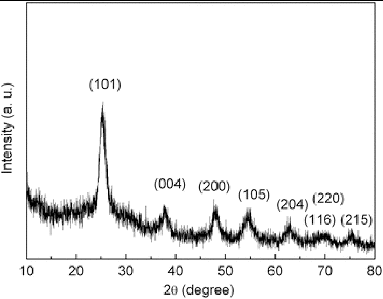
The commercially available standard Degussa-P25 TiO2 nanoparticles have an average particle size of ∼25 nm. It shows mixed rutile/anatase phase. The optical properties of TiO2 nanoparticles are studied by UV-vis spectroscopy which is shown in . The figure clearly shows the absorption peak at ∼430 nm with wide distribution. shows XRD pattern of Degussa-P25 TiO2 nanoparticle which exhibits sharp reflections at 25.32°, 37.83°, 47.81°, 54.55° and 62.71° in the 2θ range of 10°–80°, which can be indexed as (101), (004), (200), (105) and (204). The calculated crystallite size was found to be ∼6.0 nm by Scherer formula at the plane (101).
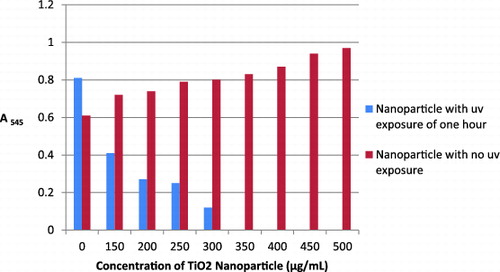
The commercial Degussa-P25 TiO2 nanoparticle is the most widely used photocatalyst.[Citation18] The irradiation of UV light on TiO2 nanoparticle generates an electron hole pair on the TiO2 surface. The hole in the valence band can react with H2O or hydroxide ions adsorbed on the surface to produce hydroxyl radicals (OH−), and the electron in the conduction band can reduce O2 to produce superoxide ions (O2−). Both holes and OH− are extremely reactive with contacting organic compounds. Detection of other ROS, such as hydrogen peroxide (H2O2) and singlet oxygen, has also been reported.[Citation10] Based on this information, the MDR strains of P. aeruginosa were then treated with TiO2 nanoparticles and then exposed to UV light for 0, 30 and 60 minutes. According to Kim et al. in 2008, the optimal initial concentration of microorganism, light intensity and light irradiation time for the photocatalytic reaction of TiO2 nanoparticles were 106 CFU/mL, 1.0 mW/cm2, 3 hours, respectively. The inoculum and light intensity were kept as above whereas the light irradiation time was varied. The MIC value of the nanoparticle was determined using broth dilution assay and confirmed by well-diffusion assay. No visible zone of inhibition was obtained for 0–500 μg/mL concentrations of TiO2 nanoparticle with 0 and 30 minutes UV irradiation. Further, with broth dilution assay, there was no change in turbidity for 0 and 30 minutes UV irradiation confirming the absence of antibacterial activity.
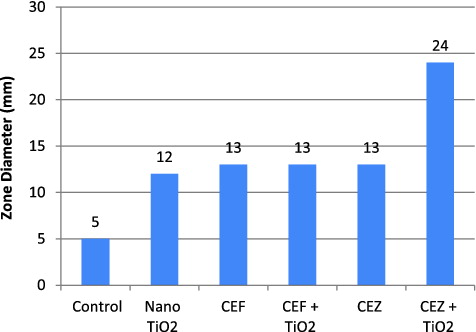
TiO2 nanoparticle (0–500 μg/mL) when pre-exposed with one hour UV irradiation, showed antibacterial action against P. aeruginosa. MIC for TiO2 nanoparticles was obtained at 350 μg/mL as shown in . An exposure to UV light on TiO2 nanoparticles for 60 minutes may lead to the formation of ROS.[Citation19] These ROS are known to bring about bacterial cell wall lysis, which is the fundamental reason for the antimicrobial activity of TiO2 nanoparticles.[Citation20] This antimicrobial activity is inferred on TiO2 nanoparticles by UV light exposure for not less than one hour. At one-hour exposure, these nanoparticles obtained very strong inhibitory action against P. aeruginosa control strain, Isolate 1 (12 mm, zone of inhibition), Isolate 2 (14 mm, zone of inhibition), Isolate 4 (10 mm, zone of inhibition) and Isolate 3 (8 mm, zone of inhibition) at 350 μg/mL by well-diffusion assay.
MICs are most often used as a research tool to determine the in vitro activity of new antimicrobials and data from such studies have been used to determine MIC breakpoints.[Citation21,22] In the present study MIC values for the antibiotics CEF and CEZ was found to be 130 μg/mL (13 mm, zone of inhibition) and 20 μg/mL (13 mm, zone of inhibition), respectively, against P. aeruginosa.
The antibacterial activity was not enhanced when TiO2 nanoparticles was used in combination with the antibiotic CEF against Pseudomonas sp. On the other hand, the antibiotic CEZ as compared to antibiotic CEF showed an excellent synergistic effect (24 mm, zone of inhibition), when combined with TiO2 nanoparticles as shown in . The other four isolates also showed the similar inhibitory effect.
Figure 4. (Colour online) Determination of MIC of Ceftazidime (10–100 μg/mL) against multi-drug resistant P. aeruginosa. On the right, arrows showing CEZ alone (13 mm, zone of inhibition) and CEZ + nanoparticle (24 mm, zone of inhibition).
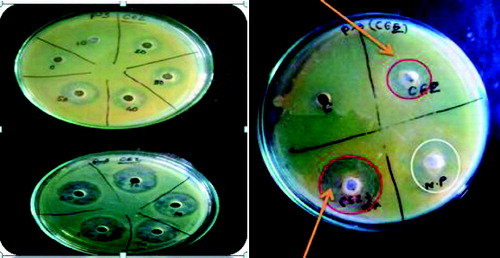
TiO2 nanoparticles when used in combination with the antibiotic CEZ thus can act as a potential antibacterial agent which is shown in . The change in MIC of CEZ in combination with TiO2 nanoparticle can be attributed to the fact that eitherthe sensitivity of antibiotic is enhanced by the presence of nanoparticles or none of the nanoparticle/CEZ in combination is suppressed.
Figure 5. Effect of TiO2 nanoparticles alone (350 μg/mL), ceftazidime (CEZ) alone (20 μg/mL), cefotaxime (CEF) alone (130 μg/mL), CEF and TiO2 nanoparticle in combination, CEZ and nanoparticle on multi-drug resistant P. aeruginosa shown as zone diameter (mm). For control 5 mm diameter shown is the well diameter.
The increasing frequency of MDRPA strains is a matter of concern as efficacious antimicrobial options are severely limited. As known nosocomial infections caused by P. aeruginosa are frequently life threatening and often challenging to treat. One potential strategy is to target the regulation of bacterial resistance mechanisms as a pathway to enhance the potency of available drugs and perhaps, restore the efficacy of available drugs.[Citation23] In such conditions, combination therapy might prove useful for countering drug resistance.
4. Conclusion
In the current scenario, multi-resistant infectious disease organisms are difficult and sometimes impossible to treat successfully. Further no new antimicrobial agents, active against MDR strains of P. aeruginosa, are in advanced stages of development as therapeutic options. In the present study, an exposure of UV irradiation of 60 minutes has shown to greatly enhance the antibacterial efficacy of TiO2 nanoparticle against MDR P. aeruginosa. Further, the antibiotic CEF potency was increased when used in combination with 60 minutes UV-irradiated TiO2 nanoparticles. This synergistic study against the pathogens offers its application potential in clinical diagnostics as an attempt to find a solution to the growing problem of antibiotic resistance. This approach is promising and thus can broaden the repertoire of useful anti-pseudomonal drugs.
Acknowledgements
The authors would like to thank the Principal, Abasaheb Garware College, Pune for necessary lab support and help. Thanks to Sahayadri Hospital, Pune for providing the clinical isolates.
References
- Obritsch MD, Fish DN, MacLaren R, Jung R. Nosocomial infections due to multidrug-resistant pseudomonas aeruginosa: epidemiology and treatment options. Pharmacotherapy. 2005;25(10):1353–1364.
- Mesaros N, Nordmann P, Ple´siat P, Roussel-Delvallez M, Van Eldere J, Glupczynski Y, Van Laethem Y, Jacobs F, Lebecque P, Malfroot A, Tulkens PM, Bambeke FV. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect. 2007;13:560–578.
- National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System report, data summary from October 1986 to April 1998. Am J Infect Control. 1998;26:522–533.
- Finch R, Hunter PA. Antibiotic resistance–action to promote new technologies: report of an EU Intergovernmental Conference held in Birmingham, UK. J Antimicrob Chemother. 2006;58:i3–i22.
- Gottlieb T, Nimmo GR. Antibiotic resistance is an emerging threat to public health: an urgent call to action at the Antimicrobial Resistance Summit 2011. Med J Aust. 2011;194(6):281–283.
- Falagas ME, Bliziotis IA. Pan drug-resistant Gram-negative bacteria: the dawn of the post-antibiotic era?. Int J Antimicrob Agents. 2007;29:630–636.
- Pal S, Tak YK, Song JM. Does the antibacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium, Escherichia coli. Appl Environ Microbiol. 2007;73(6):1712–1720.
- Fujishima A, Rao TN, Tryk DA. Titanium dioxide photocatalysis. J Photochem Photobiol C: Photochem Rev. 2000;1:1–2.
- Amézaga-Madrid P, Nevárez-Moorillón GV, Orrantia-Borunda E, Miki-Yoshida M. Photo induced bactericidal activity against Pseudomonas aeruginosa by TiO2 based thin film. FEMS Microbiol Lett. 2002;211:183–188.
- Maness P, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA. Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol. 1999;65(9):4094–4098.
- Ravikumar S, Gokulakrishnan R, Selvanathan K, Selvam S. Antibacterial activity of metal oxide nanoparticles against ophthalmic pathogens. Int J Pharm Res Dev. 2011;3(5):122–127.
- Stoimenov PK, Klinger RL, Marchin GL, Klabunde KJ. Metal oxide nanoparticles as bactericidal agents. Langmuir. 2002;18:6679–6686.
- Fresta M, Puglisi G, Giammona G, Cavallaro G, Micali N, Furneri PM. Pefloxacine mesilate loaded and ofloxacin-loaded polyethylcyanoacrylate nanoparticles–characterization of the colloidal drug carrier formulation. J Pharm Sci. 1995;84:895–902.
- Hamouda T, Hayes M, Cao Z, Tonda R, Johnson K, Craig W, Brisker J, Baker J. A novel surfactant nanoemulsion with broad-spectrum sporicidal activity against Bacillus species. J Infect Dis. 1999;180:1939–1949.
- Bauer AW, Kirby WM, Sherris JC, Turek M. Antibiotic susceptibility testing by standardized single disc method. Am J Clin Pathol. 1966;45:493–496.
- National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disc susceptibility tests. Villanova, PA: National Committee for Clinical Laboratory Standards; 2006 (9th ed.; Approved standard M2-A9).
- Kikuchi Y, Sunada K, Iyoda T, Hashimoto K, Fujishima A. Photocatalytic bactericidal effect of TiO2 thin films: dynamic view of the active oxygen species responsible for the effect. J Photochem Photobiol A Chem. 1997;106:51–56.
- Hoffman MR, Martin ST, Choi W, Behnemann DW. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95:69–96.
- Kim JY, Park C, Yoon J. Developing a testing method for antimicrobial efficacy on TiO2 photocatalytic products. Environ Eng Res. 2008;13(3):136–140.
- Blake DM, Maness PC, Huang Z, Wolfrum EJ, Huang J, Jacoby WA. Application of the photocatalytic chemistry of titanium dioxide to disinfection and the killing of cancer cells. Purif Methods. 1999;28:1–50.
- Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48(S1):5–16.
- Furtado GL, Medeiros AA. Single-disk diffusion testing (Kirby–Bauer) of susceptibility of Proteus mirabilis to chloramphenicol: significance of the intermediate category. J Clin Microbiol. 1980;12(4);550–553.
- Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance. Clin Microbiol Rev. 2009;22(4);582–610.