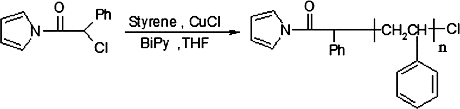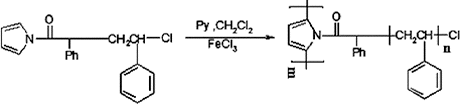Abstract
A synthetic route for the preparation of a novel solution copolymer derived from styrene (St), pyrrole (Py) and its organoclay nanocomposite with conductive and mechanical properties is demonstrated. The electroactive copolymer of polystyrene-g-polypyrrole (PSt-g-Ppy) nanocomposite was successfully prepared by atom transfer radical polymerisation (ATRP) and chemical polymerisation methods. First, potassium pyrrole was reacted by α-chlorophenyl acetyl chloride to prepare an initiator that can polymerise styrene by ATRP technique. Then, polypyrrole was prepared by chemical polymerisation using FeCl3 as an oxidant in dichloromethane,s solvent. Nanocomposites of the copolymer with modified montmorillonite were prepared with a solution intercalation method. The conductivity of the copolymer was measured by the four-point probe method. The structures of the intermediate, copolymer and nanocomposite were investigated by Fourier transform infrared spectroscopy, 1H-NMR and X-ray diffraction techniques. The molecular weight of the copolymer was determined by gel permeation chromatography. Their thermal behaviour was examined by differential scanning calorimetry and thermogravimetric analyses.
1. Introduction
Polymer/clay nanocomposites (PCNs) have attracted a great deal of attention with their improved physical and chemical properties due to the small size and large surface area of the clay particles with nanoscale dimensions.[Citation1] These materials have shown enhanced mechanical properties, such as increased stiffness and strength,[Citation2] thermal stability and flame retardancy [Citation3] and gas barrier properties [Citation4] relative to the pure polymers. There are two distinct nanostructures identified in these nanocomposites: intercalated and exfoliated/delaminated.[Citation5] The exfoliated nanostructure is more desirable than the intercalated one due to the stronger synergistic effects between polymer matrix and silicate layers. The synthesis methods of PCNs are divided into three main groups according to the starting materials and processing techniques: solution exfoliation, melt intercalation and in situ intercalative polymerisation.[Citation6] Disadvantages of the first two methods are the co-intercalation of solvents in the case of solution reaction and slow polymer transport into the interlayer space.[Citation7] Nanocomposite materials derived from polymers associated with layered inorganic solids is a line of investigation of increasing interest, not only for producing improved structural materials, but also for the preparation of new functional materials. A group of organic polymers having conjugated double bond on their backbone is known to be electroconductive. The π-electron structure in this polymer allows the enhanced electrical conductivity at certain oxidation state.[Citation8,9]
After introducing high electronic conductivity to the conducting polymer, they attracted lot of research interest and became popular basic material for advanced application such as plastic batteries, gas separation membranes, smart windows, sensors and so on.[Citation10]
Conducting polypyrrole (PPy) has increasing scientific and technological interest in the synthesis of a broad variety of promising new materials due to their unique electrical and optical properties as well as the ease of preparation and environmental stability. Polypyrrole and some of its derivatives have high antioxidative stability and are considered to belong to the most useful conductive polymers for practical application. They can be obtained in various solvents by chemical or electrochemical oxidation. Then by reductive coupling, it leads to insoluble and infusible powders.[Citation11,12]
The development of controlled radical polymerisation (CRP) permitted to achieve well-defined polymers using a radical process easy to carry out. Various methods for CRP have been proposed; however, the most successful methods include atom transfer radical polymerisation (ATRP), nitroxide-mediated polymerisation (NMP) and reversible addition–fragmentation transfer polymerisation.[Citation13–16]
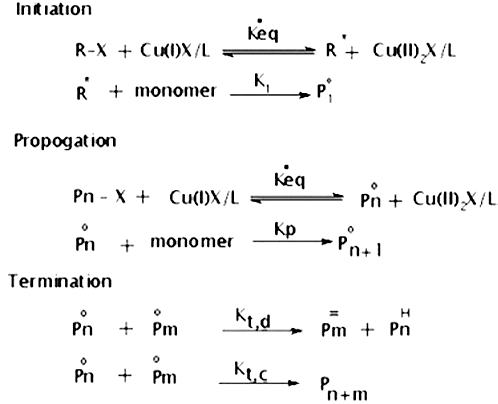
ATRP catalysed by transition metal (such as copper, ruthenium, nickel, etc) halide complexes with 2, 2′-bipyridine (bpy) derivatives for polymerisation of various monomers, such as styrene, methacrylates, acrylates, etc.[Citation17–19] ATRP employs equilibrium between dormant alkyl halides and active propagating radicals, to maintain a low concentration of active species. The activated radical species can either propagate or be deactivated to reform the dormant species ().
ATRP also has an advantage in utilising a wide range of initiators. Alkyl halides with radical stabilising substituents, such as carbonyl, cyano, or aryl group, adjacent to the C–X can be used as initiator. Any compound, including macromolecular species, can potentially be used to initiate ATRP as long as they contain activated halogen atoms.
Polymers thus obtained are rather difficult to process; therefore it limits considerably their application in industrial technology. One way to bypass these difficulties is to prepare blends, composites or graft of conductive polymers with classic ones, which has the great advantage in improving both their mechanical properties and their process ability.[Citation20,21]
During the last decade there has been widespread interest in conducting polymers both for academic purposes and for potential applications. The insolubility in common solvents and infusibility of conducting polymers, in general, make them poorly process able either by solution technique or by melt processing methods.[Citation22,23] Improvement of these material properties can be achieved either by forming copolymers, or by forming conductive polymeric composites or blends with commercially available polymers or inorganic materials which offer better mechanical and optical properties, stability and process ability.[Citation24–27]
From the beginning, interest in conducting polymers has its origins in the possible commercial applications of these materials. Foremost among the current commercial ventures are applications of conducting polymers in energy storage devices such as rechargeable batteries,[Citation28] conductive paint,[Citation29] removal of heavy metals,[Citation30,31] electromagnetic interference shielding,[Citation32] biomedical applications,[Citation33] etc.
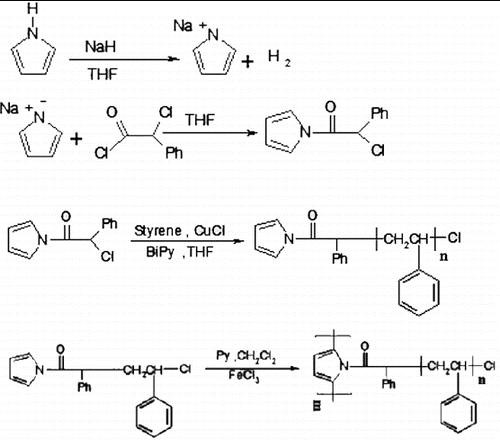
In this work, in order to improve solubility and process ability of PPy, we have prepared polystyrene-graft-polypyrrole (Ps-g-Ppy) by using ATRP and chemical polymerisation methods. First, potassium pyrrole was reacted by α-chlorophenyl acetyl chloride to prepare an initiator that can polymerise styrene by ATRP technique. Then, PPy was prepared by chemical polymerisation using FeCl3 as an oxidant. Finally, the synthesis of the nanocomposite was carried out by a solution intercalation method with tetrahydrofuran (THF) as a solvent. The route of the preparation of Ps-g-Ppy is shown in .
2. Experimental
2.1 Materials
The pyrrole monomer (Merck, Germany) was dried by distillation with calcium hydride under reduced pressure and stored in a refrigerator before use. Styrene (Aldrich, China) was purified by passing through a column of activated neutral alumina to remove the inhibitor. THF was distilled with sodium and benzophenone. 2, 2′-bipyridine (bpy), cuprous chloride, α-chlorophenyl acetyl chloride and all other chemicals were purchased from Merck and used without further purification. Sodium montmorillonite (MMT) was obtained from Southern Clay Products (Modsburg, Germany) under the trade name Cloisite NaC. MMT was reported to have an approximate aspect ratio of 250:1 and was a 2:1 tetrahedral/octahedral aluminium silicate smectite mineral with an idealised chemical formula of Na0.33[Mg0.33Al11.67Si4O10] (OH)2 and a cation exchange capacity of 95 mequiv/100 g.
2.2 Instrumentation
Fourier transform infrared (FT-IR) spectra were recorded with a Shimadzu FT-IR-8101M (Tokyo, Japan). The samples were prepared by the grinding of the dry powder with KBr and compression of the mixture to form disks. The disks were stored in a desiccator to prevent moisture absorption. 1H-NMR spectra were recorded on a Fourier transform NMR (400 MHz) Bruker instrument in CDCl3. The conductivity of the polymers was measured by a four-probe apparatus made by Azar Electric, Iran. The molecular weight of the resulting polymer was obtained with a Maxima 820 gel permeation chromatography (GPC) analysis instrument with a PSt (106, 105 and 104 Å) calibration standard with a THF mobile phase at a flow rate of 1 mL/min and a column temperature of 30 °C. The measurement of the thermal properties of the PSt-co-PPy/MMT nanocomposites was performed with a thermogravimetric analysis (TGA) spectroscopy instrument (England). Samples of about 10 mg were heated from 25 to 600 °C at the rate of 10 °C/min under a nitrogen flow. Differential scanning calorimetry (DSC) analyses were carried out with a Netzsch instrument (Bavarian, Germany). The sample was first heated to 200 °C and kept for five minutes to eliminate the heat history. The sample was then cooled down at a rate of 10 °C/min. The sample was then reheated to 200 °C at a rate of 10 °C/min. The entire test was performed under nitrogen purging at a rate of 50 mL/min. X-ray diffraction (XRD) spectra were obtained with a Siemens D 5000 X-ray generator (CuKα radiation with χ = 1.5406 A)with a 2θ scan range of 20–80 °C at room temperature. The dispersion state and layered structure of MMT were observed with a VEGA//TESCAN KX5000 scanning electron microscope (Shanghai, China).
2.3 Preparation of α-chlorophenyl acetyl pyrrole
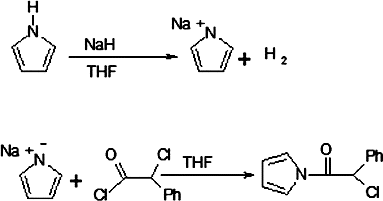
In a two-neck flask with a reflux condenser and magnetic stirring, sodium pyrrole salt was synthesised by the reaction of pyrrole with 1.6 g (0.004 mole) of sodium hydride for four hours at room temperature under N2 in THF. THF solution of 6.46 mL (0.04 mole) α-chlorophenyl acetyl chloride was added to the flask which contain sodium pyrrole salt in THF at 0 °C. The reaction was contained for four hours at room temperature under N2. α-Chlorophenyl acetyl pyrrole dried at 60 °C at reduced pressure ().
2.4 Preparation of Pst-g-Py via ATRP method
The ATRP graft polymerisation was carried out under nitrogen in a dried schlenk flask equipped with a magnetic stirring bar. The flask was charged with 5 mL of dried THF and 0.064 g (0.31 mmol) α-chlorophenyl acetyl pyrrole. After complete dissolution under stirring, CuCl (0.015 g, 0.158 mmol) and bpy (0.07 g, 0.47 mmol) were added. The system was degassed to remove oxygen by stirring the solution under nitrogen for one hour. Then styrene (10 mL, 0.87 mol) was added and the flask was immersed in an oil bath and stirring was continued at 90 °C for 40 hours. The mixture was precipitated into methanol and obtained product was dried at room temperature under vacuum, then the obtained powder was extracted by refluxing it in cyclohexane overnight, in order to remove PS homopolymer ().
2.5 Chemical preparation of polystyrene-g-polypyrrole
A two-neck flask with a reflux condenser and magnetic stirring was charged with solution of 0.5 g of Ps-g-Py in 20 mL dichloromethane fitted a dropping funnel contain (2.4 g, 14.77 mmol) of anhydrous ferric chloride in 10 mL dichloromethane. Ferric chloride added dropwise to the solution of Ps-g-Py, and then the 0.455 mL (6.5 mmol) pyrrole solution was added to FeCl3 solution dropwise, then vigorously stirred and cooled. Chemical polymerisation time varied one hour. Ps-g-Ppy dried at 60 °C at reduced pressure overnight ().
2.6 Surface modification of MMT
Organophilic montmorillonite (OMMT) was obtained after treatment with a hexadecyl trimethyl ammonium chloride salt ion-exchange process. MMT was first dispersed in deionised water under ultrasound for two hours. The modifier was prepared in deionised water separately and was added to the clay dispersion at an amount a little higher than the cation-exchange capacity of MMT. The resulting suspension was intensively stirred for 10 hands then filtered with deionised water three times. The final product was dried in vacuo at room temperature for 48 hours, after which it was ground into powder.
2.7 Preparation of the PSt-co-PPy/MMT nanocomposites
The organoclay MMT (0.1 g, 3 wt%) was dispersed in 30 mL of tetrahydrofuran for 60 minutes under ultrasound, after which 2 g of PSt-g-Ppy was dissolved in 50 mL of tetrahydrofuran. Then, the clay suspension was slowly added to the polymeric solution with constant stirring for six hours, after which the mixture was poured into 300 mL of methanol for rapid precipitation. The precipitate was filtered and dried at 60 °C in vacuum for two days.
3. Results and discussion
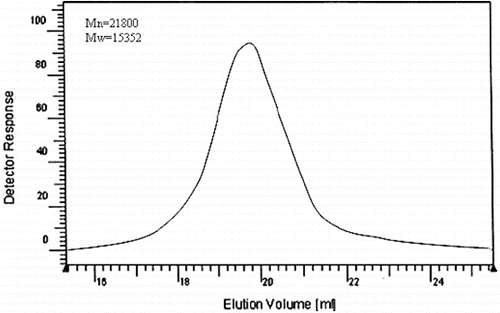
Living polymerisation is characterised by a linear increase of the molecular weight with conversion and reaction time, and a narrow molecular weight distribution is evidenced by a polydispersity index (PDI = (Mw)/(Mn)) approaching 1. represents the GPC chromatograms of the PSt and PPy copolymer, whose molecular weight distribution by GPC was 1.42. Single GPC curves of the homopolymer clearly indicated that it was the copolymer; if so, the GPC curve should have appeared as two peaks, one for the PSt homopolymer and the other for the PPy homopolymer.
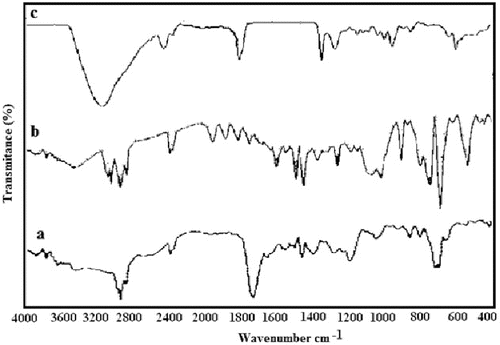
The results are summarised in . The GPC trace of the polystyrene phenyl acetyl pyrrole (Mn = 21800, Mw = 15352, PDI = 1.42) was unimodal and showed no tailing or broadening of the molecular-weight distribution; this confirmed that no cross linking or chain scission occurred during the acetylation procedures. shows the FT-IR spectra of α-chlorophenyl acetyl chloride (a) polystyrene phenyl acetyl pyrrole (b) and poly (St-co-Py) (c). Identification of the reaction products was done by FT-IR analysis. FT-IR spectra were obtained from KBr pellets. In (a), α-chlorophenyl acetyl pyrrole shows characteristic peaks at 719 cm−1 responsible for ν(C–Cl) and 1726 cm−1 responsible for amidic carbonyl group ν(N–C = O). A peak at 1193 cm−1 due to ν(C–N) of the pyrrolyl group. This fact implies that some of the chloride in α-chlorophenyl acetyl chloride converted into the pyrrolyl group by the substitution reaction. (b) shows the FT-IR spectra of polystyrene phenyl acetyl pyrrole. The characteristic peaks at 719 cm−1 were responsible for ν(C–Cl) and those at 1743 cm−1 were responsible for amidic carbonyl group ν(N–C = O). A peak at 1074 cm−1 is due to ν(C–N) of the pyrrolyl group. In (c) polystyrene-g-polypyrrole shows the peak at 751 cm−1 responsible for ν(C–Cl) with decreased intensity. A new peak at 3406 cm−1 is due to ν(N–H) of the polypyrrole chain.
Table 1. GPC of polystyrene phenyl acetyl pyrrole.
Figure 2. FT-IR spectrum of α-chlorophenyl acetyl pyrrole (a) polystyrene phenyl acetyl pyrrole (b) and spectrum of polystyrene-g-polypyrrole (c).
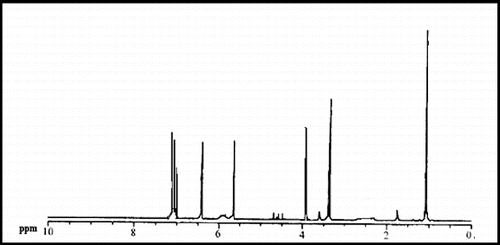
The 1H-NMR spectra of polystyrene phenyl acetyl pyrrole was shown in . The spectrum displays a characteristic peak at δ = 3.81 ppm, which is related to the CHPhCl groups, and a peak at δ = 1 ppm, which is related to the aliphatic protons of polystyrene and peaks at δ = 6.5–8 ppm, which is related to the aromatic protons of polystyrene. A pair of peaks at 5.6 ppm indicates the existence of the pyrrole groups.
Conductivity measurements were carried out by a four-point probe method. The blends of St-co-PPy nanocomposites were compacted into pellets for measurements. The reported conductivity values were the averages of four pairs of readings at different parts on both sides of the pellets. All measurements were done in air at room temperature and converted to conductivity by the following equation:
where ρ is the resistivity (Ώ cm), δ is the conductivity (s/cm), V is the potential difference (mV), I is the applied constant current (mA) and ω is the thickness (cm).
The conductivity of the nanocomposite was low compared to that of PPy. The main reason seemed to be stereochemical differences between these two conducting polymers. The results of conductivity are summarised in .
Table 2. Electrical conductivity of PPy and Ps-g-Ppy.
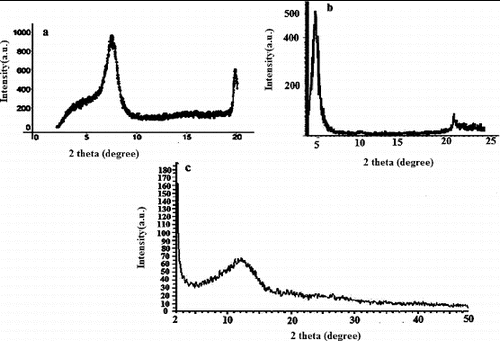
(a)–(c) shows the XRD patterns of the parent MMT (a), the modified MMT (b) and PSt-g-PPy/MMT nanocomposite (c). An increase of the basal spacing (d001) of the modified clay was observed after the insertion of the modifier. More specifically, the pristine MMT showed a d001-spacing of 12.6 Å; this corresponded to an interlayer spacing (D) of 12.6−9.6 = 3 Å, where 9.6 Å is the thickness of the individual clay sheet. In the case of the organoclay, the basal spacing (d001) became 18.38 Å with a corresponding interlayer spacing of D = 8.66 Å. XRD provided information on the changes of the interlayer spacing of the clay upon the formation of the nanocomposite. The formation of an intercalated structure should result in a decrease in 2θ, which indicates an increase in the d-spacing; the formation of an exfoliated structure usually results in the complete loss of registry between the clay layers, and no peak was seen in the XRD trace. In some cases, a disordered immiscible system was obtained, and this also showed no peaks, so the absence of an XRD peak could not be taken as definitive evidence for the formation of an exfoliated nanocomposite. For the nanocomposite, no peaks were observed related to the organophilic clay nanocomposite; this could have meant that either an exfoliated or an immiscible nanocomposite was formed.
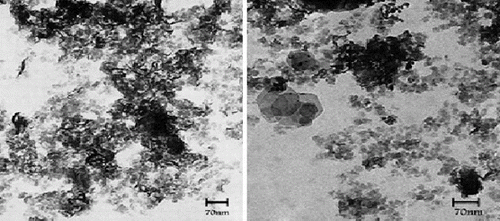
Transmission electron microscopy (TEM) provides an actual image of the morphology of the nanocomposite. TEM is complementary to XRD, especially when peaks are not observed in XRD. shows the TEM images for the PSt-g-PPy/MMT nanocomposite, which indicated that clay was well dispersed in the polymeric matrix and showed the formation of an exfoliated nanocomposite. reveals that the nanocomposites were in a much exfoliated state. The dark lines indicate the MMT layers with a thickness of about 1 nm. In , nanosized MMT aggregates could be detected in various degrees; this might have been due to intercalated or partially exfoliated structures. This observation was consistent with the XRD results. If the silicates were dispersed randomly and homogeneously in the polymer matrix, the interface area would have been enormous, and pronounced interaction could be expected.
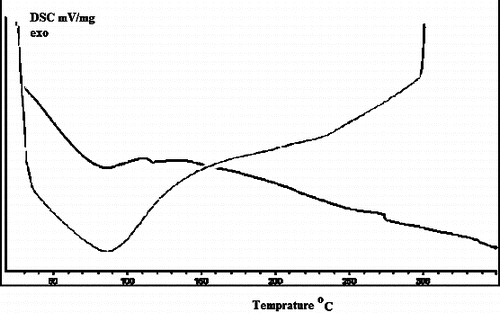
DSC is most commonly used to determine transition temperatures such as glass transitions, melting cross-linking reactions, and decomposition. However, it measures only the total heat flow and the sum of all thermal transitions in the sample. shows the DSC thermogram of PSt-g-PPy/MMT nanocomposites. The glass transition temperature (Tg) value of the PSt-g-Ppy/MMT nanocomposite is 90 °C, which is slightly higher than that of pure PPy. The DSC curve of PSt-g-Ppy/MMT nanocomposite shows that the decomposition of the nanocomposite occurs in two steps: the first step corresponds to the decomposition of the PPy chain (110–150 °C), whereas the second step is associated with PSt chain scission (270–450 °C). This is tentatively ascribed to the confinement of the polymer chains adjacent to the MMT layers, which prevents segmental motion of the polymer chains. Because OMMT layers are dispersed in the polymer matrix on a nanoscale, strong interfacial forces are created between the silicate layers and the polymer chains which may confine the movement of chain segments, resulting in an increase in Tg and the decomposition temperature. As a kind of inorganic material, the excellent thermal stability of MMT can be fully displayed; this leads to an improvement in the heat resistance of the nanocomposite, which is obvious upon addition of OMMT.
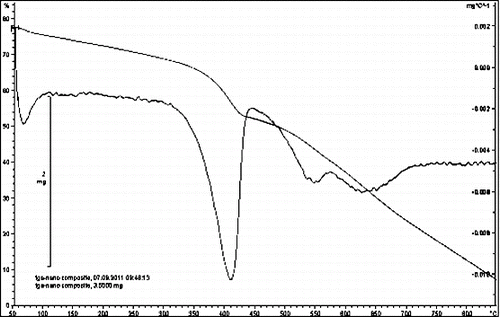
Characteristic TGA curves of the MMT, PSt-g-Ppy and PSt-g-Ppy/MMT nanocomposite are shown in . TGA of the original MMT was found to have 5.2 wt% volatile materials, with most of the organics decomposing around 200–300 °C. The TGA curve of the PSt-g-Ppy graft copolymer exhibits a two-step weight loss process with onset temperature at about 180 °C. The first weight loss increases rapidly from the onset temperature to about 320 °C and the second increases even more rapidly from this temperature to about 450 °C, after which the loss rate slows down. Obviously, the thermal stability of the PSt-g-Ppy is affected by the presence of clay particles. The increased thermal stability of the PSt-g-Ppy/MMT nanocomposite might be attributed to enhanced interface interactions. During the course of thermal decomposition, the homogeneously dispersed OMMT forms protective oxide layers on the nanocomposite surface, which impedes the burning process by reducing the oxygen supply to the bulk and preventing the emission of degraded small gaseous molecules. Moreover, according to , we can conclude that the weight loss of the nanocomposite between 250 and 400 °C is accelerated, mainly because of the dehydration reaction of OMMT followed by early degradation of the hexadecyl trimethyl ammonium cation molecules.
Figure 7. The TGA image of the montmorilonite (MMT) (a), PSt-co-PPy/MMT nanocomposite (b), PSt-co-PPy (c).
The solubility of the copolymer achieved for different percent weight of PPy in common organic solvents are shown in . Polystyrene has excellent solubility in nonpolar solvents, because of incorporation of polystyrene to the polypyrrole, solubility of PS-g-Ppy samples are increased in the low boiling point solvents such as THF, CH2Cl2 and CHCl3, whereas PPy is not soluble. The concentration used in the solubility tests was 10 mg of copolymers in 1 mL of solvents (10%). The increase in solubility can be attributed to the incorporation of the polystyrene chains. But the copolymer are not soluble in highly polar solvents such as dimethyl formamide and dimethyl sulfoxide.
Table 3. Solubility of the PS-g-Ppy samples in common organic solvents.
4. Conclusion
Polypyrrole-g-polystyrene clay nanocomposites had been prepared by living polymerisation via ATRP and chemical polymerisation method. For this purpose, first, potassium pyrrole was reacted by α-chlorophenylacetyl chloride to prepare an initiator that can polymerise styrene by ATRP technique. Then, PPy was prepared by chemical polymerisation using FeCl3 as an oxidant in dichloromethane,s solvent. Nanocomposites of the copolymer with modified montmorillonite were prepared with a solution intercalation method. The conductivity of the copolymer was measured by the four-point probe method. The structures of the intermediate, copolymer, and nanocomposite were investigated by FT-IR,1H-NMR, GPC, XRD, TEM, DSC and TGA.
Acknowledgements
The authors thank Tabriz and Saghez Payame, Noor University for supporting this project.
References
- Ray S, Okamoto M. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog Polymer Sci. 2003;28:1539–1641.
- Ke Y, Long C, Qi Z. Crystallization, properties, and crystal and nanoscale morphology of PET–clay nanocomposites. J Appl Polymer Sci. 1999;71:1139–1146.
- Su S, Wilkie C. Exfoliated poly (methyl methacrylate) and polystyrene nanocomposites occur when the clay cation contains a vinyl monomer. J Polymer Sci Part A: Polymer Chem. 2003;41:1124–1129.
- Lan T, Kaviratna P, Pinnavaia T. On the nature of polyimide–clay hybrid composites. Chem Mater. 1994;6:573–575.
- Burnside S, Giannelis E. Synthesis and properties of new poly dimethylsiloxane nanocomposites. Chem Mater. 1995;7:1597–1600.
- Alexandre M, Dubois P. Polymer-layered silicate nanocomposite preparation, properties and use of a new class of materials. Mater Sci Eng. 2000;R 28:1–63.
- Zeng Q, Wang D, Yu A, Lu G. Synthesis of polymer-montmorillonite nanocomposites by in situ intercalative polymerization. Nanotechnology. 2002;13:549–553.
- Asavapiriyanont S, Chandler K, Gunawardena G, Plectcher D. The electrodeposition of polypyrrole films from aqueous solutions. J Electroanal Chem. 1984;177:229–232.
- Takakubo M. Electrochemical polymerization of pyrrole in aqueous solutions. Synthetic Metals. 1986;16:167–172.
- Heeger A, Gustafsson G, Cao Y. Flexible light-emitting diodes made from soluble conducting polymers. Nature 1992;357:477–478.
- Arsalani N, Geckeler K. Novel electrically conducting polymer hybrids with polypyrrole. React Funct Polymers. 1997;33:166–172.
- Bozkurt A, Akbulut U, Toppare L. Conducting polymer composites of polypyrrole and polyindene. Synthetic Metals. 1996;82:41–46.
- Abbasian M, Rahmani S, Mohammadi R, Entezami A. Graft copolymerization of styrene or methyl methacrylate onto syndiotactic polystyrene by ATRP technique. J Appl Polymer Sci. 2007;104:611–619.
- Abbasian M, EsmaeilyShoja S, Shoaeifar P, Entezami A. Synthesis and characterization of amphiphilic methoxypoly (ethylene glycol)-polystyrene diblock copolymer by ATRP and NMRP techniques. J Elastomers Plast. 2012;44:205–220.
- Abbasian M, Entezami A. Metal-catalyzed living radical graft copolymerization of styrene initiated from arylated poly (vinyl chloride). Iranian Polymer J. 2006;15:395–404.
- Abbasian M, Shahparian M, EsmaeilyShoja S. Well-defined poly (methyl methacrylate) grafted to isotactic polypropylene by metal-catalyzed living radical polymerization. J. Elastomers Plast. 2013;45:317–331.
- Tizpar S, Abbasian M, Afshar Taromi F, Entezami A. Grafting of poly(methyl methacrylate) or polyacrylonitrile onto polystyrene using ATRP technique. J Appl Polymer Sci. 2006;100:2619–2627.
- Alipour M, Massoumi B, Dindar Safa K, Entezami A. Living radical polymerization of methyl methacrylate, methacrylate and their block copolymer with acrylonitrile by atom transfer radical polymerization. Iranian Polymer J. 2001;10:99–116.
- Abbasian M, Shahparian M, EsmaeilyShoja S. Chemical modification of polypropylene by nitroxide mediated living radical graft polymerization of styrene. Iranian Polymer J. 2013;22:209–218.
- Park H, Park B. Preparation and electroactivity of poly(methylmethacrylate-co-pyrrolylmethylstyrene)-g-polypyrrole. Synthetic Metals. 2002;128:229–234.
- Pearce C, Gardner W, Bartlett N, Blair N. Electronic nose for monitoring the flavour of beers. Analyst. 1993;118:371–377.
- Yin W, Li J, Li Y, Wu J, Gu T. Conducting composite film based on polypyrrole and crosslinked cellulose. J Appl Polymer Sci. 2001;80:1368–1373.
- Machado J, Karasz E, Lenz R. Electrically conducting polymer blends. Polymer. 1988;29:1412–1417.
- Paoli M, Waltman R, Diaz A, Bargon J. Conductive composites from poly(vinyl chloride) and polypyrrole. J Chem Soc Chem Commun. 1984;15:1015–1016.
- Lindsey S, Street G. Conductive composites from poly(vinyl alcohol) and polypyrrole. Synthetic Metals. 1984;10:67–69.
- Cassignol C, Cavarero M, Boudet A, Ricard A. Microstructure conductivity relationship in conducting polypyrrole/epoxy composites. Polymer. 1999;40:1139–1151.
- Bhat N, Gadre A, Bambole V. Structural and electrical properties of electropolymerized polypyrrole composite film. J Appl Polymer Sci. 2001;80:2511–2517.
- Scrosati B. Conducting polymers: advanced materials for new design: rechargeable lithium batteries. Polymer Int. 1998;47:50–55.
- Eisazadeh H, Spinks G, Wallace G. Conductive electroactive paint containing polypyrrole colloids. Mater Sci Forum. 1993;17:241–245.
- Eisazadeh H. Removal of chromium from waste water using polyaniline. J Appl Polymers. 2007;104:1964–1967.
- Eisazadeh H. Removal of mercury from water using polypyrrole and its composites. Chin J Polymer Sci. 2007;25:393–397.
- Dhawan S, Singh N, Venkatachalam S. Shielding effectiveness of conducting polyaniline coated fabrics at 101 GHz. Synthetic Metals. 2002;125:389–393.
- Benabderrahmane S., Bousalem S, Mangeney C, Azioune A, Vaulay M, Chehimi M. Interfacial physicochemical properties of functionalized conducting polypyrrole particles. Polymer. 2005;46:1339–1346.