Abstract
The present work reports study on antimicrobial activity of pure and doped ZnO nanocomposites. Polyvinyl pyrrolidone capped Mn- and Fe-doped ZnO nanocomposites were synthesised using simple chemical co-precipitation technique. The synthesised materials were characterised using transmission electron microscope (TEM), X-ray powder diffraction (XRD), energy dispersive X-ray fluorescence (EDXRF), Fourier transform infrared (FTIR) spectroscopy and ultraviolet (UV) visible spectroscopy. The XRD and TEM studies reveal that the synthesised ZnO nanocrystals have a hexagonal wurtzite structure with average crystalline size ∼7–14 nm. EDXRF and FTIR study confirmed the doping and the incorporation of impurity in ZnO nanostructure. The antimicrobial activities of nanoparticles (NPs) were studied against fungi, gram-positive and gram-negative bacteria using the standard disc diffusion method. The photocatalytic activities of prepared NPs were evaluated by degradation of methylene blue dye in aqueous solution under UV light irradiation. Experimental results demonstrated that ZnO NPs doped with 10% of Mn and Fe ions showed maximum antimicrobial and photodegradation efficiency in contrast with that of the 1% loading. The enhancement in antimicrobial effect and photocatalytic degradation is attributed to the generation of reactive oxygen species due to the synergistic effects of Mn and Fe loading.
Keywords:
1. Introduction
Pathogenic microbial contaminations and eradication of organic pollutants have been a major threat to mankind as well as to the environment. Therefore, the development of more efficient material with enhanced antimicrobial and photocatalytic activity is of great significance. Despite the great progress in antimicrobial development, many infectious diseases like intracellular infections are difficult to treat.[Citation1,Citation2] Major reasons of difficulty are transportation through cell membranes, low activity in the cells, antimicrobial toxicity to healthy tissues and acquired resistance of infectious microbes.[Citation3–Citation6] To address these issues, nanoscale materials have been emerged up as novel antimicrobial agents. Nanoparticles (NPs) are ideal forms of antimicrobial agents because these materials exhibit large surface to volume ratio and high reactivity in comparison to bulk form.[Citation7,Citation8] Many NPs have antimicrobial properties and used to control drug-resistant microbial populations.[Citation9] Various inorganic metal oxide NPs viz., ZnO, MgO, TiO2 and SiO2 exhibit considerable antimicrobial activities and used in therapeutics, diagnostics and nanomedicine-based antimicrobial agents.[Citation7,Citation10–Citation12] Inorganic NPs show greater effectiveness on resistant strains of microbial pathogens, less toxicity, heat resistance and provide mineral elements essential to human cells.[Citation13–Citation15] Ariga et al. proposed the new concept of bioinspired nanoarchitectonics as emerging drug delivery systems.[Citation16] The polymer-based NP drug delivery system can also be designed to improve the pharmacokinetics and bio distribution of the drug.[Citation17] Nakanishi et al. have summarised the biological applications, especially cell growth, sensing, and control using nanoarchitectures of nanocarbons.[Citation18] Wang et al.[Citation19] proposed that nanodiamond–epirubicin drug delivery complex is capable of killing chemoresistant cancer stem cells and preventing secondary tumour formation in liver cancer. It has been noticed that compound naphthalocyanine encapsulated in water soluble polymer dendrimer can be used to kill the tumour and cancer cells in ovarian cancer.[Citation20] The applications of nanomaterials in the food processing and shipping industries have also been reported in literature.[Citation21]
Among metal oxide NPs, ZnO NPs have many significant features such as chemical and physical stability, high catalytic activity, effective antibacterial activity as well as intensive ultraviolet (UV) and infrared (IR) adsorption.[Citation22,Citation23] In addition to this, ZnO NPs are most efficient photocatalysts used in the photodegradation of environmental organic pollutants and toxic.[Citation24,Citation25] The most common technique used to produce defects in pure ZnO NPs is based on the choice of synthesis methods,[Citation26,Citation27] use of composite photocatalysts [Citation28,Citation29] and the doping of pure ZnO with suitable metal ion impurities.[Citation30,Citation31] Various transition metal ions [Citation32–Citation35] have been used to increase the interfacial charge transfer kinetic of ZnO nanostructures. ZnO NPs have wide range of applications. It is found in paints, cosmetics, plastic and rubber manufacturing, electronics and pharmaceuticals. It is also widely used to treat a variety of skin conditions, in products such as baby powder, antidandruff shampoos and antiseptic ointment.[Citation36] It is also a component in tape used by athletes as a bandage to prevent soft tissue damage during workouts.[Citation37] These materials have been successfully used for water disinfection and purification.[Citation38] The present work reports the antimicrobial and photocatalytic activities of Mn- and Fe-doped ZnO NPs synthesised using chemical co-precipitation method.
2. Experimental
2.1. Materials
Following bacteria and fungus were used in the present study.
Bacteria: Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Salmonella typhi, Pseudomonas aeruginosa, Bacillus subtilis.
Fungi: Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, Trichophyton mentegrophytes.
Clinical isolates (C) of bacteria and fungi were procured from Gian Sagar, Medical College, Rajpura, Punjab. Standard cultures (S) of bacteria and fungi were procured from Institute of Microbial Technology, Chandigarh.
2.2. Preparation of pure and doped ZnO NPs
The polyvinyl pyrrolidone (PVP) capped pure ZnO NPs and doped with Fe (1% and 10%) and Mn (1% and 10%) were prepared using bottom-up wet chemical precipitation technique through hydrolysis and oxidising process. In a typical synthesis of Mn-doped ZnO nanocrystals, 1.0 M ZnCl2, 0.1 M MnCl2.4H2O, 2.0 M NaOH and 2% PVP were prepared separately in deionised water. The Zn and Mn precursor solutions were mixed in the stoichiometries proportion under vigorous stirring. 4 ml of 2% PVP solution was added to total 50 ml volume, before dropwise addition of 1.0 M ZnCl2 solution into 2.0 M NaOH solution. Likewise, the Fe-doped ZnO NPs were prepared using 1.0 M ZnCl2, 0.1 M FeCl3 and 2.0 M NaOH precursor solutions in the stoichiometries proportion. The resulting precipitates were filtered, thoroughly washed and then dried in air at ambient temperature to obtain NPs in powder form. Finally, the synthesised powder was calcined in a muffle furnace at 600 °C for 2 h under normal atmospheric conditions.
2.3. Characterisation
2.3.1. X-ray diffraction (XRD) analysis
The crystalline size of pure and doped ZnO NPs has been determined from XRD spectra recorded using powder XRD (PAN-Analytic) set-up equipped with 3050/60 goniometer and Cu anode X-ray tube. The XRD scans for the powder samples were performed in the 2 (range 20°–80°) keeping step size 0.001 for the Cu K X-ray radiation (λ = 1.5418 Å).
2.3.2. Transmission electron microscopy (TEM)
Formation and particle sizes of the synthesised materials were confirmed from TEM by placing a drop of the NPs dissolved in methanol on carbon coated grids and air drying. TEM images were taken using Hitachi (H-7500) TEM facility available at Punjab University, Chandigarh, India, at an accelerating voltage of 160 kV with magnification of ×30 K and ×30 K.
2.3.3. Fourier transform infrared (FTIR) spectroscopy
The presence of various chemical functional groups and the formation of pure ZnO NPs doped with Fe (1% and 10%) and Mn (1% and 10%) are also supported by FTIR spectra of NPs encapsulated with potassium bromide (KBr) salt. The characteristic IR absorption was recorded in the frequency range of 4000–4500 cm−1 using a TAG FTIR spectra.
2.3.4. Ultraviolet–Visible (UV–Vis) spectroscopy
The optical absorption measurements were carried out using UV–Vis absorption spectrophotometer (Systronic PC based Double Beam Spectrophotometer: 2202). For UV–Vis absorption studies 0.01 g of pure and doped ZnO NP powder was dispersed in 10 ml of methanol solution, and further used for characterisation.
2.3.5. Photocatalytic measurements
The photocatalytic activities of NPs were examined by studying the degradation of methylene blue (MB) dye aqueous solution under UV exposure. The photocatalytic studies under UV light were performed using UV-photoreactor equipped with two 18 W UV tubes (Orial made 66001). The photocatalytic reaction under UV light were carried out with ∼112 mg nanopowder mixed with 250 ml of 32 ppm MB dye solution in a glass reactor with surface area 200 cm2. The aqueous suspension was put under constant stirring in dark for 1 h, so that the MB dye atoms were adsorbed on the surface of nanocrystals. The stable suspension was then exposed to the UV radiation with continuous magnetic stirring. About 10 ml of suspension solutions was sampled at different time intervals and centrifuged. The supernatant after centrifugation was analysed by the UV-Vis spectrophotometer.
The rate of decolourisation of the test contaminant was noticed from the optical absorption spectra. The degradation efficiency (ϵ) of dye is calculated from the equation
(1) where C0 is the initial concentration of the dye and C is the concentration after UV photons irradiation. The photocatalytic process of MB can be expressed as apparent pseudo-first-order kinetic equation:
(2) where κ is the apparent pseudo-first-order rate constant, C0 is original MB concentration and C is MB concentration in aqueous solution at time t.
2.4. Determination of antimicrobial activity
2.4.1. Disc diffusion assay
Antibacterial and antifungal activities of the synthesised NPs were evaluated by the standard disc diffusion method described by Bauer et al. [Citation39] and modified according to clinical and laboratory standards institute guidelines.[Citation40] The chosen concentration range was 1mg/ml to 100 mg/ml in methanol and activity was determined by measuring the zone of inhibition.
2.4.2. Determination of MIC, MBC and MFC
Minimum inhibitory concentration (MIC) of the NPs for various bacteria was calculated by Broth micro dilution reference method (CLSI M7-A7) [Citation41] after determination of minimum effective antibacterial concentration from the disc diffusion method. The MIC of NPs for filamentous fungi was done by Broth micro dilution reference method (CLSI M38-A2) [Citation42] and for yeast by (CLSI M27-A3).[Citation43] Resazurin reagent was used as indicator. The lowest concentration that prevented colour change was taken as a minimum inhibitory concentration. Maximum bactericidal concentration (MBC) and maximum fungicidal concentration (MFC) were determined by plating a particular volume of sample from the wells in the microtitre plate on the nutrient agar and sabouraud dextrose agar plates of bacteria and fungi, respectively.
3. Results and discussion
3.1. Characterisation
3.1.1. X-ray diffraction (XRD) analysis
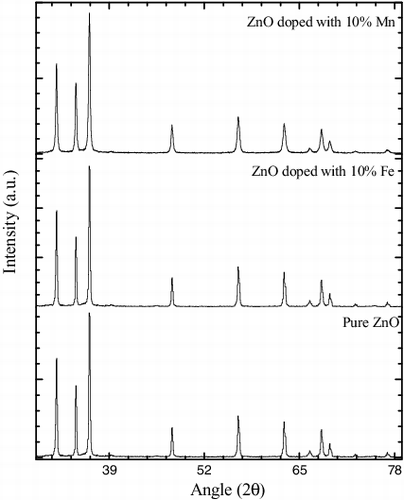
The XRD patterns for pure and doped ZnO powder samples are shown in . All recorded reflections support the hexagonal wurtzite structure of ZnO with lattice parameters a = b = 3.249 Å and c = 5.206 Å. The average crystalline size (D) of the synthesised NPs was calculated using the Scherrer's formula,[Citation44]
where β is full width half maxima of the diffraction peak, θ is the Bragg peak angle and λ is the wavelength of Cu Kα radiation. The average crystalline sizes calculated using the Scherrer formula for ZnO nanomaterial doped with different contents of Mn and Fe impurities are given in .
Table 1. Decolourisation rate constants of MB dye using ZnO photocatalysts doped with Mn and Fe impurities under UV exposure.
3.1.2. Transmission electron microscopy (TEM)
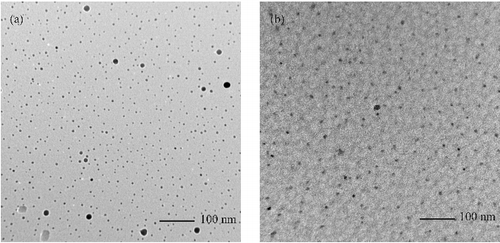
The particle size and morphology of the synthesised ZnO NPs were analysed from TEM micrographs (a) and (b)). The micrographs reveal that the product consists of spherical particles with the average size ∼9–15 nm, which is in good agreement with that estimated by Scherer formula based on the XRD pattern.
3.1.3. Fourier transform infrared (FTIR) spectroscopy
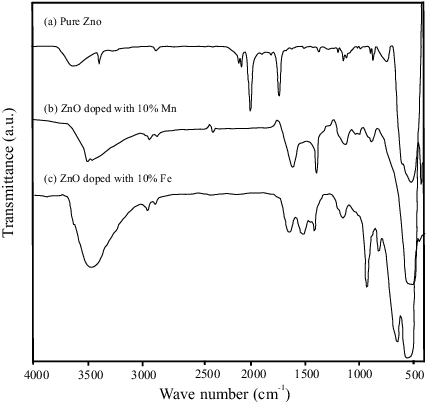
The presence of various chemical functional groups and the formation of PVP capped ZnO NPs are also supported by FTIR spectra () in KBr matrix. Similar spectra were obtained for the undoped ZnO as well as for the Mn- and Fe-doped ZnO samples. The absorption peaks in the range of 400–700 cm−1 could be attributed to the ZnO stretching modes.[Citation45] Also observed were weak absorption peaks in the range of 1100–1600 cm−1 corresponding to the OH bending mode,[Citation46] C–OH plane bending and C–OH out-of-plane bending.[Citation47] We observed a broad band in the 2900–3700 cm−1 region, which can be explained as overlapping O–H stretching modes and C–H stretching modes. Another major characteristic peak is due to PVP capping agent and coupling agent (KH-570) used in the IR spectroscopy.
3.1.4. Ultraviolet–Visible (UV–Vis) spectroscopy
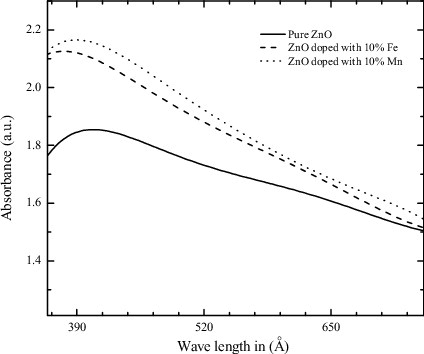
shows the UV–Vis absorption spectra of pure ZnO and ZnO doped with 10% Mn and Fe impurities. The absorption peaks of pure ZnO, Mn- and Fe-doped ZnO NPs are 399, 378 and 389 nm, respectively. It has been noticed that the position of absorption spectra in case of doped ZnO nanomaterial shifts towards the lower wavelength side with increasing dopants concentration. This indicates that band gap of pure ZnO material increases with doping concentration. The increase in band gap or blue shift can be explained by the Burstein–Moss effect.
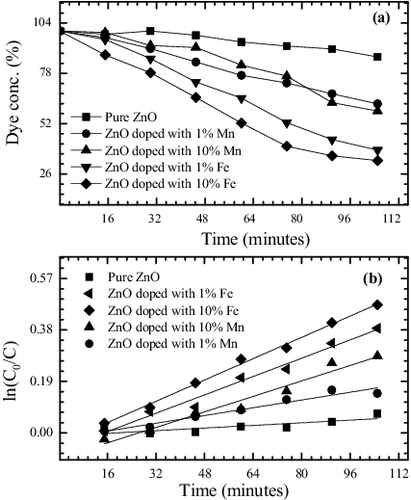
3.1.5. Photocatalytic measurements
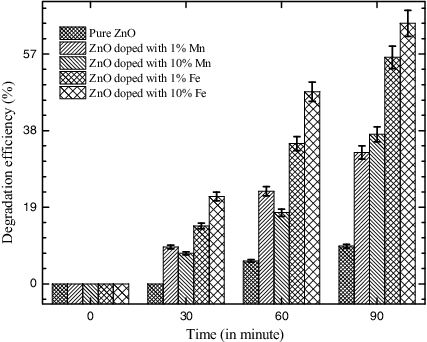
Photocatalytic performances of the synthesised nanostructures were evaluated by photodegradation of MB dye under UV light using optical absorption spectroscopy. The sensitised photodegradation curves and plot of Ln (C0/C) versus the UV irradiation time (t) in presence of different photocatalysts are shown in (a) and b), respectively. A near linear relationship between Ln (C0/C) and ‘t’ divulges that photodegradation of MB dye follows the pseudo-first-order kinetics. The photocatalytic degradation of MB dye was evaluated from the apparent rate constants (k) calculated from the linear curves using Equation (2). Higher the value of apparent rate constants (k) more will be the photocatalytic performance. The obtained first-order rate constants (k) and degradation efficiency (ϵ) of the synthesised NPs under UV exposure are also listed in . Degradation efficiency of pure and doped ZnO NPs is also shown in a bar diagram (). The experimental results clearly show the significance of Mn- and Fe-doping for the photocatalytic degradation of the MB dye solution. It has been noticed that the MB dye solution degraded to maximum extent (ϵ = 65% and k = 6.65 × 10−3 s−1) in case of ZnO nanocrystals doped with 10% Fe impurity, whereas it degraded to the minimum extent (ϵ = 9% and k = 6.08 × 10−4 s−1) by pure ZnO photocatalyst after 105-min UV light irradiation.
3.2. Antimicrobial activity
3.2.1. Disc diffusion assay
The antimicrobial activity of pure and doped NPs is given in –. It is clear from the tables that ZnO NPs doped with 10% Mn and Fe ions impurities exhibit higher antibacterial activities as compared to 1% loading and pure ZnO. The experiment results indicate that doping in the nanomaterials plays a significant role in the antibacterial activity. Our results are well supported by the earlier studies reported by Sharma et al. [Citation48] and Rekha et al. [Citation49] that transition metal doping in ZnO enhances the antibacterial activity. Thus, in this report, doped ZnO NPs have shown the best antibacterial behaviour compared to ZnO NPs. The antifungal effect of NPs has received only marginal attention and just a few studies on this topic have been published. In case of fungi the activity was reported only against T. mentagrophytes (S) and C. neoformans (C) and no activity was seen in the case of A. fumigatus and C. albicans. Our results showed better antifungal activity against T. mentagrophytes as compared to the earlier work of Kim et al. who reported antifungal effect of Ag NPs on T. mentagrophytes and C. albicans at 1mg/ml.[Citation50] However, in our study no activity has been observed against C. albicans and A. fumigatus. The absence of activity against Aspergillus may be due to the complex cell wall structure with high percentage of chitinous layer. Owing to the chitin layer, OH produced by NPs was not able to cause the destruction of cell wall.[Citation51] Though at high concentration (100 mg/ml) activity against C. neoformans was observed in our study, which is quite least studied. However, the exact mechanism of resistance to the fungus is not known till now. The interaction between NPs and the membrane results changes in the membrane observed as ‘pits’ on the membrane surfaces. The formation of pores subsequently leads to cell death.[Citation52] It may also be the case through which ZnO NPs showed activity against T. mentagrophytes and C. neoformans.
Table 2. Antimicrobial activity of ZnO NPs against various microorganisms.
Table 3. Antimicrobial activity of Fe-doped ZnO NPs against various microorganisms.
Table 4. Antimicrobial activity of Mn-doped ZnO NPs against various microorganisms.
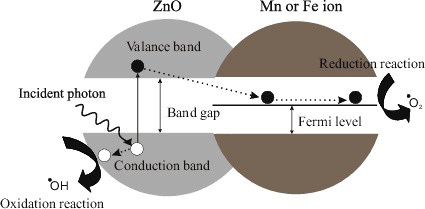
In this study, different concentrations of nanoscale Fe- and Mn-doped ZnO and pure ZnO were tested to find out the best concentration that can have the most effective antibacterial property. With an increase in concentration of doping antimicrobial activity increased. Our data are in accordance with the previous studies, dealing with the antimicrobial effects of NPs.[Citation53] If the concentration of doped metals in nano-ZnO increases in culture medium, interaction between oxygen and dehydrogenase enzyme increases too which enhances antimicrobial activity.[Citation54] The increase in antibacterial and photocatalytic activity to increase in dopants concentration degradation is attributed to the generation of reactive oxygen species (ROS) due to coupling or synergistic effects of Mn and Fe loading. The ROS generation is attributed to the creation of photoinduced charge carriers in ZnO NPs and their interactions with oxygen and water molecules on the surface of particles. It is proposed that photogenerated electron migrates to the surface of dopant ions from the conduction band of excited ZnO (). Likewise, the photogenerated hole is also transferred to the surface of ZnO NPs. An electron in the conduction band on the catalyst surface can reduce molecular oxygen to superoxide anion. The superoxide anion can then react with H2O to form H2O2, which further forms •OH radicals. On the other hand, migrated hole reacts with chemisorbed H2O molecules to form reactive species such as O2− and •OH radicals. Such an efficient charge separation increases the lifetime of the charge carriers and increases the efficiency of the interfacial charge transfer to adsorbed substrates. The presence of ROS such as O2− and •OH radicals is the primary cause of cell death and organic matter mineralisation. Another possible explanation for this could be the abrasive surface texture of ZnO as doping of metal oxide and/or transition metals increases the surface defects.
3.2.2. Minimum inhibitory concentration (MIC)
MIC and MBC/MFC for the NPs were calculated against gram-negative as well as gram-positive bacteria and fungal strains (). In this case also doped ZnO NPs were found to be better than pure. Excellent MIC of 0.09 mg/ml against B. subtilis (S) was obtained in the case of 10% Fe doping. Overall, in the present study gram-negative bacterial strains were more sensitive in comparison to gram-positive strains against the NPs tested. Except for 10% Fe which showed more activity towards B. subtilis, a gram-positive bacteria. However, all other NPs were more sensitive to S. typhi and P. aeruginosa. Previous studies have also shown good activity of ZnO NPs against gram-negative bacteria.[Citation55,Citation56] More activity towards gram-negative bacteria may be because the cell wall of gram-positive bacteria has more peptidoglycan than gram-negative bacteria cell wall, which is negatively charged, and more ZnO, Fe and Mn ions may get trapped to peptidoglycan in gram-positive bacteria.[Citation57] Whereas in case of gram-negative bacteria there are electrostatic interactions between NPs and cell surface followed by cell morphological changes, increase in cell permeability and their accumulation in the cytoplasm.[Citation58]
Table 5. Minimum inhibitory concentration (mg/ml) of pure ZnO and doped ZnO NP against various microorganisms.
Table 6. Minimum bactericidal/fungicidal concentration (mg/ml) of pure ZnO and doped ZnO NP against various microorganisms.
MIC followed the same pattern as antimicrobial activity. MIC observed in T. mentegrophytes (S) and C. neoformans (C) was 0.33 ± 0.14 and 26.6 ± 14.4 mg/ml, respectively. MIC values for both the fungal strains were 0.25 ± 0.21 and 11.5 ± 11.8 mg/ml in case of 10% Fe doping and 0.33 ± 0.14 and 13.5 ± 10.9 mg/ml in case of 1% Fe doping, respectively. Similarly, MIC values of T. mentegrophytes and C. neoformans were 0.21 ± 0.07 and 9.4 ± 5.4 mg/ml, respectively () in case of 10% Mn doping. MIC of T. mentegrophytes against 1% doping (s) was 0.25 ± 0.24 mg/ml whereas for C. neoformans was 14.5 ± 9.5 mg/ml (). However, less activity of NPs against fungus may be attributed to the complex cell wall structure of the fungus.
3.2.3. Maximum bactericidal/fungicidal concentration (MBC/MFC)
Results of MBC/MFC were on the same line as MIC. The values obtained for MBC were at least the double of the counterpart MIC which suggests the bactericidal nature of NPs. Optimum MBC was obtained for 10% Fe-doped ZnO NP against B. subtilis (S) (0.14 ± 0.43 mg/ml) followed by 10% Mn against S. typhi (S) (0.17 ± 0.28 mg/ml). Several investigations have suggested the possible mechanisms involving the interaction of NPs with the biological macromolecules. The production of H2O2 from the surface of ZnO is considered as an effective means for the inhibition of bacterial growth.[Citation59] Another possible mechanism is the release of Zn2+ ions, resulting in the damage of the bacterial membrane and direct cellular internalisation of ZnO NPs.[Citation60] A recent study suggested the generation of ROS by ZnO NPs may lead to oxidative stress and lipid peroxidation, and as a result NPs get internalised resulting in oxidative DNA damage.[Citation61] NPs are known to deactivate cellular enzymes and DNA by coordinating with the electron-donating groups such as carboxylates, amides, imidazoles, indoles, hydroxyls and so forth. They create pits in bactericidal cell walls, leading to increased permeability and eventually the cell death.[Citation62] The metal ion-based nanomaterials exhibit broad-spectrum biocidal activities towards different bacteria, fungi and viruses.[Citation63] Previous studies of NPs have shown that the greater the size of NPs, the greater the efficacy in inhibiting the growth of bacteria, involving both the production of ROS and the accumulation of NPs.[Citation64] The high rate of generation of surface oxygen species from ZnO leads to the death of the bacteria once it kills/captures the bacteria. The ZnO NPs presumably remain tightly adsorbed on the surface of the leftover/dead bacteria preventing further antibacterial action. However, ZnO NPs continue to release peroxides into the medium even after the surface of the dead bacteria is completely covered by ZnO NPs, thereby showing high bactericidal efficacy.
4. Conclusions
As clear from the results that a higher percentage of doping leads to a significant rise in antimicrobial potential, still higher content of metal ion doping (including Fe, Mn and others) needs to be studied further. However, based on the findings of present studies, it cannot be denied that Fe-doped ZnO possess an enormous potential as an antimicrobial agent and therefore can be pursued as an important candidate for future studies.
Acknowledgements
The authors are grateful to Shoolini University, Department of Biotechnology, Bajhol, Solan, Himachal Pradesh, India, for support and institutional facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Matthews L, Kanwar RK, Zhou S, Punj V, Kanwar JR. Applications of nanomedicine in antibacterial medical therapeutics and diagnostics. Open Trop Med J. 2010;3:1–9.
- Zhang L, Pornpattananangkul D, Hu MJ, Huang CM. Development of NPs for antimicrobial drug delivery. Curr Med Chem. 2010;17:585–594.
- Tenover FC. Mechanism of antimicrobial resistance in bacteria. Am J Med. 2006;119:S3–S10.
- Deotale V, Mendiratta DK, Raut U, Narang P. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Ind J Med Microbiol. 2010;28:124–126.
- Wright GD. Antibiotic resistance in the environment: a link to the clinic. Curr Opin Microbiol. 2010;13:589–594.
- Mandell BP, Tillotson G. Antimicrobial safety and tolerability: differences and dilemmas. Clin Infect Dis. 2001;32:S72–S79.
- Reddy KM, Kevin F, Jason B, Denise GW, Cory H, Alex P. Selective toxicity of zinc oxide NPs to prokaryotic and eukaryotic systems. J Appl Phys Lett. 2007;90:1–3.
- Mukherjee A, Sadiq M, Prathna TC, Chandrasekaran N. Antimicrobial activity of aluminium oxide NPs for potential clinical applications. In: Méndez-Vilas A, editor. Science against microbial pathogens communicating current research and technological advances. Badajoz, Spain: FORMATEX; 2011. p. 245–251.
- Rai M, Yadav A, Gade A. Silver NPs as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83.
- Laura KA, Delina YL, Pedro JJA. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. J Water Res. 2006;40:3527–3532.
- Mohsen J, Zahra B. Protein nanoparticle: a unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7:4926–4934.
- Sobha K, Surendranath K, Meena V, Jwala KT, Swetha N, Latha KSM. Emerging trends in nanobiotechnology. J Biotechnol Mol Biol Rev. 2010;5:001–012.
- Nagarajan P, Rajagopalan V. Enhanced bioactivity of ZnO NPs – an antimicrobial study. J Sci Technol Adv Mater. 2008;9:035004.
- Toshiaki O, Osamu Y, Yasuhiro I, Zenbe-e N. Antibacterial activity of ZnO powder with crystallographic orientation. J Mater Sci Med. 2008;19:1407–1412.
- Zakaria ZA, Mat Desa A, Ramasamy K, Ahmat N, Mohamad AS, Israf DA, Sulaiman MR. Lack of antimicrobial activities of Dicranopteris linearis extracts and fractions. Afr J Microbiol Res. 2010;4:071–075.
- Ariga K, Kawakami K, Ebara M, Kotsuchibashi Y, Ji Q, Hill JP. Bioinspired nanoarchitectonics as emerging drug delivery systems. New J Chem. 2014;38:5149–5163.
- Chu KS, Hasan W, Rawal S, Walsh MD, Enlow EM, Luft JC, Bridges AS, Kuijer JL, Napier ME, Zamboni WC, DeSimone JM. Plasma, tumor and tissue pharmacokinetics of Docetaxel delivered via nanoparticles of different sizes and shapes in mice bearing SKOV-3 human ovarian carcinoma xenograft. Nanomedicine. 2013;9:686–693.
- Nakanishi W, Minamia K, Shrestha LK, Ji Q, Hill JP, Ariga K. Bioactive nanocarbon assemblies: nanoarchitectonics and applications. Nanotoday. 2014;9:378–394.
- Wang X, Low XC, Hou W, Abdullah LN, Toh TB, Rashid MMA, Ho D, Chow EKH. Epirubicin-adsorbed nanodiamonds kill chemoresistant hepatic cancer stem cells. ACS Nano. 2014;8:12151–12166.
- Taratula O, Schumann C, Duong T, Taylor KL, Taratula O. Dendrimer-encapsulated naphthalocyanine as a single agent-based theranostic nanoplatform for near-infrared fluorescence imaging and combinatorial anticancer phototherapy. Nanoscale. 2015;7:3888–3902.
- Feng G, Cheng Y, Wang SY, Hsu LC, Feliz Y, Borca-Tasciucb DA, Worobo RW, Moraru CI. Alumina surfaces with nanoscale topography reduce attachment and biofilm formation by Escherichia coli and Listeria spp. Biofouling. 2014;30:1253–1268.
- Kalyani G, Anil VG, Bo-Jung C, Yong-Chien L. Preparation and characterization of ZnO NPs coated paper and its antibacterial activity study. J Green Chem. 2006;8:1034–1041.
- Matei A, Cernica I, Cadar O, Roman C, Schiopu V. Synthesis and characterization of ZnO – polymer nanocomposites. Int J Mater Form. 2008;1:767–770.
- Gouvea CA, Wypych F, Moraes SG, Duran N, Nagata N, Peralta-Zamora P. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere. 2000;40:433–440.
- Lai Y, Meng M, Yu Y, Wang X, Ding T. Photoluminescence and photocatalysis of the flower-like nano-ZnO photocatalysts prepared by a facile hydrothermal method with or without ultrasonic assistance. Appl Catal B. 2011;105:335–345.
- Li B, Wang Y. Facile synthesis and enhanced photocatalytic performance of flower-like ZnO hierarchical microstructures. J Phys Chem C. 2010;114:890–896.
- Pawinrat P, Mekasuwandumrong O, Panpranot J. Synthesis of Au-ZnO and Pt-ZnO nanocomposites by one-step flame spray pyrolysis and its application for photocatalytic degradation of dyes. Catal Commun. 2009;10:1380–1385.
- Cho S, Jang JW, Kim J, Lee JS, Choi W, Lee KH. Three dimensional type II ZnO/ZnSe heterostructures and their visible light photocatalytic activities. Langmuir. 2011;27:10243–10250.
- Qin H, Li W, Xia Y, THe. Photocatalytic activity of heterostructures based on ZnO and N-doped ZnO. Appl Mater Interfaces. 2011;3:3152–3156.
- Li Y, Zhao X, Fan W. Structural, electronic, and optical properties of Ag-doped ZnO nanowires: first principles study. J Phys Chem C. 2011;115:3552–3557.
- YeC, Bando Y, Shen G, Golberg D. Thickness-dependent photocatalytic performance of ZnO nanoplatelets. J Phys Chem B. 2006;110:15146–15151.
- Murugesan V, Sivamurugan K, Ariga. Photocatalytic activity of La-doped ZnO for the degradation of monocrotophos in aqueous suspension. J Mol Catal A. 2007;266:149–157.
- Lu F, Cai WP, Zhang YG. ZnO hierarchical micro/nanoarchitectures: solvothermal synthesis and structurally enhanced photocatalytic performance. Adv Funct Mater. 2008;18:1047–1056.
- Ullah R, Dutta J. Photocatalytic degradation of organic dyes with manganese doped ZnO NPs. J Hazard Mater. 2008;156:194–200.
- Akhavan O, Ghaderi E. Enhancement of antibacterial properties of Ag nanorods by electric field. Sci Technol Adv Mater. 2009;10:015003.
- Hughes G, McLean NR. Zinc oxide tape: a useful dressing for the recalcitrant finger-tip and soft-tissue injury. Arch Emerg Med. 1988;5:223–227.
- Hu H, Zhang W. Synthesis and properties of transition metals and rare-earth metals doped ZnS Nanoparticles. Opt Mater. 2006;28:536–550.
- Li Q, Mahendra S, Lyon DY, Brunet L, Liga MV, Li D, Alvarez PJJ. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42:4591–4602.
- Bauer AW, Kirby WMN, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45:493–496.
- National Committee for Clinical Laboratory Standards (NCCLS). Performance standard for antimicrobial disc susceptibility test. Approved standard. Villanova (PA): NCCLS; 2006.
- Chemical Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard – seventh edition. Wayne (PA): CLSI; 2006. (CLSI document M7-A7).
- Chemical Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard – second edition. Wayne (PA): CLSI; 2008. (CLSI M38-A2).
- Chemical Laboratory Standards Institute (CLSI). Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard – third edition. Wayne (PA): CLSI; 2008. (CLSI document M 27-A3).
- Cullity BD. Elements of X-ray diffraction. Reading (MA): Addison-Wesley; 1978.
- Hernández A, Maya L, Sánchez-Mora E, Sánchez EM. Sol-Gel synthesis, characterization and photocatalytic activity of mixed oxide ZnO-Fe2O3. J Sol Gel Sci Technol. 2007;42:71–78.
- Das J, Evans IR, Khushalani D. Zinc glycolate: a precursor to ZnO. Inorg Chem. 2009;48:3508–3510.
- Suwanboon S, Ratana T, Ratana WT. Effect of Al and Mn dopant on structural and optical properties of ZnO thin film prepared by sol-gel route. J Sci Technol. 2007;4:111–121.
- Sharma D, Rajput J, Kaith BS, Kaur M, Sharma S. Synthesis of ZnO NPs and study of their antibacterial and antifungal properties. Thin Solid Films. 2010;519:1224–1229.
- Rekha K, Nirmala M, Nair MG, Anukaliani A. Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide NPs. Phys B. 2010;405:3180–3185.
- Kim KJ, Sung WS, Suh BK, Moon SK, Choi JS, Kim JG, Lee DG. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Biometals. 2009;22:235–242.
- Gajbhiye MJ, Kesharwani A, Ingle A, Rai M. Fungus- mediated synthesis of silver NPs and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine. 2009;5:382–386.
- Holt KB, Bard AJ. Interaction of silver (I) ions with the respiratory chain of Escherichia coli: an electrochemical and scanning electrochemical microscopy study of the antimicrobial mechanism of micromolar Ag+. Biochemistry. 2005;44:13214–13223.
- Hranisavljevic J, Dimitrijevic N, Wurtz G, Wiederrecht G. Photoinduced charge separation reactions of J-aggregates coated on silver NPs. J Am Chem Soc. 2002;124:4536–4537.
- Sikong L, Kongreong B, Kantachote D, Sutthisripok W. Photocatalytic activity and antibacterial behavior of Fe3+-doped TiO2/SnO2 NPs. Energy Res. 2010;1:120–125.
- Li WR, Xie XB, Shi QS, Zeng HY, OU-Yang YS, Chen YB. Antibacterial activity and mechanism of silver NPs on Escherichia coli. J Appl Microbiol Biotechnol. 2009;85:1115–1122.
- Kawahara K, Tsuruda K, Morishita M, Uchida M. Antibacterial effect of silver-zeolite on oral bacteria under anaerobic conditions. Dent Mater. 2000;16:452–455.
- Sinha R, Karana R, Sinha A, Khare SK. Interaction and nanotoxic effect of ZnO and Ag NPs on mesophilic and halophilic bacterial cells. Bioresour Technol. 2011;102:1516–1520.
- Sawai J, Yoshikawa T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductimetric assay. J Appl Microbiol. 2004;96:803–809.
- Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mater. 2001;3:643–646.
- Brayner R, Ferrari-lliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 2006;6:866–870.
- Lin W, Xu Y, Huang CC, Ma Y, Shannon KB, Chen DR, Huang YWJ. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J Nanopart Res. 2009;11:25–39.
- Greenberg C, Steffek C. Bio-adhesion to thin films in relation to cleaning. Thin Solid Films. 2005;484:324–327.
- Raghupati KR, Koodali RT, Manna AC. Size dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide NPs. Langmuir. 2011;27:4020–4028.
- Jan T, Iqbal J, Ismail M, Zakaullah M, Naqvi SH, Badshah N. Sn doping induced enhancement in the activity of ZnO nanostructures against antibiotic resistant S. aureus bacteria. Int J Nanomed. 2013;8:3679–3687.