ABSTRACT
The bioreduction method employed for the synthesis of colloidal AgNPs and AuNPs is reported here. Methanolic and aqueous extracts of Dolichos biflorus Linn seed was used as the bio-reducing agent. The structural and morphological aspects of the synthesised metal nanoparticles were investigated using X-ray diffraction (XRD), energy-dispersive spectroscopy (EDX), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). XRD, revealed crystalline nature of the synthesised particles, UV–vis spectrophotometric analysis showed characteristic absorption peak for both AgNPs and AuNPs. EDX analysis confirmed the presence of elemental silver and gold particles and the average size and morphology were determined by SEM and TEM. The synthesised AgNPs exhibited good antibacterial potential whereas AuNPs showed poor activity against human pathogenic, gram-positive bacteria such as Staphylococcus aureus, Bacillus subtilis and gram-negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa.
1. Introduction
Nowadays nanoparticles are gaining tremendous popularity because of its outstanding significance of optical, chemical and biological properties. Application of nanoparticles are widely distributed across various range of areas, such as agriculture, waste management, pollution control, solar cell and biomedical approach for antimicrobial activity, anticancer, antitumour, etc. Research has been intensified for the development of antibacterial material containing different metal nanoparticles. Among them, silver and gold (either in metal or in ionic form) have long been known to have strong inhibitory and bactericidal effect as well as a broad spectrum of antimicrobial qualities.[Citation1–3] These two noble metals have no toxic effect to human health.
Various chemicals and physical methods are listed for the synthesis of nanoparticles, such as chemical reduction,[Citation4] photochemical reduction [Citation5,Citation6] and electrochemical reduction.[Citation7,Citation8] These processes sometimes depend on the toxic chemicals used as reducing agent, or capping agent which may not be harmful for the synthesis of nanoparticles in industrial purposes. But it is always expected to synthesise the nanoparticles with a greener approach [Citation9] especially for the environmental and biological sectors. Therefore, there is a growing need to develop environment friendly processes to avoid the toxic chemicals in their synthesis.
To overcome the problem of toxicity in synthesis, extracts (aqueous, organic) of various medicinal plants and their different parts are the most alternative efficient choice in the synthesis of nanoparticles. The plant extracts contain a variety of poly-ol (aromatic, nonaromatic and polysaccharides) and water or alcohol soluble heterocyclic compounds which have both protective and reductive activity, and are mainly responsible for the reduction of silver and gold ions. Along with that the ease of synthesising Au and Ag nanoparticles and their affinity for the binding with many biological molecules, make them attractive candidates for study. In recent years, the greener approach for the synthesis of silver and gold nanoparticles (AuNPs) using various plant-mediated extracts as a reducing agent has been a new dimension of research over conventional one.
Dolichos biflorus Linn is commonly known as ‘Horse gram’. In India, the seeds are used in the treatment of constipation, piles, pain, cough, oedema, asthma, wounds and urinary calculi.[Citation10,Citation11] This study was therefore designed for the evaluation of complete green nano synthesis with two different types of extracts of D. biflorus Linn seed and their characterisation and study of antimicrobial activity. This study presents an overview on the preparation of silver nanoparticles (AgNPs) and AuNPs by greener approaches which are benign for human health.
2. Experimental
2.1. Materials
Dry seeds of D. biflorus Linn were purchased from the market. Methanol (≥99.9%) and silver nitrate (GR) was commercially purchased from Merck Limited, India and chloroauric acid (∼52% Au basis) from Sigma-Aldrich, India. Agar powder, beef extract powder and peptone were purchased from HiMedia, India. Throughout the experiments, milli Q water was used.
2.2. Preparation of Dolichos biflorus Linn extract
Seeds of D. biflorus were sieved and sun dried to remove the residual moisture. 25 g of seeds were extracted in 100 ml sterile distilled water and 100 ml methanol separately. Both the extracts were centrifuged at 3000 rpm for 15 mins and filtered the supernatant, and preserved at 4 °C for further studies.
2.3. Synthesis of AgNPs
The aqueous and methanol extract of seeds of D. biflorus (1 mg/ml) were used as the bio-reducing agent for the synthesis of AgNPs separately using AgNO3 (1 mM) prepared by dissolving in double distilled water and methanol, respectively, at room temperature. Different reaction concentrations of D. biflorus seed extract and AgNO3 solution (1:4, 2:3, 3:2, 4:1) were prepared. The progress of the reaction was continuously monitored by ultraviolet–visible spectrophotometry (UV–vis). At regular intervals, aliquot samples were taken to measure the optical density in the range 300–800 nm and its colour was found to change from transparent to grey in case of water extract and transparent to brown for methanolic extract.
2.4. Synthesis of AuNPs
The reduction into AuNPs was carried out by mixing of aqueous and methanol extract of seeds, D. biflorus (1 mg/ml) with aqueous and methanolic solution of HAuCl4 (1 mM), respectively, at room temperature. D. biflorus seed extract and HAuCl4 solution (1:4, 2:3, 3:2, 4:1) were prepared in various proportions. The pale yellow coloured solution turned to ruby red colloid solution for both the extracts, indicating the formation of AuNPs. The reduction of metal ions was continuously monitored by UV–vis measurement in the range 300–800 nm.
2.5. Characterisation
Nanoparticles are synthesised in different reaction concentrations which are as follows 1:4; 2:3; 3:2; 4:1 of D. biflorus seed extract and salt solution (AgNO3, HAuCl4 separately) and then all of them are characterised by different instruments. Spectrophotometer, JASCO V- 530 UV–vis, was used for the measurement of optical properties of synthesised colloidal particles within the 300–800 nm range. The phase and crystal structures of the deposited metal NPs were characterised by a Bruker D-8 Advanced X-ray diffractometer (XRD) with θ–2θ scanning mechanism and Ni-filtered Cu Kα X-radiation with wavelength 1.540598 Å. Scanning electron microscopy (SEM) observations were carried out on a ZEISS EVO 18 Electron microscope. The energy-dispersive spectroscopy (EDX) of the nanoparticles was measured on ZEISS EVO 18 Electron microscope. The surface morphology measurements of the deposited metal NPs were carried out by fabricating a drop of suspension onto a clean glass slides and allowing water to completely evaporate with platinum coating.
2.6. Antimicrobial activity by well diffusion method
Nanoparticles are synthesised in different reaction concentrations which are as follows 1:4; 2:3; 3:2; 4:1 of D. biflorus seed extract and salt solution (AgNO3, HAuCl4 separately) and then all of them are measured for antimicrobial activities. The disc diffusion method was applied to evaluate the AgNPs and AuNPs with four human pathogenic bacteria, Gram positive bacteria, such as Staphylococcus aureus, Bacillus subtilis Gram negative bacteria, such as Escherichia coli, Pseudomonas aeruginosa grown on nutrient agar plate separately. The test bacterial cultures were inoculated on solidified agar plate and incubated at 37 °C for 24 hrs.
3. Results and discussions
3.1. Optical properties by UV–vis analysis
Synthesised colloidal AgNPs showed prominent absorption band within 420–430 nm, which corresponds to the wavelength of the surface plasmon resonance of AgNPs. The absorbance peak within 420–430 nm () for the synthesised AgNPs [Citation12] was sharp when aqueous extract used as the reducing agent and broad when methanol extract was used as the reducing agent.
Figure 1. UV–vis spectrum of (I) AgNPs and (II) AuNPs obtained from (A) aqueous and (B) methanolic extracts of D. biflorus.
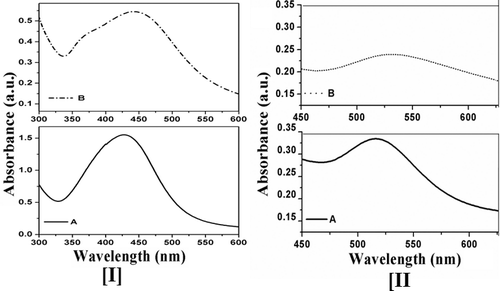
Surface plasmon resonance of synthesised colloidal AuNPs that appeared at 530 nm indicates the formation of AuNPs [Citation13] from both aqueous and methanolic extract. However, the absorption peak for the aqueous suspension of AuNPs was steeper in comparison to the methanolic one.
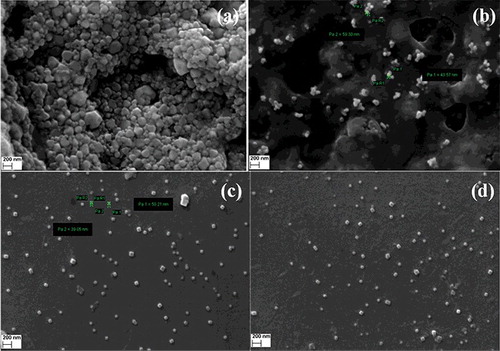
3.2. Energy-dispersive X-ray spectroscopy (EDX) and scanning electron microscopy (SEM)
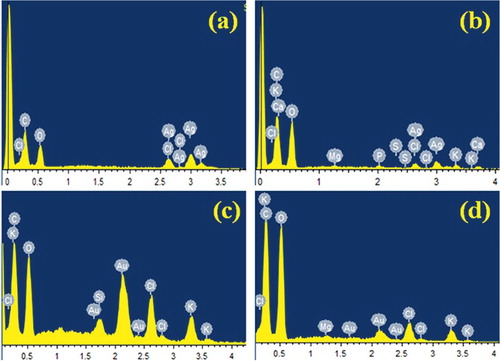
Analysis through EDX illustrated the presence and elemental composition of synthesised AgNPs and AuNPs (). The main peaks obtained were for Ag, Au and some other weaker peaks for C, O, Cl, Al, K and Na.
Figure 2. EDX spectra of synthesised (a) AgNPs in aqueous medium, (b) AgNPs in methanolic medium, (c) AuNPs in aqueous medium and (d) AuNPs in methanolic medium.
The SEM image () further ascertains that the morphology of AgNPs and AuNPs are predominantly spherical in shape. The SEM image confirmed the particles were agglomerated and they formed irregular shape.
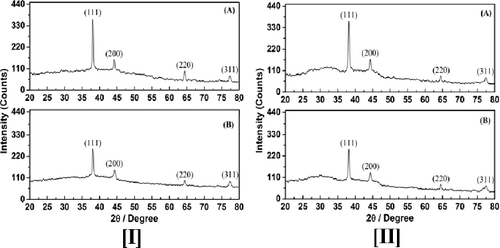
3.3. X-ray diffraction (XRD) technique
The structural properties of prepared nanoparticles were determined by X-ray diffraction (XRD) technique. The diffraction angles corresponding to different peaks were matched with those in standard JCPDS data (04-0783 for Ag, 04-0784 for Gold) files to identify the phases formed.
Analysis of XRD was performed to confirm the crystalline nature of the AgNPs and AuNPs (). The XRD pattern showed numbers of Bragg reflections that may be indexed on the basis of the fcc structure of Ag and Au. Four distinct peaks at 2θ values of 38.11, 44.27, 64.42 and 77.42° were indexed with the planes (111), (200), (220) and (311) for the fcc cubic Ag. The well resolved and intense XRD pattern clearly showed that the AgNPs formed by the reduction of Ag+ ions using aqueous and methanol extracts are crystalline in nature.[Citation14–16] Similar results were reported for AgNPs in the literature. Four diffraction peaks at 2θ values of nearly 38.18, 44.39, 64.57 and 77.54 were observed in each of the figures which correspond to (111), (200), (220) and (311) planes, respectively, of fcc Au in AuNPs.[Citation17–19] From XRD of Ag and Au, it is clear that grains are strongly oriented along the (111) plane. The peaks corresponding to the (111) plane is stronger than the other planes.
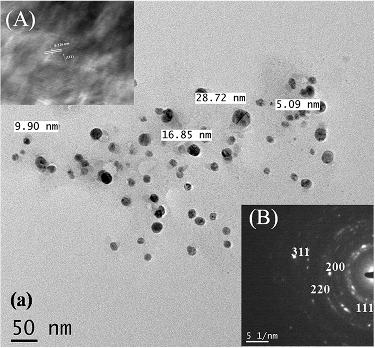
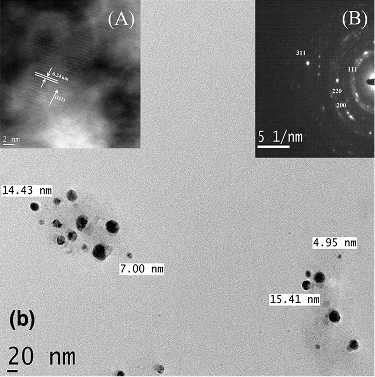
3.4. Transmission electron microscopy (TEM)
Transmission electron microscopy (TEM) was employed to characterise the morphology and distribution of particle sizes of synthesised AgNPs and AuNPs. The average particle size was found as 5.33 ± 1.18 and 6.93 ± 2.88 nm ( and ). The inset figures show the (A) high-resolution transmission electron micrograph (HRTEM) image and (B) selected area diffraction (SAED) pattern of the NPs. The distinct rings that appeared in the SAED pattern confirmed the polycrystalline nature of the samples. The d-value calculated from the ring diameters confirmed the presence of Ag and Au for individual samples and the corresponding crystal planes are labelled. The SAED patterns of the AuNPs and AgNPs indexed to the (111), (200), (220), and (311) Bragg reflections of the fcc structure which are in agreement with the result of X-ray diffraction which confirmed high crystalline nature of the samples. The observed lattice fringes clearly revealed the growth of NPs preferentially along the (111) plane with high degree of crystallinity. Also, HRTEM revealed the line spacing (0.223 nm for AgNPs and 0.24 for AuNPs) corresponding to the (111) crystal plane for Ag and Au. Therefore, the clear lattice fringes in HRTEM and typical SAED pattern with bright circular rings corresponding to the (111), (200), (220) and (311) planes show that the NPs obtained are highly crystalline in nature.[Citation20–22]
3.5. Antimicrobial activity by well diffusion method
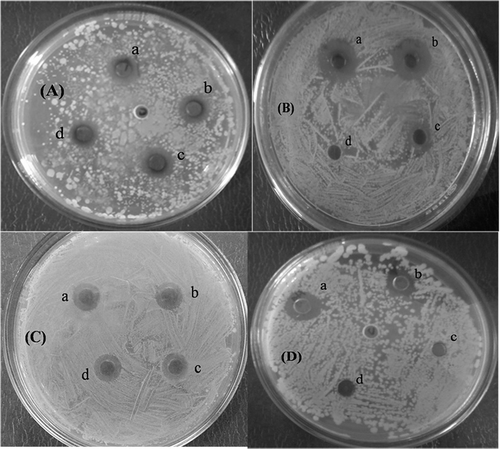
AgNPs and AuNPs which were synthesised by D. biflorus Linn seed extract showed zone of inhibitions against several human pathogenic bacteria. Probable reasons for reduced antibacterial efficiency of AuNPs compared to AgNPs are because silver itself has higher antimicrobial effect than gold. This effect is dependent on superficial contact, where silver can inhibit enzymatic systems of the respiratory chain and alter DNA synthesis. The replication of bacterial DNA was reduced and the protein became inactivated after adherence of silver (AgNPs) treatment in comparison with gold.
Antimicrobial activity conducted against S. aureus, B. subtilis, E. coli, P. aeruginosa with synthesised AgNPs showed a high inhibitory effect on human pathogenic Gram positive and Gram negative bacteria (). The activity against bacteria was carried out by measuring the observed zone of inhibition by well diffusion method. Synthesised AuNPs showed poor antimicrobial activity with Gram positive and Gram negative bacteria.
Figure 7. Antimicrobial activity against (A) Staphylococcus aureus, (B) Bacillus subtilis, (C) Escherichia coli, (D) Pseudomonas aeruginosa where, (a) AgNPs synthesised in aqueous mediam; (b) AgNPs synthesised in methanol medium; (c) AuNPs synthesised in aqueous medium and (d) AuNPs synthesised in methanolic medium.
4. Conclusions
In conclusion, spherical-shaped AgNPs and AuNPs were synthesised successfully by the bioreduction method using aqueous and methanol extract of D. biflorus Linn seed from aqueous and methanolic solution of AgNO3 and Aucl4, respectively, which is a template-less method. The major constituents in extract are mainly biomolecules like poly-ol (aromatic, nonaromatic and polysaccharides) which are responsible for the bioreduction of stable AgNPs and AuNPs.
The characteristic plasmon resonance peak that appeared on UV–vis for the synthesised colloidal AgNPs and AuNPs indicates the formation of nanoparticles from both aqueous and methanolic extract. TEM images clearly revealed that AgNPs and AuNPs have been formed in the range between 5.33 ± 1.18 and 6.93 ± 2.88 nm, respectively. Their polycrystalline nature was confirmed by the XRD and TEM analysis. Antimicrobial potentiality against human pathogenic bacteria showed a promising result. The production of these NPs in the proposed method is eco-friendly, cost effective and single step as well as less time consuming which could be commercialised for large-scale production and many potential biomedical applications.
Acknowledgements
All authors participated in the experiments and read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Agnihotri S, Mukherji S, Mukherji S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: elucidation of the mechanism of bactericidal action of silver. Nanoscale. 2013;5:7328–7340.
- Dong PV, Ha CH, Binh LT, et al. Chemical synthesis and antibacterial activity of novel-shaped silver nanoparticles. Int Nano Lett. 2012;2:9. doi:10.1186/2228–5326–2–9.
- Zhou Y, Kong Y, Kundu S, et al. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnol. 2012;10:19. doi:10.1186/1477–3155–10–19.
- Kholoud MM, Noura AE, Eftaihab A, et al. Synthesis and applications of silver nanoparticles. Arab J Chem. 2010;3(3):135–140.
- Kshirsagar P, Sangaru, SS, Brunetti V, et al. Synthesis of fluorescent metal nanoparticles in aqueous solution by photochemical reduction. Nanotechnology. 2014;25(4):045601. doi:10.1088/0957–4484/25/4/045601.
- Scaiano JC, Billone P, Gonzalez CM, et al. Photochemical routes to silver and gold nanoparticles. Pure Appl Chem. 2009;81(4):635–647.
- Roldán MV, Pellegri N, Sanctis OD. Electrochemical Method for Ag-PEG Nanoparticles Synthesis. J Nanoparticles. 2013;7. doi:10.1155/2013/524150.
- Yanga J, Shengyuan D, Jianping L, et al. Electrochemical synthesis of reduced graphene sheet–AuPd alloy nanoparticles composites for enzymatic biosensing. Biosens Bioelectron. 2011;29:159–166.
- San NO, Kursungöz C, Tümtas Y, et al. Novel one-step synthesis of silica nanoparticles from sugarbeetbagasse by laser ablation and their effects on the growth offreshwater algae culture. Particuology. 2014;17:29–35.
- Saha S, Verma RJ. Efficacy study of Dolichos biflorus in the management of nephrotoxicity. Asian Pacific J Tropic Biomed. 2012;S1471–S1476.
- Suralkar AA, Kasture SB. Inhibitory effect of Dolichos biflorus extract on allergic airway inflammation and hyperresponsiveness in animal model of ovalbumin-induced asthma. Int J Phytomed. 2013;5(2):197–206.
- Bhui DK, Bar H, Sarkar P, et al. Synthesis and UV–vis spectroscopic study of silver nanoparticles in aqueous SDS solution. J Mol Liquids. 2009; 145(1):33–37.
- Tsutsui Y, Hayakawa T, Kawamura G, et al. Tuned longitudinal surface plasmon resonance and third-order nonlinear optical properties of gold nanorods. Nanotechnology. 2011;22(27):275203. doi:10.1088/0957–4484/22/27/275203.
- Dipankar C, Murugan S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized fromIresineherbstii leaf aqueous extracts. Colloids Surf B Biointerf. 2012;98:112–119.
- Edison TJ, Sethuraman MG. Biogenic robust synthesis of silver nanoparticles using Punica granatum peel and its application as a green catalyst for the reduction of an anthropogenic pollutant 4-nitrophenol. Spectrochim Acta A. 2013; 104:262–264.
- Khan MAM, Kumar S, Ahamed M, et al. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res Lett. 2011;6:434.
- Narayanan KB, Sakthivel N. Synthesis and charactirization of nano-gold composits using Cylindrocladium floridanum and its heterogeneous catalysis in the degradation of 4-nitrophenol. J Hazardous Mater. 2011;189:519–525.
- Tsenga KH, Liaoa CY, Huanga JC, et al. Characterization of gold nanoparticles in organic or inorganic medium (ethanol/water) fabricated by spark discharge method. Mater Lett. 2008;62(19):3341–3344.
- Yana W, Petkovb V, Mahurina SM, et al. Powder XRD analysis and catalysis characterization of ultra-small gold nanoparticles deposited on titania-modified SBA-15. Catal Commun. 2005;6(6):404–408.
- Kumar B, Smita K, Cumbal L, et al. Sonochemical synthesis of silver nanoparticles using starch: a comparison. Bioinorg Chem Applicat. 2014;8. doi:10.1155/2014/784268.
- Philip D. Green synthesis of gold and silver nanoparticles using Hibiscus rosasinensis. Physica E. 2010;42:1417–1424.
- Philip D. Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim Acta Part A. 2009;73:374–381.