ABSTRACT
Silver nanoparticles stabilised with anionic polymeric polyelectrolytes were successfully synthesised by high-energy UV reduction. Three types of polyelectrolytes were used including poly(methacrylic acid) (PMA), poly(acrylic acid) (PAA) and poly(4-styrenesulphonic acid-co-maleic acid) (CoPSS). The formation of the prepared solutions exhibited surface plasmon resonance at the wavelength of 475, 730 and 408 nm by using PMA, PAA and CoPSS as the stabilising agents. UV–visible spectrophotometer, transmission electron microscope (TEM) and zeta potential analyser were employed to characterise the formation of the prepared solutions. The silver nanoparticles stabilised with anionic polyelectrolytes were immobilised on polyester air filters using a layer-by-layer technique. This is the sequential dipping of polyester air filters in a dilute solution of cationic poly(diallyldimethylammonium chloride) and anionic polymeric polyelectrolytes capped silver. The surface topography of the polyester air filters were measured by field emission scanning electron microscope. Results showed that silver nanoparticles had the highest surface coverage on the polyester air filters probably because it is a good bonding candidate and insures strong film growth. The multilayers polyester air filters coated silver nanoparticles were tested against the gram positive pathogen Staphylococcus aureus. The deposition of silver nanoparticles onto the polyester air filters resulted in 92.18%, 84.32% and 71.19% of bacteria removal using PMA, PAA and CoPSS as the stabilising agent.
1. Introduction
Silver nanoparticles have generated a lot of interest among researchers from various disciplines over recent decades [Citation1] due to the unique optical, electrical and antimicrobial properties.[Citation2–4] The antimicrobial activity of silver nanoparticles has been known for a long time. It is believed that the mechanism of silver nanoparticles effect on antimicrobial activity involves the adsorption and accumulation of silver nanoparticles in the bacterial cells and shrinkage of the cytoplasm membrane or detachment from the cell wall. Using this strategy, DNA molecules become condensed and lose their ability to replicate upon the infiltration of silver nanoparticles.[Citation5,Citation6] In recent years, silver nanoparticles have been proposed as an antimicrobial agent to coat onto air filters. An air filter is a device composed of fibrous materials which removes solid particulates such as dust, pollen, mold and bacteria from the air. Most of the published work has mainly focused on the preparation of silver nanoparticles using chemical reduction of silver ions by sodium borohydride or trisodium citrate [Citation7,Citation8] and tested for their antimicrobial activity. The disadvantage of the chemical synthesis route is the toxicity of the reagent used. However, relatively little research has been published regarding the preparation of silver nanoparticles using green synthesis (UV-reduction) to study the antimicrobial activity on air filters.
In this article, the green synthesis of silver nanoparticles using different types of stabilising agents such as poly(methacrylic acid) (PMA), poly(acrylic acid) (PAA) and poly(4-styrenesulphonic acid-co-maleic acid) (CoPSS) are presented. The surface plasmon resonance (SPR), surface charges, size and morphology of silver nanoparticles were characterised by UV–visible spectrophotometer, zeta potential analyser and transmission electron microscopy (TEM), respectively. Subsequently, the silver nanoparticles were deposited on polyester air filters using the layer-by-layer (LbL) technique. The deposition efficiency of the silver nanoparticles air filter films was confirmed using a field emission scanning electron microscope (FESEM) and antimicrobial activity investigated against Staphylococcus aureus. Additionally, in this study, the synthesis of silver nanoparticles using sodium borohydride, chemical-reduction was also explored. Much work has been done to compare the chemical characteristics of silver nanoparticles and their antimicrobial activity on air filters.
2. Experimental section
2.1. Chemicals
PMA (sodium salt, molecular weight = 9500 gmol−1), silver nitrate (AgNO3), sodium borohydride (NaBH4), CoPSS (sodium salt, molecular weight = 20,000 gmol−1), PAA (molecular weight = 5100 gmol−1), poly(diallyldimethylammonium chloride) (PDADMAC, molecular weight = 200,000–350,000 gmol−1) and poly(sodium 4-styrene sulphonate) (PSS, molecular weight = 70,000 gmol−1) were purchased from Sigma-Aldrich, Co., Ltd USA. All chemicals used were analytical grade and used without further purification. All solutions were prepared with 18 MΩ resistance double-distilled water.
2.2. Synthesis of silver nanoparticles
Green synthesis: silver nanoparticles were prepared by a high-energy UV reduction using PMA, PAA and CoPSS as the stabilising agents. In the UV-assisted synthesis, the aqueous 5 mM silver nitrate solutions were mixed with different types of 10 mM anionic polymeric polyelectrolytes, PMA, PAA and CoPSS in 5 mM acetic acetate buffer pH 4.75. The mixed solutions were exposed to UV light (256 nm) for approximately 5, 15, 30, 45 and 60 min. During this process, the colour of the mixed solutions were changed from colourless to deep purple, deep blue and light orange colours by using PMA, PAA and CoPSS as the stabilising agent, respectively. The resultant nanoparticles suspensions were kept at 4 °C in closed containers for further use.
Chemical reduction: silver nanoparticles were synthesised by a well-described method using sodium borohydride as the reducing agent. For the stabilisation of these particles, various anionic polymeric polyelectrolytes (PMA, PAA and CoPSS) were used as the capping agents in water. The preparation steps can be summarised as follows: a 10 mM solution of anionic polymeric polyelectrolytes was mixed with an equal volume of 5 mM silver nitrate. Then, 20 ml of a freshly prepared 100 mM sodium borohydride solution was quickly added to the silver/polyelectrolytes mixture under vigorous stirring. The colour of the solutions changed from colourless to yellow-orange, which confirmed the formation of spherical silver nanoparticles. The suspensions were stored at 4 °C in closed containers.
2.3. Layer-by-layer deposition of silver nanoparticles on polyester air filters
For LbL thin film build up on polyester air filters, the substrates were first immersed for 5 min in a solution containing 10 mM of polycationic PDADMAC. The substrates were then rinsed three times using deionised water in order to remove the excess and loosely bound polyelectrolytes from the substrate surface. The surface, with a polycationic polymer top layer, was then immersed for 5 min in a solution containing 10 mM polyanionic PSS followed by rinsing three times in water. At that point, the polyester air filters were dipped in a silver nanoparticle solution. The self-assembled polycationic PDADMAC and negatively silver nanoparticles were achieved by repeating this process until the desired number of layers was reached.
2.4. Antimicrobial assay
The antimicrobial activity against S. aureus in polyester air filters was evaluated by ASTM E2722-09 standard method. Briefly, each air filter of 2 cm×2 cm was placed in 5 ml of nutriment broth and 0.5 ml of pathogenic S. aureus. The bacteria/broth was shaken for 1 hour. At that point, 1 ml of the mixture was diluted with deionised water. Then 0.1 ml of each dilute solution was placed into solid agar and incubated at 25 °C for 24 hours. The number of variable S. aureus bacteria was counted as the number of colony forming unit per millilitre.
3. Results and discussion
Silver nanoparticles were synthesised by high-energy UV reduction of silver ions in different types of anionic polymeric polyelectrolytes. PMA, PAA and CoPSS anionic polymeric polyelectrolytes were used to stabilise silver nanoparticles. A stabilising agent is usually added to the formulation of silver nanoparticles in order to prevent nanoparticle–nanoparticle interaction by electrostatic repulsion or steric hindrance. Without the action of the stabilising agent, the nanoparticles would grow into large particles up to micron size by aggregation and Ostwald ripening. The UV–visible spectra of silver nanoparticles solution prepared by different types of anionic polymeric polyelectrolytes stabiliser are shown in (a–c). Anionic polymeric polyelectrolytes-stabilised silver nanoparticles were formed after exposure to UV light. The increase in absorbance as the function of time can be seen. The appearance of the characteristic SPR was 475, 730 and 408 nm for the stabilising agents PMA, PAA and CoPSS, respectively. The obtained silver nanoparticles show deep purple, deep blue and light orange colours. The author speculates that the difference in spectral SPR peak is due to the incompleteness of silver ion reduction, which disturbs the dielectric surrounding medium of the silver nanoparticles. The water molecules around the metallic nanoparticles were displaced from the formation of Ag+/Coo− complexes and modified the new dielectric constant of the medium. Less hydrated subjects have been demonstrated to induce a red-shifted SPR spectrum.[Citation9] In order to know the surface charges of silver nanoparticles, a zeta potential analyser was used to investigate. The values of surface charges of silver nanoparticles stabilised with anionic polymeric polyelectrolytes PMA, PAA and CoPSS are given in , revealing the average zeta potential of –37.90, –33.12 and –27.36 mV, respectively. The freshly prepared silver nanoparticles demonstrated negative charges confirming the presence of the anionic polymeric polyelectrolytes. In contrast, the solution prepared with a chemical reduction using NaBH4 had a yellow–orange colour characteristic ((d)) with the maximum absorbance peak position of each solution found to be located around 392 nm. Depending on the nature of NaBH4 used, the strong reducing agent (NaBH4) can provide full reduction of silver ions. Upon reduction, the zero valency silver atoms (Ag0) aggregate into nanoparticles, whose growth is controlled by the stabilising agent. The zeta potential of silver nanoparticles stabilised with PMA, PAA and CoPSS were found in the range of –34.22––25.13 mV, which confirms the successful preparation of silver nanoparticles.
Figure 1. Absorbance spectra of green synthesised silver nanoparticles stabilised with (a) PMA, (b) PAA, (c) CoPSS and (d) chemical reduction of silver nanoparticles stabilised with PMA, PAA and CoPSS.
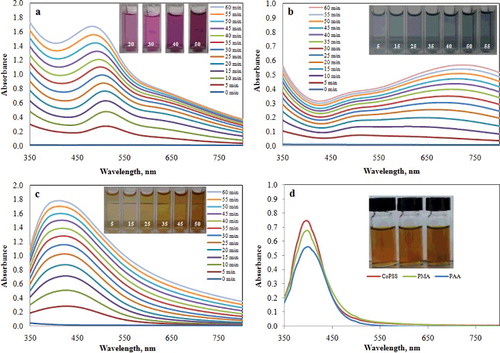
Table 1. Zeta potential of silver nanoparticles.
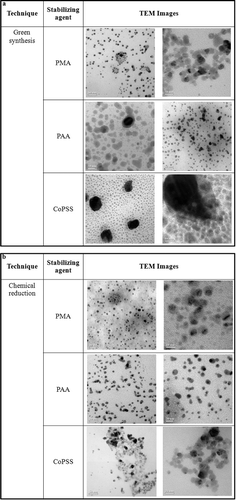
Anionic polymeric polyelectrolytes stabilised silver nanoparticles using a concentration ratio of AgNO3: anionic polymeric polyelectrolytes: acetic acetate buffer at 5:10:5 mM were chosen to investigate their particle size and morphology using TEM. The UV synthesised silver nanoparticles seen in (a) appear spherical in shape and have a narrow particle distribution. The average particle size of PMA, PAA and CoPSS stabilised silver nanoparticles were 13.75 ± 0.87 (n = 100), 20.13 ± 0.54 (n = 100) and 11.94 ± 0.73 nm (n = 100), respectively. For reference, (b) shows the particle size and morphology of PMA, PAA and CoPSS stabilised silver nanoparticles using chemical reduction. The experiment represents typical silver nanoparticles synthesis from NaBH4 which leads to having a spherical shape characteristic with an average particle size of 5–10 nm (n = 100). As seen from this result, chemical reduction of silver nanoparticles was responsible for the formation of smaller particles than in photo reduction.
Figure 2. TEM images of (a) green synthesised silver nanoparticles and (b) chemical reduction of silver nanoparticles.
An interesting point in these experiments was to study the antimicrobial activity of polyester air filters coated with green synthesised silver nanoparticles. The anionic polymeric polyelectrolytes stabilised silver nanoparticles were immobilised on polyester air filters using a LbL self-assembly technique. The advantage of the electrostatic interaction between negatively charged silver nanoparticles capped anionic polymeric polyelectrolytes can then be self-assembled into thin film in the sequence with positively charged PDADMAC. The sequential dipping of polyester air filters in PDADMAC and silver nanoparticles capped with PMA, PAA and CoPSS led to the appearance of purple, blue and yellow colours, respectively.
In (a), the FESEM images of polyester air filters coated with green synthesised silver nanoparticles are shown. Three different images represent the adsorption of nanoparticles capped with PMA, PAA and CoPSS. It is interesting to observe that each polyelectrolyte capping led to a different loading of nanoparticles onto the polyester air filters. PMA capping provided the highest surface coverage whilst CoPSS capping had the lowest. The hydrophobic properties of polyelectrolyte argument can be used to explain such differences. shows the contact angle of silver nanoparticles stabilised with PMA, PAA and CoPSS onto a polyester air filter. As seen in this table, the contact angle of green synthesised silver nanoparticles were approximately 84, 79 and 70 degrees for PMA, PAA and CoPSS, respectively. As suggested in the previous work from Kotov et al.[Citation10] more hydrophobic nanoparticles tend to adsorb in a more packed and denser fashion. This is probably due to a low electrostatic charge and low electrostatic repulsion between particles. On the other hand, more hydrophilic carboxylic as well as sulphonic groups of CoPSS possess the lowest adsorption of the nanoparticles into polyester air filters due to its low carboxylic content, might be a poor stabilising agent in the nanoparticles preparation. Based on these observations, it seems possible that unreacted CoPSS polyelectrolytes would be present in the solution and induced a competitive adsorption mechanism during the adsorption into the polyester air filters. For reference, the contact angle of silver nanoparticles synthesised by chemical reduction was also explored in this study. The contact angle of silver nanoparticles coated on polyester air filters were 77, 72 and 69 degree for PMA, PAA and CoPSS, respectively.
Figure 3. FESEM images and EDS of (a) green synthesised silver nanoparticles and (b) chemical reduction of silver nanoparticles onto polyester air filters.
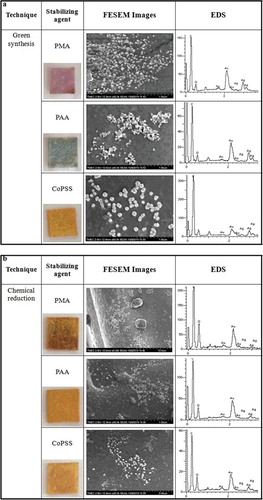
Table 2. Contact angle of 10 layers of silver nanoparticles coated on polyester air filters.
In order to further confirm the growth of silver nanoparticles onto polyester air filters, elemental information of nanoparticles was obtained by FESEM/energy dispersive X-ray spectrometer (EDS). The presence of EDS images of polyester air filters coated with the green synthesis and chemical reduction of silver nanoparticles are shown in (a,b). The EDS measurements were indicated the Ag element on polyester air filters. All of these observations tend to confirm a well-defined LbL deposition of the silver nanoparticles onto polyester air filters.
Upon studying the antimicrobial activity of green synthesised silver nanoparticles coated onto polyester air filters, the multilayer silver nanoparticles were tested against the gram positive pathogen S. aureus. The method of testing was applied from ASTM E2722-09 test method. The antimicrobial efficiency is summarised in . This table displays that the polyester air filters coated with 10 layers of green synthesised silver nanoparticles using PMA as the stabilising agent have excellent antimicrobial activity with 92.18% of bacteria removed. The PAA and CoPSS capped silver nanoparticles show a lower antimicrobial activity with 84.32% and 71.19%, respectively. The percentage of bacteria reduction was decreased as the function of the deposition number of layers decrease. The five layers of green synthesised silver nanoparticles using PMA, PAA and CoPSS found the percentage reduction of S. aureus. in 83.94%, 76.12% and 66.59%, respectively.
Table 3. Antimicrobial activity of polyester air filters coated with silver nanoparticles.
The FESEM images of the surface coverage of the LbL silver nanoparticles on polyester air filters and the antimicrobial activity clearly demonstrated that higher nanoparticles packing leads to higher antimicrobial activity. The higher nanoparticles packing on polyester air filters can be attributed to the attachment of silver nanoparticles to the surface of cell membranes disturbing the permeability and respiration function of the cell. In contrast, the antimicrobial activity of polyester air filters coated with a chemical reduction of silver nanoparticles solution was investigated. Results show that the anionic polymeric polyelectrolyte capped silver nanoparticles were less efficient on bacterial removal when compared with the green synthesised version. This was correlated with FESEM micrographs.
4. Conclusions
In summary, anionic polymeric polyelectrolytes (PMA, PAA and CoPSS) were used to encapsulate silver nanoparticles. A simpler method for the preparation of silver/anionic polymeric polyelectrolytes nanoparticles using UV reduction has been demonstrated. The electrostatic properties of anionic polymeric polyelectrolytes were used to primarily provide the stabilisation of the nanoparticles by electrostatic repulsion and then allow their assembly using the LbL technique. Polyelectrolye multilayers thin films from PDADMAC and anionic polymeric polyelectrolytes capped silver were successfully coated on to polyester air filters using the LbL self-assembly technique. The anionic polymeric polyelectrolytes capped silver nanoparticles were deposited on polyester air filters, which led to the highest packing of nanoparticles appearance. The coated PDADMAC/anionic polymeric polyelectrolytes capped silver on the polyester air filter showed antimicrobial properties towards S. aureus bacteria. These silver nanoparticles coated polyester air filters could easily be transferred to industry.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Haes AJ, Van Duyne RP. A nanoscale optical biosensor: sensitivity and selectivity of an approach based on the localized surface plasmon resonance spectroscopy of triangular silver nanoparticles. J Am Chem Soc. 2002;124:10596–10640.
- Shen Y, Prasad PN. Nanophotonics: a new multidisciplinary frontier. Appl Phys B. 2002;74:641–645.
- Guo H, Tao S. Silver nanoparticles doped silica nanocomposite coated on an optical fiber for ammonia sensing. Sens Actuators B. 2007;123:578–582.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27:76–83.
- Kvitek L, Panacek A, Soukupova J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008;112:5825–5834.
- Kittler S, Greulich C, Diendorf J, et al. Toxicity of silver nanoparticles increases during storage because of slow dissolution under release of silver ions. Chem Mater. 2002;22:4548–4554.
- Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol. 2007;3:95–101.
- Henglein A, Giersig M. Formation of colloidal silver nanoparticles: capping action of citrate. J Phys Chem B. 1999;103:9533–9539.
- Link S, El-Sayed MA. Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals. Int Rev Phys Chem. 2000;19:409–453.
- Kotov NA, Dekany I, Fendler JH. Layer-by-layer self-assembly of polyelectrolyte-semiconductor nanoparticle composite films. J Phys Chem. 1995;99:13065–13069.
