?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Microsize Powders of Ni and Cu were prepared by water atomization technique to fabricate metal matrix composites containing various percentages of nanosized boron nitride particles (1, 2, 3, 4, 5 wt. % of BN in a matrix containing (20 wt. %Ni and 80 wt. %Cu). The prepared mixtures were cold compacted under 400 MPa, and sintered for 2 h at 1000 °C in a controlled atmosphere of 3:2 N2/H2 gas mixtures. The microstructure and the chemical composition of the prepared powders as well as the consolidated composites were investigated by X-ray diffraction as well as field emission scanning electron microscope (FESEM) equipped with an energy dispersive spectrometer (EDS). The produced Cu and Ni powders have spheroid shape of size less than 100 microns, but the investigated BN has an equiaxed particle shape and particle size of ∼ 500 nm. It has been also observed that BN and Ni particles were homogeneously distributed in the Cu matrix of the present BN/Ni-Cu composites. The density, electrical resistivity, saturation magnetization and hardness of the composites were measured. It was observed that, by increasing BN content, the relative density was decreased, while the saturation magnetization, electrical resistivity and hardness were increased.
1. Introduction
Metal matrix composites (MMCs) are of high specific strength, specific modulus, damping capacity and good wear resistance, compared to unreinforced alloys. Ceramic reinforced MMCs have a great mechanical performance [Citation1]. There has been an increasing interest in composites containing low density and low cost ceramic reinforcements. It offers a possibility to tailor the properties of a metal by adding an appropriate ceramic reinforcement and to meet demands in physical and mechanical management. Metals as Al, Mg, Fe, Ti, Ni, Cu, Ag, Co and Nb or alloys as Ni-Cu, Zn-Co, Co-Ni, Ni-Fe were mainly used as metallic matrices [Citation2]. Among others nickel as a durable and tough metal has been widely used, due to it's resistant to corrosion and abrasion [Citation3]. There are several techniques for the fabrication of MMCs. Powder Metallurgy (P/M) is one of these methods. Metal matrix composites produced by P/M have unique properties. Because composites are sintered at lower temperatures in P/M method, undesirable compounds are less often formed at the interfaces. Accordingly, such composites have superior mechanical properties. In addition; P/M technique is a continually and rapidly evolving technology embracing most metallic and alloy materials and a wide variety of shapes, it has been frequently used to manufacture a variety of mechanical components owing to its low energy consumption, high materials utilization, and low production cost [Citation4,Citation5].
There are several types of particles, whiskers or fibers ceramics that can be used as reinforcement in MMCs (as SiC, Al2O3, TiC, WC and diamond) according to the required application of the materials. Although diamond is the hardest known material in nature, it is rare and too expensive to be used as reinforcement. Cubic boron nitride ‘cBN’ is synthesized in 1957 [Citation6–8], and it is the second hardest known material and it possesses numerous excellent physical and chemical properties, high corrosion resistance and mechanical properties. Boron nitride has two crystal structures; hexagonal (hBN) similar to graphite and cubic (cBN) similar to diamond. However, cBN is much harder than hBN. Similar to the synthesis of diamond at high temperature and high pressure; cBN can also be formed at high temperature under high pressure (ultra hP/hT). A catalyst might lower the required temperature and pressure [Citation9,Citation10].
Using cBN as a cutting tool at severe conditions of temperature and cutting speed [Citation11] is referred to its high properties, such as high hardness, high thermal and chemical stability. It is used mainly for machining hard ferrous materials, especially when diamond reacts chemically with these materials at temperatures higher than 700 °C. However, pure cBN compact is difficult to prepare even using (hP/hT) techniques [Citation12]. Several research works succeeded to fabricate cBN using sintering additives. The sintering conditions depend on the used additives and/or binding materials. It requires a pressure of 6 GPA and a temperature of 1500 °C. Powders of cBN used to fabricate tools of difference shapes are usually mixed with metal binders. Such sintering conditions are attained using ultrahigh pressure devices. In addition, when additives such as powders of Ni, Al and Cu, are added to cBN particles for solid phase sintering, their effect as sintering additives or binding materials is limited [Citation13–16].
Copper base composites offer excellent strength properties for several applications. It is currently produced by P/M technique, in which cold compaction and/or hot pressing are performed at high pressure and temperature, because the expansion of compacts during sintering leads to the formation of pores and the weakening of the bond between the ceramic powder and the metal matrix [Citation17,Citation18]. Alloying elements as Ni, Sn, Pb or Al can be added to the Cu base composites to improve its resistance, ductility and thermal stability without causing considerable damages of its form, electric and thermal conductivity and corrosion resistance [Citation19,Citation20].
As an inference, composite formation limited by compatibility problems of dissimilar materials may be expected to realize by the P/M technique and composites prepared in such a manner are expected to have improved properties. In fact, BN has long been interesting to us with its unique characteristics particularly some polymer like traits. Although it has been widely used in applications, there still exits new areas for research. It should be ideal if BN could be incorporated into metal matrix and its composites with desired properties could be obtained. On the other hand, we presently have little consideration in evaluating the potentials of formed BN metal matrix composites for any practical uses.
In the present research, Boron Nitride / Nickel-Copper Matrix Composites BN/ (Ni-Cu) with a uniform distribution of the BN nanoparticles in the (Ni-Cu) metal matrix were fabricated by P/M technique. In addition, studying the effect of BN nano-particles content on the composite microstructure, physical properties and hardness. In addition; try to construct a stable structure involved these three dissimilar materials (BN, Ni and Cu). Such an effort may have importantly significance in advanced materials design which is currently underway.
2. Experimental methods and procedures
2.1. Materials
Powders of Ni and Cu are prepared by the atomization technique at the Central Metallurgical Research and Development Institute, Cairo, Egypt using water atomizer model PSI; UK. Copper and Nickel powders are prepared separately by induction melting in silicon carbide crucibles in air. In case of Cu it is superheated to1300 °C, and in case of Ni to 1700 °C. After complete melting bottom pouring is carried out through a ceramic melt delivery nozzle of 6 mm diameter into a confined water atomizer operating at a pressure of 20 MPa. The high-pressure water jets were directed against the molten stream. The melt flow rate, estimated from the operating time and weight of the atomized melt, is about 4 kg/min. The water flow rate, calculated from the water consumption rate, was about 200 l/min. A preliminary study was performed to determine the suitable atomization conditions for the fabrication of copper and nickel. reports the atomization conditions applied for Cu and Ni powders fabrication. The size distribution of Cu and Ni powders is determined by conventional mechanical sieving vibrator model ControLab FRANCE. Sieved powders with a specific size range of 100 µm are used for present investigation. Boron nitride powder with an average particle size of 500–700 nm is purchased from (ILGIN Co. LTD- South Korea). Different mixtures of up to 5 wt. % BN and a mixture containing 20 wt. % Ni and 80 wt. % Cu are prepared by mechanical mixing for 30 min in a ceramic mortar using 0.5 wt. % paraffin wax dissolved in 20 ml acetone as a binding agent. The obtained milled mixtures are heated at 60 °C for 120 min. followed by cooling to room temperature.
Table 1. Applied conditions for the atomization of Cu and Ni powders.
2.2. Consolidation of BN/20Ni-Cu powders
The produced BN/Ni-Cu powder mixtures are cold compacted under 400 MPa using a uniaxial hydraulic pressing machine in a 9 mm diameter cylindrical hardened steel mold. A preliminary study was takes place to optimize the compaction pressure and the sintering conditions of the prepared BN/Ni-Cu powder mixtures according to our previous work [Citation17,Citation21–26]. The obtained compacts are sintered for 2 h in a closed furnace in controlled atmosphere of 3:2 N2/H2 gas mixtures at 1000 °C. Finally, the furnace is turned off and the sintered specimens are maintained in the furnace for cooling in N2 atmosphere.
2.3. Methods of assessment of powders and BN/20Ni-Cu composites
An extensive microstructural and morphological study is preformed to investigate Cu, Ni, BN powders and BN/Ni-Cu mixtures, as well as the grinded and polished BN/Ni-Cu sintered compacts by QUANTA FEG250-EDAX Genesis field emission scanning electron microscope equipped with an energy dispersive X-ray micro analyzer (EDS). Also, the chemical composition and phase analysis are investigated by X-ray diffraction (XRD) on a Brukur advanced X-ray diffractometer model D8 kristalloflex (Ni-filtered Cu Kα).
The expected theoretical densities of designed compositions are calculated by the rule of mixtures using theoretical density values of the elements Cu (8.96 g/cm3), Ni (8.90 g/cm3) and BN (3.48 g/cm3) [Citation27,Citation28]. The theoretical densities of produced composites are calculated by using the rule of mixtures (EquationEquation 1(1)
(1) ) [Citation29].
(1)
(1)
where, dc, dm, df densities of the composite, matrix and dispersed phase respectively and Vm, Vf volume fraction of the matrix and dispersed phase, respectively.
The relative green density of the produced compacts is measured using a micrometer and a three-digit balance. On the other hand, the densities of sintered materials are determined using water as a floating liquid, and sintered density (ρ) are calculated by Archimedes principal given by equation [Citation30];
(2)
(2)
where, Wair and Wwater represent the specimen weight in air and water, respectively.
The electrical resistivity of the samples is measured using digital micro-ohmmeter (GDM- 8145) and the resistivity (ρ) is calculated in μΩ.cm according to the equation;
(3)
(3)
where, R is the resistance in μΩ, L is the length in cm and A is the cross-sectional area in cm2.
Magnetic hysteresis loops at room temperature (MH curves) of prepared BN/20Ni-Cu mixtures, and sintered compacts using Lake-Shore model 7400, Japan. The saturated magnetizations of investigated specimens are measured as functions of sample weight.
Vickers hardness of BN/20Ni-Cu sintered composites are measured using Vickers hardness tester model Shimadzu JAPAN by applying 50 g load and 15 sec loading time at room temperature (25 °C). The test is repeated for each specimen and the average of at least 5 readings along the specimen cross section is calculated.
3. Results and discussion
3.1. Assessment of powders and BN/Ni-Cu mixtures
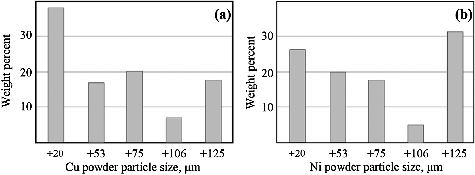
shows the particle size distribution by sieve analysis of the produced copper and nickel powders using Sieve analysis tester Model Contro Lab, France. The amount of tested copper powder is 100 gm and that of nickel is 10 gm. Only fine particles are used here, and the largest particle size of copper is −20 µm, while nickel powder shows different sizes between 125 µm and −20 µm. Because of the magnetic behavior of nickel, fine copper powder and large nickel particles are used.
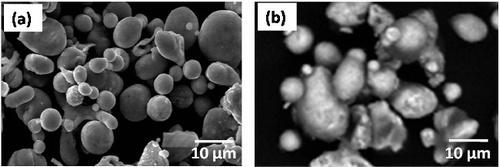
shows the FESEM micrographs of as atomized Cu and Ni powders. It could be observed that, the particle shape of as-solidified powders is mostly spherical in case of Cu and cubic to irregular in case of Ni, and is of a rough surface morphology. A preliminary study was performed in our previous work to determine the atomization conditions of metal powders. The study revealed that, the particle size, shape and morphology of the atomized metal powders are affected by several parameters such as the angle between the water jet and the stream of the molten metal, the flow rate of the molten stream and the water flow rate. In addition; the viscosity and surface tension of the molten metal which are affected by the overheating of the molten metal before atomization [Citation31]. shows FESEM images of cubic boron nitride and a mixture of 5 wt. % cubic boron nitride with 20 wt. % nickel and copper powders.
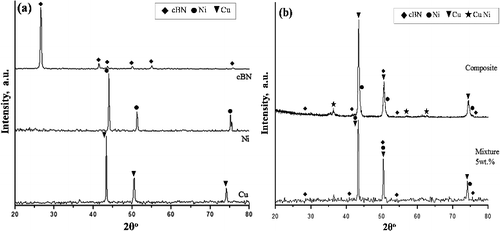
(a) shows XRD patterns for the investigated powders of Cu, Ni and BN particles. Both Cu and Ni have fcc crystal structure and show (111), (200) and (220) reflection lines. On the other hand, the BN has a cubic crystal structure and shows (002), (111), (200) and (220) reflection lines. Moreover, sintered BN/Ni-Cu composites consist of (fcc) Cu and (fcc) Ni as a major phase and the coexistence of boron nitride. One of the important features here is the absence of any peak of improper or impurity phase. This implies that there is no reaction between the cBN particles and Ni or Cu metals at the applied sintering conditions (1000 °C for 2 h). Only the intensification of the lines (111), (200) and (220), due to the incorporation of BN nanoparticles in the metal matrix was observed. In addition, the appearance of three peaks implies the presence of a new phase. It is due to the formation of the Ni-Cu solid solution between the copper and nickel fine powder during the sintering at 1000 °C [Citation32–34]. On the other hand, as reported in our previous report, the boron nitride can be interacting at high temperature with some metal matrix to form a compound layer composed of metal borides as well as metal nitrides in the interface region between the surface of the boron nitride nanoparticles and the metal matrix [Citation14]. The formation of the compound layer is due to the in-situ transformation reaction between the metal matrix and the surface of the nanosized boron nitride particles under the high temperature during the sintering process. From previous reports [Citation35–40], the possible chemical interaction between the nanosized boron nitride and the Ni-Cu matrix during the sintering process can be expected as follows:
(4)
(4)
(5)
(5)
The interaction that occurs between the boron nitride and the metal matrix during the sintering is a poly-step process and the slowest step is the rate determining step which is controlled by diffusion. Previous work reported that there is no reaction between nitrogen with Cu at temperatures below 1173 K and the solubility of nitrogen neither in solid Cu nor in liquid Cu up to 1673 K. [Citation38,Citation41]. However, the intermetallic compounds CuN3, Cu3N and CuN6 may be formed due to the interaction between the fine copper powder and the nanosized boron nitride. Due to the low detection limit of the used XRD technique we cannot be observe any phase for the interaction between the boron nitride and the copper-nickel matrix. Future studies will be started to investigate the interaction between the nanosized boron nitride and the Ni-Cu metal matrix.
3.2. Microstructure of consolidated BN/Ni-Cu powders
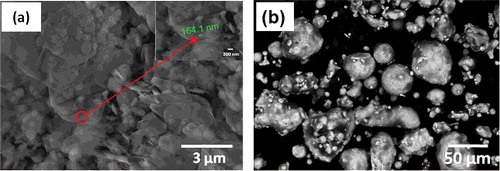
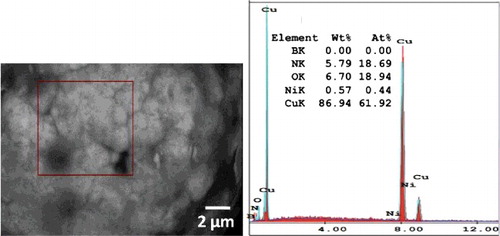
EDS analysis of mixed 5 wt. % BN/20Ni-Cu using FESEM is presented in . Spot analysis of the marked position is performed and the results show the characteristic peaks for mixed 5 wt. % BN/20Ni-Cu. Almost uniform distribution of the BN nano-particles in Cu/Ni mixture is observed. However, by increasing the BN content in the metal matrix and under the prolonged sintering time may increase the chance for the BN grain growth and enhancing the BN grain coarsening [Citation42].
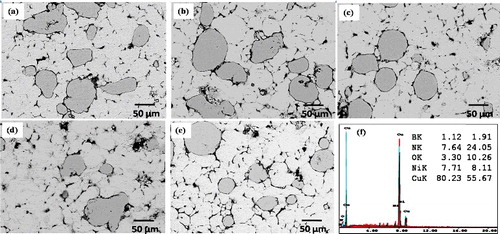
In the Backscattered electron (BSE) images BN particles are appeared to be gray, and this is confirmed with EDS analysis. In order to determine the distribution of elements in the structure, surface analysis is performed by Electron Dispersive Spectrum (EDS). shows the images of FESEM and EDS of sintered 5 wt. % BN/20Ni-Cu nanocomposite. The EDS analysis indicates the main spectrums of B, N, Ni, Cu and O elements at different locations, which correspond to the existing of BN, Ni and Cu. shows the distribution BN in metal matrix leading to a homogenous microstructure of the sintered BN/20Ni-Cu materials.
3.3. Physical properties of powders and BN/Ni-Cu mixtures
The theoretical densities of the produced composites are calculated by using the rule of mixtures (EquationEquation 1(1)
(1) ), which is an approximate approach to estimate composite properties. The relative density (actual density by a theoretical one) of the investigated sintered composites is measured using a method based on Archimedes' principal.
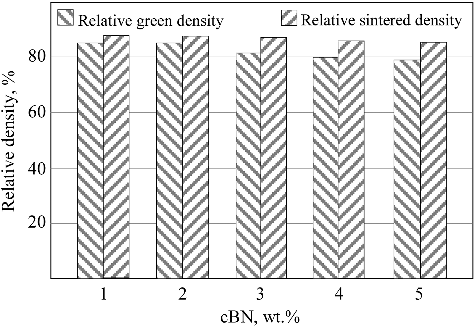
shows the relative sintered density in comparison with the relative green density of BN/Ni-Cu sintered materials with different weight contents of BN. By increasing the BN weight percent, the green as well as the sintered densities were decrease due to the poor interaction between the BN reinforcement particles and the Ni and metal matrix which increase the chance to pore formations on the interface between the BN particles and the metal matrix.
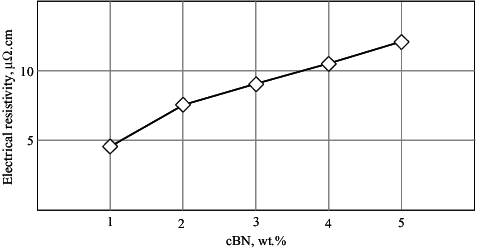
Measured values of electrical resistivity of the sintered BN/Ni-Cu composites are plotted in . The electrical resistivity of Cu-Ni composites is important, especially for electrical or electronic applications. The electrical resistivity increases with increasing the cBN content of the composite, due to the high resistivity of cBN reinforcement. Besides, increasing the cBN content, the sintered density decreases due to the increase of porosity, as could be observed in .
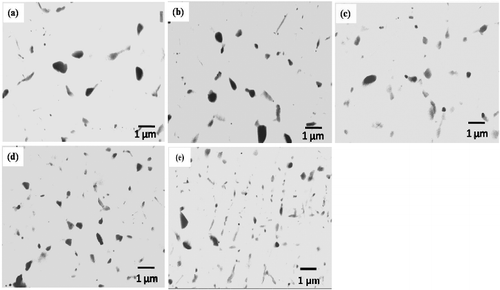
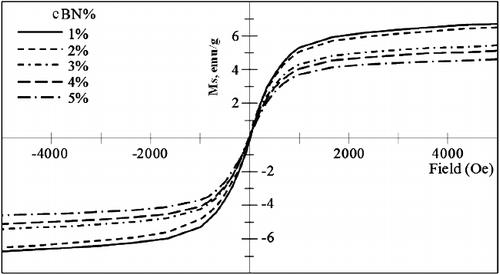
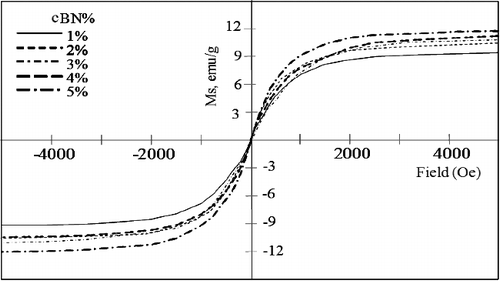
shows the magnetic hysteresis loops of the investigated mixtures of BN, Ni and Cu powders, and shows the hysteresis loops of the investigated BN/20Ni-Cu composites, under a magnetic field of 0.5 Tesla. In addition, shows the effect of the BN content on the saturation magnetization. It is obvious from the results in that the saturation magnetization (Ms) of the powder mixtures, decreases with increasing BN content, due to the nonmagnetic properties of BN powder [Citation43]. However, in case of the BN/Ni-Cu sintered composites the saturation magnetization increases with increasing of the BN content. This could be attributed to the configuration of Ni-Cu compound during the sintering process [Citation44]. The ratio of Ni-Cu decreases with increasing of BN contents, which is in agreement with XRD results.
Figure 12. Effect of BN content on saturation magnetization of mixed powders and sintered BN/20Ni-Cu composites.
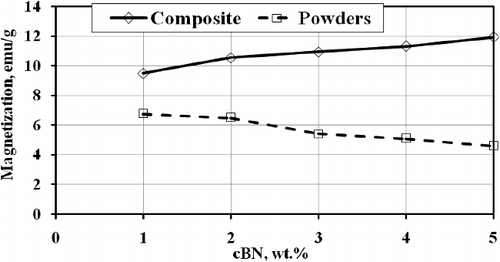
The saturation magnetization of sintered Ni is 51.94 emu/g is lower than the standard value (54.8 emu/g). This could be due to the presence of traces of contaminates as O2 and P in the prepared Ni [Citation45,Citation46].
3.4. Hardness of sintered BN/Ni-Cu composites
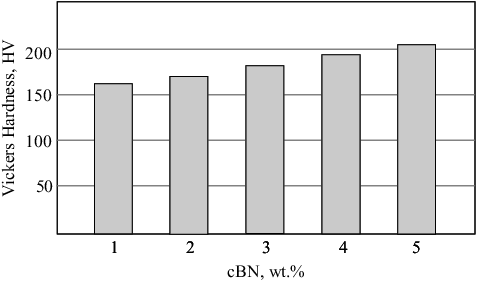
The effect of BN content on the hardness of present composites is shown in . By increasing BN (hard phase) content, the hardness of the composite increases. It is due to the contribution of BN hard phase in the Ni-Cu matrix, and the tight adhesion between BN particles and Ni-Cu matrix, as well as the higher densification and low porosity of the sintered BN/20Ni-Cu composites. However, the increase of hardness is not pronounced, which may be due to the nickel and copper metallic binder effect. By increasing the soft metallic binder content (Ni-Cu) the densification increases leading to hardness increase.
4. Conclusions
Powder metallurgy technique is applied to fabricate Ni-Cu metal matrix composites reinforced with different contents of boron nitride. Based on present results it could be concluded that increasing BN content leads to:
| 1- | It decreases the density and increases the hardness. | ||||
| 2- | The green density and sintered density of BN/20Ni-Cu composites slightly decrease. | ||||
| 3- | Both electrical resistivity and hardness of BN/20Ni-Cu composites increase. | ||||
| 4- | It decreases the saturation magnetization of the BN/20Ni-Cu powders mixture, and increases the saturation magnetization of the BN/20Ni-Cu composites. | ||||
Copper based composites with improved physical and mechanical properties are produced for several applications.
Acknowledgement
The authors wish to thank the researchers and the technicians of the Central Metallurgical R&D Institute (CMRDI) in Cairo, Egypt for their cooperation. The authors thank Prof. Soon H. Hong, professor of materials science and engineering at the Korean advanced institute of science and technology (KAIST) for his guidance during the work.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Soy U. Metal matriks kompozit malzemeler. Turkey: SAÜ Teknoloji Fakültesi; 2009.
- Chawla N, Chawla KK. Metal matrix composites. New York: Springer; 2006.
- Shabani A, Toroghinejad MR, Shafyei A. Fabrication of Al/Ni/Cu composite by accumulative roll bonding and electroplating processes and investigation of its microstructure and mechanical properties. Mater Sci Eng A. 2012;558:386–393.
- Liu S, Zhang H, Hu J. Effect of carbusintering on densification behavior and mechanical properties of Fe-2%Ni-x%Cu alloys. Mater Design. 2011;32:3686–3691.
- Dong X, Hu J, Wang H, et al. A study on carbon concentration distribution and microstructure of P/M materials prepared by carbusintering. J Mater Process Technol. 2009;209:3776–3782.
- Chiou SY, Ou SF, Jang YG, et al. Research on CBN/TiC composites Part1: effects of the cBN content and sintering process on the hardness and transverse rupture strength. Ceram Int. 2013;39:7205–7210.
- Kitiwan M, Ito A, Zhang J, et al. Densification and mechanical properties of cBN–TiN–TiB2 composites prepared by spark plasma sintering of SiO2-coated cBN powder. J Eur Ceram Soc. 2014;34:3619–3626.
- Yaman B, Mandal H. Spark plasma sintering of Co–WC cubic boron nitride composites. Mater Letters. 2009;63:1041–1043.
- Sigalas I, Caveney RJ, Bailey MW. Diamond materials and their applications. In: Ralf Riedel, editor. Handbook of ceramic hard materials. 2. Weinheim, Germany: Wiley-VCH Verlag GmbH; 2008. p. 478–572.
- Umer MA, Sub PH, Lee DJ, et al. Polycrystalline cubic boron nitride sintered compacts prepared from nanocrystalline TiN coated cBN powder. Mater Sci Eng A. 2012;552:151–156.
- Xu CH, Wu GY, Xiao GC, et al. Al2O3/(W,Ti)C/CaF2 multi-component graded self-lubricating ceramic cutting tool material. Int J Refract Metal Hard Mater. 2014;45:125–129.
- Lv R, Liu J, Li Y, et al. High pressure sintering of cubic boron nitride compacts with Al and AlN. Diamond Related Mater. 2008;17:2062–2066.
- Casanova CA, Balzaretti NM, Voronin G, et al. Experimental study of plastic deformation during sintering of cubic boron nitride compacts. Diamond Related Mater. 1999;8:1451–1454.
- Daoush WM, Park HS, Hong SH. Fabrication of TiN/cBN and TiC/diamond coated particles by titanium deposition process. Trans Nonferrous Metal Soc China. 2014;24:3562–3570.
- Arsecularatne JA, Zhang LC, Montross C, et al. On machining of hardened AISI D2 steel with PCBN tools. J Mater Process Technol. 2006;171:244–252.
- Kurt A, Seker U. The effect of chamfer angle of polycrystalline cubic boron nitride cutting tool on the cutting forces and the tool stresses in finishing hard turning of AISI 52100 steel. Mater Des. 2005;26:351–356.
- Moustafa SF, Rashad WM, El- Shereafy E. Cu/Matrix composites produced with either coated or uncoated reinforcement powders. Canadian Metall. 2001;40(4):533–537.
- Zhang J, Tu R, Goto T. Spark plasma sintering of Al2O3–cBN composites facilitated by Ni nanoparticle precipitation on cBN powder by rotary chemical vapor deposition. J Eur Ceram Soc. 2011;31:2083–2087.
- Anklekar RM, Bauer K, Agrawal DK, et al. Improved mechanical properties and microstructural development of microwave sintered copper and nickel steel PM parts. Powder Metall. 2005;48:39–46.
- Rubio WM. Paulino GH and Silva EC, Analysis, manufacture and characterization of Ni/Cu functionally graded structures. Mater Design. 2012;41:255–265.
- Daoush WM, Elsayed E, El Kady O, et al. Enhancement of physical and mechanical properties of oxide dispersion strengthened tungsten heavy alloys. Metall Mater Trans A. 2016;47(5): 2387–2395.
- Daoush W, Swidan A, El-Aziz GA, et al. Fabrication, microstructure, thermal and electrical properties of copper heat sink composites. Mater Sci Appl. 2016;7:542–561.
- Ahmed MA, Daoush WM, El-Nikhaily AE. Fabrication and characterization of Copper/Silicon Nitride composites. Adv Mater Res. 2016;5(3):131–140.
- Daoush WM, Moustafa SF. Coated powders ‘a good base’ for intermetallics. Metal Powder Report. 2008;63(11):16–18 .
- Daoush WM. Processing and characterization of CNT/Cu nanocomposites by powder technology. J Powder Metall Met Ceram. 2008;47(9-10):531–537.
- Elsayed A, Li W, El Kady OA, et al. Experimental Investigations on the Synthesis of W-Cu nanocomposite through spark plasma sintering. J Alloys Compd. 2015;639:373–380.
- Kır D, Islak S, Çelik H, et al. Effect of the cBN content and sintering temperature on the transverse rupture strength and hardness of cBN/Diamond cutting tools. Sci Sintering. 2012;44:235–243.
- Haynes WM, editor. CRC handbook of chemistry and physics. 92nd ed. Boca Raton, FL: (Internet Version); CRC Press/Taylor and Francis; 2012.
- Yellappa M, Puneet U, Krishnareddy GV, et al. Fabrication and characterisation of aluminium-fly ash composite using stir casting method. Int J Mech Eng Rob Res. 2014;3(2):2278–0149.
- Selfridge AR. Approximate Material Properties in isotropic Materials,IEEE Trans Sonics Ultrason SU. 1985;32(2):381–394.
- Moustafa SF, Daoush WM, Ibrahim A, et al. Hot forging and hot pressing of AlSi powder compared to conventional powder metallurgy route. J Mater Sci Appl. 2011;2:1127–1133.
- Soliman HM, Abdel-Rahman HH. The use of rotating cylinder electrode to study the effect of 1,3-Dihydroxypropane on the production of copper powder. J Braz Chem Soc. 2006;4:705–714.
- Pavlatou EA, Raptakis M, Spyrellis N. Synergistic effect of 2-butyne-1,4-diol and pulse plating on the structure and properties of nickel nanocrystalline deposits. Surf Coat Technol. 2007;201:4571–4577.
- Hao XP, Cui DL, Shi GX, et al. Synthesis of cubic boron nitride at low-temperature and low-pressure conditions. Chemistry Mater. 2001;13(8): 2457–2459.
- Cao X, Wang X, Cui L, et al. Strongly coupled nickel boride/graphene hybrid as a novel electrode material for supercapacitors. Chem Eng J. 2017;327:1085–1092.
- Gage SH, Ruddy DA, Pylypenko S, Richards RM. Deep eutectic solvent approach towards nickel/nickel nitride nanocomposites. Catalysis Today 2018 ; 306:9–15.
- Torun O. Boriding of nickel aluminide. Surf Coat Technol. 2008;202:3549–3554.
- Awasthi S, Pandey CP, Balani K. Synergistic role of carbonaceous reinforcements on multi length scale tribology of electrophoretically deposited nickel-boron nitride coatings. Mater Res Bull. 2018;99:61–72.
- Yue GH, Yan PX, Wang J. Study on the preparation and properties of copper nitride thin films. J Cryst Growth. 2005;274:464–468.
- Pompei E, Magagnin L, Lecis N, et al. Electrodeposition of nickel–BN composite coatings. Electrochim. Acta. 2009;54:2571–2574.
- Predel B. Cu-N (Copper-Nitrogen). In: Madelung O, editor. Cr-Cs – Cu-Zr. Landolt- Börnstein - Group IV Physical Chemistry (Numerical Data and Functional Relationships in Science and Technology). Vol. 5d. Berlin, Heidelberg: Springer material; 1994.
- Edelstein AS, Cammarata RC. Nanomaterials: synthesis, properties and applications. Bristol: Institute Physics Publication; 1996.
- Zhang WS, Zheng JG, BrÄuck E, et al. Structure and magnetic properties of boron-oxide and boron-nitride coated iron nanocapsules. J Mater Sci Technol. 2010;26(11): 1051–1056.
- López M, Gómez ME, Reyes D, et al. The structure and its dependence on the magnetic properties of Ni5CoXCu95-X alloys produced by mechanical alloying and subsequent annealing. Mater Sci. 2010;638-642:3876–3882.
- Moustafa SF, Daoush WM. Synthesis of nano-sized Fe–Ni powder by chemical process for magnetic applications. J Mater Process Technol. 2007;181:59–63.
- Daoush WM, Elkady OA. Microstructure, physical properties and hardness of alumina short fibres/nickel matrix composites fabricated by powder technology. J Composite Mater. 2014;48:3735–3746.