Abstract
In this work, by appealing to a heterometallic cluster approach, a new highly porous metal-organic framework (MOF) formulated as {(Me2NH2)[Ni3K2(TZIA)3(H2O)3]·(DMF)4}n (1) has been fabricated by a solvothermal reaction of a bifunctional organic ligand 5-(1 H-tetrazol-5-yl)isophthalic acid (H3TZIA) with the Ni(II) and K(I) salts in water and DMF mixed solvents. The resulting activated 1a has been explored in detail for BET analysis to probe its porosity. The removal of water molecule at the aim of generating 1a makes K+ ion become a heterogeneous catalyst of size-selective with high efficiency for solvent-free cyanosilylation of acetaldehyde under mild conditions. Furthermore, the prevention effect of the compound on the arrhythmia occurrences after anesthesia during operation was evaluated. The arrhythmia incidence and abnormal blood pressure was calculated and measured for the detection of compound prevention ability. Besides, the Norepinephrine (NE) and Adrenaline (A) released by the cardiomyocytes were measured by the ELISA kit.
Introduction
The incidence of perioperative arrhythmias is 60%−80%, and it can be as high as 100% in cardiothoracic, macrovascular and craniocerebral operations [Citation1]. Severe arrhythmia after anesthesia during operation is more difficult to handle. Clinical observations found that the anesthetic bupivacaine, lidocaine, propofol can cause electrocardiogram, with the character of Brugada wave or long QT interval, followed by ventricular tachycardia and ventricular fibrillation [Citation2, Citation3]. However, the mechanism is still unknown. Thus, it is urgent for us to detect the mechanism of arrhythmia after anesthesia during operation [Citation4].
Porous coordination polymers (PCPs) or metal-organic frameworks (MOFs) are the materials formed by the coordination bonds between the organic ligands and metal clusters/ions [Citation5–7]. MOFs are famous for its diverse structures and potential applications in the field of catalysis, separation and gas storage, luminescence and sensing, as well as other advanced functions [Citation8–10]. Metal ions in MOFs act as skeleton connecting sites and may be used as the surface of the heterogeneous catalyst to separate Lewis acid sites. Unluckily, in the solution, a lot of MOFs are unstable. Stability is essential for the application of industrial and recovery of heterogeneous catalysts. Therefore, preparing MOFs with stability remains challenge. MOF’ s stability is decided largely by secondary building units (SBUs) or metal ions. The recent literatures have revealed that the cluster-based units could endow MOFs with better framework stability compared with those based on the single metal ions [Citation11, Citation12].
Trialkylsilyl cyanide (TMSCN) along with carbonyl compounds reaction is an important approach of C-C bond formation in the organic synthesis. It can be used for forming the derivatives of cyanohydrin, and can also be converted into many well drugs along with chemicals, for example, β-hydroxy amino alcohols, alpha-hydroxy ketones, alpha-hydroxy acids, etc. [Citation13]. Hence, it is a hot issue in the society of chemistry to find the catalysts with high effective for carbonyl compounds cyanosilication catalyzed by TMSCN. In recent decades, a lot of the homogeneous catalysts, such as N-heterocyclic carbenes, inorganic or organic salts, Lewis bases and Lewis acids, can catalyze reaction, they have been widely researched [Citation14, Citation15]. Nevertheless, the reactions of homogeneous catalytic usually require painstaking steps of purification, so in recent years, research direction in the field are mainly focused on the use of the heterogeneous catalysts, particularly MOFs, at the aim of overcoming the problem of product catalyst separation at the conditions of without solvent [Citation16–18]. In this study, by employment of the heterometallic strategy, a new highly porous metal-organic framework {(Me2NH2)[Ni3K2(TZIA)3(H2O)3]·(DMF)4}n (1) has been successfully prepared by a solvothermal reaction of a hetero-donor organic ligand 5-(1 H-tetrazol-5-yl)isophthalic acid (H3TZIA) with the corresponding Ni(II) and K(I) salts in water and DMF mixed solvent. X-ray of single crystal study exhibits that K+ cations were immobilized on surface of pore via trinuclear Ni2+–tetrazole coordination motif. The resulting activated 1a has been explored in details for BET analysis to probe its porosity. The removal of water molecule at the aim of generating 1a makes K+ ion become a heterogeneous catalyst of size-selective with high efficiency for without solvent acetaldehyde cyanation under the conditions of mild. In the biological function research, the prevention effect of the compound on the arrhythmia occurrences after anesthesia during operation was evaluated, and the results indicated that the compound could reduce the arrhythmia incidence and abnormal blood pressure significantly. Besides, the ELISA detection of the NE and A revealed that the sympathetic excitability was obviously inhibited by the compound treatment.
Experimental
Chemicals and measurements
Solvents, reagents as well as the raw materials were all purchased from the sources of commerce and could be utilized with no deep purification. With Nicolet Avatar 360 FT-IR spectrophotometer we determine FT-IR spectra. The analysis of thermogravimetric was performed in the nitrogen flow with the equipment of Q50 TGA (TA) thermal analysis, and the heating rate is 5 °C·min−1. Powder X-ray diffraction patterns (PXRD) of samples of bulk were measured at the room temperature on a MiniFlex (Cu Kα, λ = 1.5418 Å). GC analysis was carried out on an Agilent 7890B GC analyzer. The isotherms of gas adsorption (N2) at low pressure (up to 1 bar) were detected by porosity analyzer along with ASAP 2020 specific surface area.
Preparation and characterization for {(Me2NH2)[Ni3K2(TZIA)3(H2O)3]·(DMF)4}n (1)
We synthesized complex 1 via NiCl2·4H2O which is 23.79 mg and 0.10 mmol, KF·2H2O of 14.10 mg and 0.15 mmol as well as H3TZIA which is 23.40 mg and 0.10 mmol solvothermal reaction in the mixed solvent of 2 mL H2O, N and N-dimethylformamide (7 mL, DMF) for three days at 140 °C. We acquired a light yellow crystals with block-shape. After filtration and separation from the mother solvent, we washed crystals by using anhydrous DMF for three times, then dried it in the air. The yield was approximately 62% on the basis of H3TZIA. Anal. Calcd for C41H51K2Ni3N17O19 (1): C, 37.06; H, 3.87; N, 17.92%; N, 6.16; found: C, 37.26; H, 3.99; N, 5.94%.
With Oxford Xcalibur E diffractometer we acquired compound 1’ s X-ray data. CrysAlisPro software was used to analyze the strength data, then convert strength data into the hkl files. Based on direct method, compound 1’ s primitive structure model was established via using SHELXS program, and modified via SHELXL-2014 program based on least square approach. Compound 1’ s all non-H atoms were mixed with the anisotropic parameters, and all the H atoms were geometrically fixed on their linked C atoms via utilizing the AFIX commands. exhibits the parameters of crystallography along with the information of the numerical value of compound 1 after recombination.
Table 1. Compound 1’ s parameters of crystallography and refinement details.
Arrhythmia rat model and treatment
The 30 SD rats (male, 6–8 weeks, 200–220 g) used in this experiment were obtained from Model Animal Research Center of Nanjing University (Nanjing, China). The rats were kept in the standard condition with 45% humidity and 20–25 °C temperature. All the experiments performed in this study were approved by the Ethics Committee of the Affiliated Hospital of Nanjing University (Nanjing, China). For arrhythmia model construction, the rats were divided into three different groups, the control group (shame operation + PBS treatment), model group (operation + PBS treatment) and treatment group (operation + compound treatment). The rats in the model group and treatment group were injected with Aconitine to induce the arrhythmia model, the PBS was injected into the control group rats. Then, the compound was used for the arrhythmia treatment at the concentration of 5 mg/kg. The incidence of arrhythmia was calculated and the abnormal blood pressure was measured and recorded.
ELISA assay of NE and A
After treatment by using compound for one day, Norepinephrine (NE) and Adrenaline (A) released from the cardiomyocytes were measured by the ELISA kit according to the instruction of the protocols with some modification. In brief, the arrhythmia model was established in rats, followed by the compound treatment at the concentration of 5 mg/kg. After compound exposure, the myocardial tissue was collected from the rats in different groups, and the NE and A content was measured by the ELISA detection kit. The experiment was carried out three times, and results were presented as mean ± SD.
Results and discussion
Crystal structure of complex 1
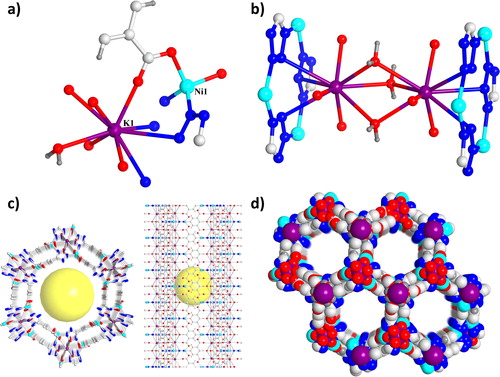
In water and DMF mixed solvent, the target complex 1 can be easily synthesized by solvothermal method. On the basis of the data of crystal collected under the room temperature, the results of refinement along with structural solution show that complex 1 crystallizes in the square P63/m space group and has a three-dimensional framework which has the nanotube channels. There is one crystallographically independent Ni2+ ion, one K+ ion, one μ2-bridging water molecule as well as a half TZIA3− ligand in the asymmetric unit (). Ni2+ ion among units of molecule have the tetrahedral coordination geometry, which is defined via two O atoms from four independent TZIA3− ligands as well as two N atoms from the tetrazole groups, which forms a distorted tetrahedral coordination environment. Three Ni2+ ions were linked by the groups of tetrazolium to form the molecular building block (MBB) of trinuclear with triangular shape. N atoms of the group of tetrazolium bind two K+ ions to MBB of trinuclear via coordination and distribute symmetrically on the triangle (). K+ ions are partially linked via isophthalic along with water acid on two independent TZIA3− ligands at the aim of generating a new MBB. MBBs are bridged via sharing the motifs of Ni2+–tetrazole to provide the building unit with rod-like Ni-K. The structure unit with rod-like is connected with the coordination of K+ and Ni2+ centres through TZIA3− ligand carboxyl group to provide the porous skeleton which is 1-D hexagonal channels with nanotube-shape (considering Van der Waals radius, pore size = 12 Å, ). Ignoring the [(CH3)2NH2]+ ions within channels as well as solvent molecules with disorder state, PLATON estimates 1’ s achievable volume is 53.2% ().
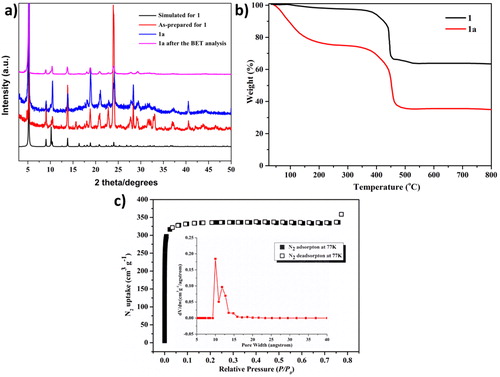
PXRD, TGA and BET analysis
At the aim of checking as-prepared complex 1’ s phase purity, pattern of PXRD of complex 1 was collected under the room temperature by using freshly prepared samples, and the results are shown in . The discovered along with calculated diffraction peaks well reflect sample purity. The discrepancy of reflective strength for discovered along with simulated results is attributed to crystals distinct orientation within powder samples. Complex 1’ s analysis for thermogravimetric was carried out and the thermodynamic stability of complex 1 was discussed. According to , the weightlessness of complex 1 is 23.8% continuously from 25 °C to 212 °C, which is related to remove four lattice DMF along with three coordinated water molecules, 1’ s framework could be thermally stable until up to 340 °C. Inspired via its latent porosity along with appropriate size of pore, we encourage deep exploration of 1’ s microporous properties. Complex 1 was assessed for 5 h at 313 k under the condition of high vacuum after full exchange of acetone, resulting in solvent removal or activation for 1a. Obviously, complex 1a maintains its microporous properties still, because for 1a’ s characteristic peaks are fundamentally consistent with the pattern of simulated XRD (). After removing solvent molecules from the newly established framework, some displacements are expected to be discovered between 1 and 1a. In addition, TGA analysis confirmed that there was no lattice solvent in 1’ s skeleton, indicating that there was no significant weightlessness between the temperature of 25 and 367 °C. According to , at 77 K, adsorption isotherm of N2 exhibits that compound 1a has the adsorption isotherm of type I, and there is a mild lag between desorption along with adsorption, it can use the dynamics characteristics of frame and multi-channel explanation. N2’ s adsorption capacity was up to 342 cm3/g at 1 atm along with 77 K (). The estimated apparent surface area of Brunauer–Emmett–Teller (BET) as well as the surface area of Langmuir surface area are 1100 along with 1477 m2/g, respectively. The distribution ranges of pore size were between 10 and 12 Å. These results are the same as those observed from the crystal structure. It shows that 1a’ s skeleton structure remains after solvent removal.
Catalytic properties of 1a
In consideration of complex 1a has coordinated unsaturated metal centres (UMCs) along with a large channel, it can be utilized as the latent catalysts of Lewis acid for the cyanosilylation of the carbonyl compounds and test its performance of catalysis. The model experiments were carried out by using the benzaldehyde as typical molecule under the conditions of without solvent by changing the amount and temperature of catalyst. According to , complex 1a indicated good activity of catalysis, providing 98% benzaldehyde conversion under a room temperature in one day, and the lower catalyst loading is 0.1 mol% K, which was the highest activity of catalysis in cyanosilylation in a quantity of only 1/15 of Cr-MIL-101 (1.5 mol% Cr-active site). In addition, 1a as the catalyst does not require the organic solvent of dichloromethane, but catalyst Cr-MIL-101 needs. Only two hours later, 1a was removed through filtration and reaction was completely stopped, and only 45% of the whole conversion was provided by standing for about 15 h. This indicated that there was no species of homogeneous catalyst within the solution of reaction, and 1a was real heterogeneous catalyst.
Table 2. Catalyst 1a’ s conditions optimization and 1’ s comparative test.
Under the conditions of majorization (2 equivalent TMSCN, catalyst of 0.1 mol% and under the room temperature), nine substrates of carbonyl such as cyclones, aliphatic aldehydes as well as aromatic were cyanated. Results are gathered in , which revealed that reaction has extensive survivability to a variety of substrates. Under the conditions of standard, the aromatic aldehydes which has substituents of electron-effect (electron-withdrawing NO2 and electron-donating CH3) or distinct substituents were used. After about 9–12 h of reaction, the yield of the product was over 90%. However, under the conditions of standard, to aliphatic aldehydes along with ketones usually have lower efficiency of catalysis than the aromatic aldehydes because of their inherent decreased reactivity. After a long time of reaction (two days), cyclobutanone conversion was only up to 83%. In order to study along with explain open K+ sites fine activity of catalysis, 1a and 1 activities of catalysis were detected at a same condition of reaction. Cyanation reaction conversion rates of 40.3% along with 38.2% were acquired, that is similar to the conversion rate with no catalyst, which may be because of the saturation geometry of the whole K+ ions within 1. The results also shown that the unsaturated K+ coordination within 1a has an important function in the process of catalysis.
Table 3. The cyanosilylation of various carbonyl compounds with TMSCN.
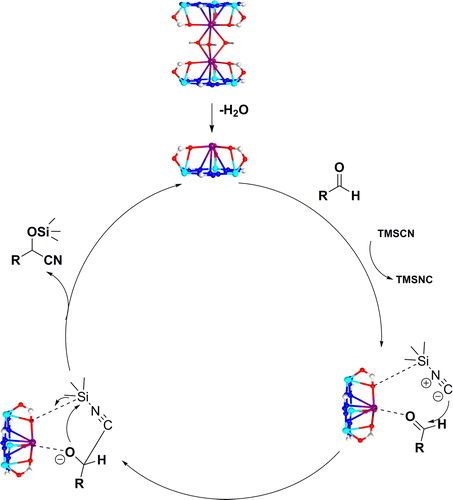
On the basis of the results of experiments and the reports of the literatures, a rational mechanism of reaction was put forward to explain the process of cyanation catalyzed by 1a. The unstable water molecules within 1a’ s channels were dislodged via heating to lay bare the metal centres of unsaturation. With coordinated K centres of unsaturation to active aldehydes and preform reaction with TMSCN (Scheme 1). via using aldehydes to displace products, and catalysts activate continually aldehydes within next cycle of catalysis.
Compound reduced the incidence of arrhythmia and abnormal blood pressure
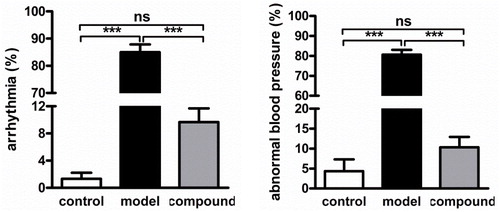
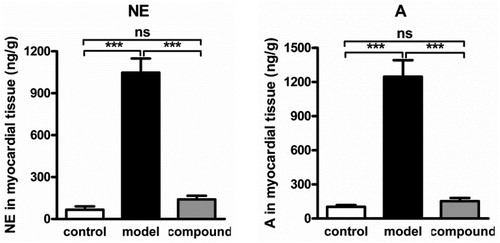
After the synthesis of the novel structure compound, its protective effect against arrhythmia occurrences after anesthesia during operation was evaluated in this section. Firstly, the arrhythmia rat model was constructed with Aconitine, then the compound was given for treatment. According to results of , we can observe that in the model group, the incidence of arrhythmia and abnormal blood pressure was obvious higher than the normal group (p < 0.01). After compound treatment, this incidence was reduced significantly, almost to the normal level. This result revealed the excellent prevention effect of compound against arrhythmia
Compound inhibited the production of NE and A in myocardial tissue
Within former research, we have confirmed the prevention function of the compound against Arrhythmia rat, but the detail mechanism of compound was still unclear. Thus, in this experiment, the NE and A in myocardial tissue was determined to explore the related mechanism of compound against arrhythmia. From the results in , we got this information, the content of NE and A released from the myocardial tissue was much higher than the normal group, which indicating the high level of sympathetic excitability. While, after compound treatment, the sympathetic excitability was significantly inhibited, reflected as the reduced level of NE and A.
Conclusion
In conclusion, we have generated a novel porous metal-organic framework by corresponding K(I)and Ni(II) salts and hetero-donor organic ligand 5-(1 H-tetrazol-5-yl)isophthalic acid (H3TZIA) solvothermal condition in water and DMF mixed solvent. X-ray of single crystal study shows that K+ ionic were immobilized on the surface of pore via trinuclear Ni2+–tetrazole coordination motif. The resulting activated 1a has been explored in detail for BET analysis to probe its porosity. The removal of water molecule at the aim of generating 1a makes K+ ion become a heterogeneous catalyst of size-selective with high efficiency for without solvent cyanosilylation of acetaldehyde under mild conditions. In biological research, we aimed to develop new candidates for the treatment of arrhythmia after anesthesia during operation. Firstly, we established the arrhythmia rat model, then the compound was given for treatment. After that, the incidence of arrhythmia and abnormal blood pressure was measured and recorded, and the results indicated the prevention ability of compound on the arrhythmia incidence and abnormal blood pressure. Besides, the ELISA detection of the of NE and A in myocardial tissue revealed that the sympathetic excitability was obviously inhibited by the compound treatment.
Authors’ contributions
Chao Ding synthesized and characterized the compounds; Weiwei Zhang and Zhenzheng Xu performed the biological experiments; Huimin Zhang, Junqi Zheng designed the study and prepared the manuscript.
| Abbreviations | ||
| A | = | adrenaline |
| ELISA | = | enzyme linked immune sorbent assay |
| NE | = | norepinephrine |
Disclosure statement
The authors declare that they have no competing interests.
Reference
- Atreya AR, Priya A, Pack QR, et al. Use and outcomes associated with perioperative amiodarone in cardiac surgery. J Am Heart Assoc. 2019;8(15):e009892.
- Staikou C, Stamelos M, Stavroulakis E. Perioperative management of patients with genetic multisystem diseases associated with pre‐excitation. Anaesthesiol Intensive Ther. 2019;51(2):133–146.
- Lin Y, Wu HK, Wang TH, et al. Trend and risk factors of recurrence and complications after arrhythmias radiofrequency catheter ablation: a nation-wide observational study in Taiwan. BMJ Open. 2019;9(5):e023487
- Echeverria-Villalobos M, Todeschini AB, Stoicea N, et al. Perioperative care of cannabis users: A comprehensive review of pharmacological and anesthetic considerations. J Clin Anesth. 2019; 57:41–49.
- Xue D-X, Cairns AJ, Belmabkhout Y, et al. Tunable rare-earth fcu-MOFs: A platform for systematic enhancement of CO2 adsorption energetics and uptake. J Am Chem Soc. 2013;135(20):7660–7667.
- Han Y-H, Zhou Z-Y, Tian C-B, et al. A dual-walled cage MOF as an efficient heterogeneous catalyst for the conversion of CO 2 under mild and co-catalyst free conditions. Green Chem. 2016;18(14):4086–4091.
- Xiong Y, Fan Y-Z, Yang R, et al. Amide and N-oxide functionalization of T-shaped ligands for isoreticular MOFs with giant enhancements in CO2 separation. Chem Commun. 2014;50(93):14631–14634.,
- Chen D-M, Zhang X-J. Stepwise and hysteretic sorption of CO2 in polycatenated metal-organic frameworks. Cryst Eng Comm. 2019;21(32):4696–4700.
- Xu W, Huang S, Luo F. A novel acylamide MOF showing self-catenated hxg-d-4-Fddd nets with 3-fold interpenetration and highly selective adsorption of CO2 over N2, CH4, and CO. Inorg Chem Commun. 2014;49:56–58.
- Liu JJ, Bin Shan Y, Fan CR, et al. Encapsulating naphthalene in an electron-deficient MOF to enhance fluorescence for organic amines sensing. Inorg Chem. 2016;55(7):3680–3684.
- Qian J, Jiang F, Su K, et al. Heterometallic cluster-based indium–organic frameworks. Chem Commun. 2014;50(96):15224–15227.,
- Schubert U. Cluster-based inorganic–organic hybrid materials. Chem Soc Rev. 2011;40(2):575–582.
- Song JJ, Gallou F, Reeves JT, et al. Activation of TMSCN by N-heterocyclic carbenes for facile cyanosilylation of carbonyl compounds. J Org Chem. 2006;71(3):1273–1276.
- Kikukawa Y, Suzuki K, Sugawa M, et al. Cyanosilylation of carbonyl compounds with trimethylsilyl cyanide catalyzed by an yttrium-pillared silicotungstate dimer. Angew Chem Int Ed. 2012;51(15):3686–3690.
- Cui X, Xu M-C, Zhang L-J, et al. Solvent-free heterogeneous catalysis for cyanosilylation in a dynamic cobalt-MOF. Dalton Trans. 2015;44(28):12711–12716.
- Bhunia A, Dey S, Moreno JM, et al. A homochiral vanadium–salen based cadmium bpdc MOF with permanent porosity as an asymmetric catalyst in solvent-free cyanosilylation. Chem Commun. 2016;52(7):1401–1404.,
- Neogi S, Sharma MK, Bharadwaj PK. Knoevenagel condensation and cyanosilylation reactions catalyzed by a MOF containing coordinatively unsaturated Zn(II) centers. J Mol Catal A Chem. 2009;299(1-2):1–4.
- Ladrak T, Smulders S, Roubeau O, et al. Manganese-based metal-organic frameworks as heterogeneous catalysts for the cyanosilylation of acetaldehyde. Eur J Inorg Chem. 2010;2010(24):3804–3812.,