?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Leucus aspera is a perennial plant traditionally used as an herbal medicine in many countries. The biosynthesis of metal nanoparticles using medicinal plants is not only economical but also environmentally friendly as well as having miscellaneous biomedical applications. In this study, Leucus aspera extract as a stabilising and reducing agent was utilised to synthesise Ag nanoparticles in the aqueous medium. In addition, the anti-alveolar cancer property of AgNPs was investigated in the in vitro condition. Various techniques containing UV–Vis. spectroscopy, FT-IR spectroscopy, X-ray diffraction (XRD), transmission electron microscopy (TEM), scanning electron microscopy (SEM), and energy dispersive X-ray spectrometry (EDS) were used to characterise the synthesised nanoparticles. On the other hand, the MTT assay was run to evaluate the cytotoxicity activity of AgNPs. The crystal size of AgNPs, according to the XRD analysis, was 34.22 nm. Moreover, the uniform spherical morphology ranging from 40.67 to 58.17 nm was detected in the SEM images for the biosynthesised nanoparticles. In the antioxidant test, the IC50 of AgNPs and BHT against DPPH free radicals were 87 and 41 µg/mL, respectively. The synthesised nanocomposite had very low cell viability and high anti-alveolar cancer activities against A549 cell line without any cytotoxicity on the normal cell line (HUVEC). The viability of malignant alveolar cell line reduced dose-dependently in the presence of Ag NPs. Perhaps notable anti-alveolar cancer activities of the synthesised nanocomposite against common alveolar cancer cell line are linked to their antioxidant activities.
1. Introduction
Cancer is a deadly disease with high mortality that leads to many psychological and economic conflicts. Lifestyle is one of the most important and effective factors in the incidence of cancer. Environmental factors such as environmental pollutants, carcinogens and mutagens, bacterial and viral infections as well as genetic susceptibility are the most important factors in the incidence of cancer [Citation1–4]. Depending on the type of cancer, the extent of the disease and the patient's condition, a combination of different methods such as surgery, radiotherapy and chemotherapy are used to fight and control cancer. Despite the possibility of side effects in advanced cancers, most of these common methods do not produce positive results and the need for research and finding new ways to fight cancer is urgently needed [Citation3–6]. Nanotechnology is the efficient production of materials, devices and systems by controlling matter at the nanometer scale and exploiting new properties and phenomena that have been developed at this scale. Nanoparticles are atomic or molecular assemblies with minimum dimensions between 1 and 100 nm with different physicochemical properties compared to the mass of their material. Nanotechnology has been instrumental in developing a new cancer treatment strategy [Citation5–9].
Today, nanoparticle technology has made great strides in the production of many drugs, and the production of nanoparticles is one of the hopes in the effective treatment and diagnosis of many diseases, including cancer [Citation10–13]. Metallic nanoparticles have long been considered as a candidate for cancer treatment. Because natural metal oxides are present in large quantities in nature, the processing and synthesis of these nanoparticles can be one of the least expensive synthesis protocols [Citation13–17]. Metallic nanoparticles are one of the new types of widely used mineral particles that have been considered by researchers due to their suitable physical and chemical properties and at the same time, it has more adsorption power than other metallic nanoparticles-containing compounds [Citation18–20]. Metallic nanoparticles are one of the therapeutic compounds recognised by the US Department of Food and Drug Administration as a safe substance. Metallic nanoparticles are biocompatible and nontoxic and have also been used as medical fillers, cosmetics and drug carriers [Citation20–24]. Among the special properties of metallic nanoparticles are high chemical stability, low dielectric constant, high catalytic activity, absorption of infrared and ultraviolet light and most importantly its antibacterial properties. If the therapeutic and anticancer effects of these compounds are confirmed, this could be a significant step in advancing cancer therapies [Citation13–19].
In recent years, biological methods that nontoxic, cost-effective, and environmentally friendly have become the focus of interest compared to physicochemical nanoparticle synthesis methods [Citation25]. Various pathways have been developed for the biogenic or biological formulation of nanomaterials from the salts of different metal ions. The synthesis of nanoparticles under purely 'green' principles can be achieved by using an environmentally compatible solvent system with environmentally friendly stabilising and reducing factors [Citation1,Citation10,Citation11,Citation26–31]. The basic principle in the biogenesis of nanoparticles is reducing metal ions of several biomolecules found in organisms. In addition to reducing the environmental impact of biological synthesis, it enables the production of large quantities of nanoparticles, which are well defined in size and morphology, independent of contamination. Microorganisms, marine algae, plant extracts, plant tissue, fruits, and all plants are administrated to formulate nanomaterials [Citation29,Citation31]. Reducing metal ions using biological materials has been a known method since the 1900s. Due to the poor understanding of the mechanisms of the reducing agents, it has increased interest in the past 30 years [Citation8]. In recent years, researchers have revealed that biological materials nanoparticles have unique anticancer potentials. Metallic nanoparticles have achieved notable consideration in the field of medicine. Some studies conducted today have shown that some nanoparticles have therapeutic properties and it is an excellent alternative to physicochemically different metal-supported nanoparticles, antibacterial, and especially anticancer drugs [Citation17–23].
In the recent study, the properties of prepared AgNPs by Leucus aspera leaf aqueous extract against alveolar cancer A549 cell line were evaluated.
2. Experimental
2.1. Material
Phosphate buffer solution (PBS), Sabouraud Dextrose Agar, Sabouraud Dextrose Medium, Muller Hinton Agar, Mueller Hinton Medium, carbazole reagent, 4-(Dimethylamino)benzaldehyde, Dulbecco's Modified Eagle Medium (DMED), Ehrlich solution, dimethyl sulfoxide (DMSO), hydrolysate, decamplmaneh fetal bovine serum, borax–sulphuric acid mixture, 2,2-diphenyl-1-picrylhydrazyl (DPPH), and antimycotic antibiotic solution all were achieved from Sigma-Aldrich company of USA.
2.2. Synthesis of AgNPs
Extraction was carried out by dissolving 50 g of Leucus aspera leaf powder in 500 mL of distilled water and kept on an orbital shaker for 48 h. Then the extract was filtered using Whattman filter paper No. 1 and the filtrate was concentrated in a rotary evaporator under reduced pressure (60 ± 100 C). Furthermore, the herbal extracts were lyophilised at −48 °C for 24 h and stored for further use.
To synthesise AgNPs, firstly, 2 g Leucus aspera was dissolved in 20 mL de-ionised water. Then, 2.5 mL of the prepared solution was added to an aqueous AgNO3 solution (5 × 10−4M). In the subsequent stage, the obtained solution was heated up to 80 °C in the oil bath under a certain stirring speed for 24 h. This led to the gradual formation of AgNPs. The similar reactions were also carried out using various concentrations of Leucus aspera.
2.3. Chemical characterisation of AgNPs
For analyzing AgNPs, the common techniques of organic chemistry, i.e. FT-IR and UV–Vis. spectroscopy, FE-SEM, and TEM were used. The biomolecules involved in the reduction of AgNPs were detected by the FT-IR spectrophotometer (Shimadzu IR affinity 1). The gold nanoparticles were primarily confirmed using UV–Vis spectroscopy at a scan range from 450 to 750 nm wavelength (Jasco V670 Spectrophotometer). The morphological features in terms of shape and sizes were analyzed by FE-SEM (Fe-SEM ZEISS EVO18) and TEM (TEM FEI-TECNAI G2-20 TWIN) microscopic techniques.
2.4. Assessment of the antioxidant potential of AgNPs by DPPH
The DPPH molecule has a stable free radical in which delocalisation of the spare electron over the molecule, results the molecules do not dimerise. This delocalisation results in the deep violet colour with a characteristic absorption peak in ethanol solution at 517 nm. When the DPPH solution was mixed with electron donating substance, which leads to the reduced form with colour loss. Later, the solution undergoes further reactions and control the stoichiometry, hence reduced (decolourised) the number of molecules of the DPPH by one molecule of the reductant [Citation32].
In this experiment, 3 mL 0.004% DPPH solution was mixed with several concentrations of AgNPs for determining antioxidant properties. A group containing 3 mL ethanol and 3 mL DPPH was considered as the control group. According to the international standards, the samples absorption rate was determined at 517 nm and the antioxidant potentials of AgNPs were assessed according to the following formula [Citation32]:
2.5. Measurement of cell toxicity of AgNPs
In this experiment, the following cell lines have been used for investing the cytotoxicity and anti-alveolar cancer effects of the AgNPs using an MTT assay:
Normal cell line: HUVEC.
Human alveolar cancer cell line: A549.
These cells (1 × 105 cells/well) were plated individually using 96 well plates in DMEM media with antimycotic antibiotic solution and 10% FBS. The cells were carried out to 96 well plates at a 1 × 103 cell concentration and incubated, then treated with several dilutions of AgNPs (0–1000 μg/mL). After 24 h at 37 °C incubation, 0.05 mg/mL of MTT reagent was added to all wells. After 24 h incubation at 37 °C, MTT reagent was discarded and cell lines were washed thrice with PBS and the value of absorbance was determined at 570 nm. The percent of cell viability was measured using the following equation [Citation33]:
2.6. Statistical analysis
The obtained results were fed into SPSS-22 software and analyzed by one-way ANOVA, followed by Duncan post hoc test (p ≤ 0.01).
3. Results and discussion
Cancer is recognised as one of the leading causes of death in today's society and several drugs have been introduced to treat this disease, but, most common cancers are not yet controllable and this disease imposes huge costs on the patient and society [Citation34–36]. The main factor in the development and progression of cancer has not yet been precisely identified, however, the available data suggest that metabolic disorders in the tissue and immune disorders may be involved in the development and exacerbation of this disease. In addition, metabolic disorders in the production and excretion of oxygen free radicals are important factors affecting cancer cells [Citation37–40]. Free radicals are destructive compounds that are produced as a by-product by the body's chemical reactions and are destroyed by the body's defense system and enzyme system and antioxidants. However, in cases where the body's metabolic disorders and the production of free radicals are high and they are not destroyed by the neutralising system, due to their instability, these compounds have a strong tendency to react with a variety of molecules in the body [Citation37–40]. It is estimated that each cell in the human body is exposed to free radicals 10,000 times a day and DNA strands 5,000 times a day. Damage to cell components includes proteins (genetic disorder), fats (lipid oxidation), and cell membranes (permeability disorder) that if the damage is not repaired, it leads to disruption of the chemical reaction and normal proteinisation of the cell and the formation of harmful compounds and sometimes cancer cells in the body [Citation41–44]. It is reported that thousands of cancer cells are produced daily in the human body that are killed by the body's defense system. In some cases, due to dysfunction of the above systems, cancer cells proliferate and conditions for cancer development in different tissues [Citation43–46]. According to the above, antioxidants play a vital role in preventing disorders caused by the effects of free radicals and thus the prevention and treatment of cancer. Antioxidants are a wide range of molecular compounds with complex properties that combine with and neutralise free radicals. The results show that more than 60,000 types of molecular antioxidants have been identified so far. Antioxidants can be effective in three known ways to prevent and treat cancer; 1. Destruction of free radicals 2. Strengthen the immune system to destroy cancer cells. Prevent the adhesion of cancer cells to other cells and prevent their proliferation [Citation43–47].
3.1. Structural characterisation of synthesised AgNPs
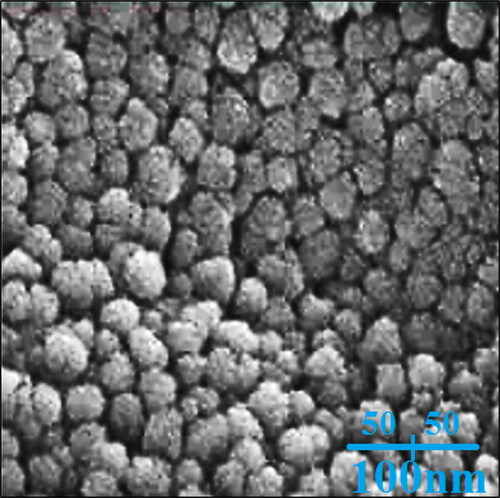
FE-SEM analysis. FE-SEM technique is sufficient method to investigate morphology of nanoparticles. The FE-SEM images of AgNPs are shown if . The nanoparticles are formed in a spherical morphology in an average size of 26.18 nm. The green synthesised AgNPs, exhibit a tendency to aggregate that is known as a general property for green synthetic metallic nanoparticles using plants extracts [Citation32]. In our review of literature, the size of silver nanoparticles, which were synthesised using plant extract, was in the range of 5–251.1 nm [Citation32].
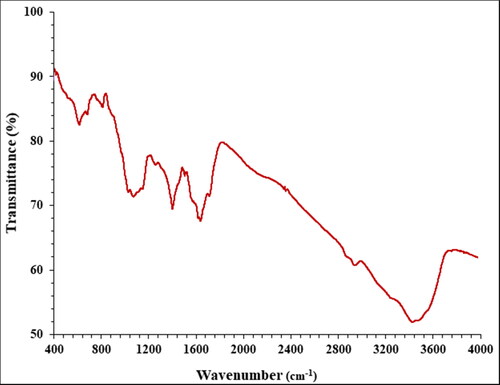
FT-IR analysis. FT-IR technique is another qualitative method in characterisation of nanoparticles. The presences of the bands in the specific wavenumber regions shows useful information about the metallic nanoparticles. For example, if the nanoparticles are formed as metal oxide, the peaks at 400–700 cm−1 belong to metal oxygen bond. The presences of the peaks at the other region are attributed to the different bonds for organic compounds in the plant extract that bind to the nanoparticles. The FT-IR spectra of AgNPs is shown in . The peaks at wavenumbers of 423, 511, and 624 cm−1 belong to Ag–O bond. A previous study has reported the peaks for green synthetic AgNPs with a little difference in wavenumber [Citation32]. Furthermore, the peaks at 3486 and 2950 cm−1 (O–H and aliphatic C–H stretching), 1403–1625 cm−1 (C = C and C = O stretching), (1025 cm−1 –C–O stretching) belong to the various bonds of organic compounds in plant extract that exist as the plant secondary metabolites. These compounds can be comprised different class of compounds such as phenolic, flavonoid, triterpenes, which were reported previously [Citation32].
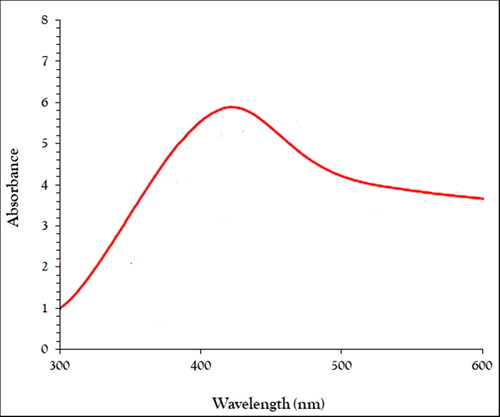
UV–Vis analysis. The surface plasmon resonance (SPR) of the nanoparticle can be analyzed using UV–Visible spectroscopy. The UV–Vis. spectrum of the green-synthetic nanoparticles of NAgNPs is presented in . The creation of the green-synthetic NiNPs was approved by the results. There is a SPR band appearance at the wavelength of 418 nm that approved the formation of the nanoparticles. The band is very close to a previously reported on the green synthesised of AgO nanoparticles using plant extracts [Citation32].
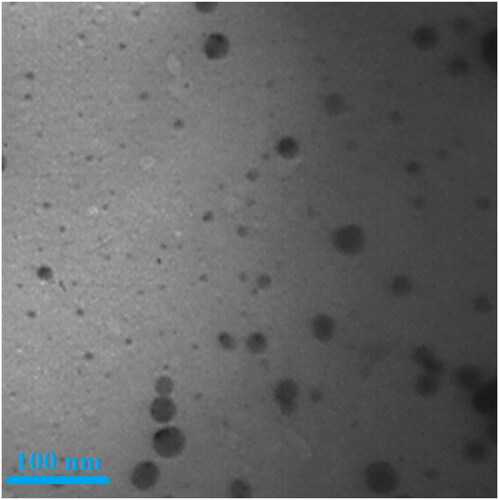
TEM analysis. TEM is the other test for determining the morphology and size of metallic nanoparticles. In our study, the range size of the nanoparticles (11–36 nm) calculated through TEM images (). Furthermore, the histogram plot from the TEM image showed the particle size distribution of biosynthesised gold nanoparticles ranges of 10–41 nm. In the previous studies, the size of gold nanoparticles formulated by aqueous extract of medicinal plants had been calculated in the ranges of 10–100 nm with the shape of spherical [Citation32]. These reports support the results of the current work.
3.2. Cytotoxicity and anti-alveolar cancer activities of Ag NPs
The unique physical and chemical properties of silver nanoparticles have attracted the attention of the scientific community due to their high plasmonic properties, heat transfer, chemical stability and antibacterial effects. Using silver is nothing new; it dates back to Hippocrates, who used it as an antibacterial to control wounds [Citation48–50]. Silver nanoparticles are used in many commercial products, including soap, food, plastics, catheters, textiles, and bandages. However, their mechanism of action is still unknown. Many factors (shape, size, surface chemistry, morphology, density, charge, and purity) affect the biological activity of silver nanoparticles [Citation48–53]. Another important feature of silver nanoparticles is their role in treating cancer. Silver nanoparticles are a promising tool as an anticancer agent in diagnosis and evaluation. They have many benefits with strong effects against different cancer cell lines. Their better penetration and ability to detect silver nanoparticles in the body make them a more effective tool in treating low-risk cancers compared to standard treatments [Citation52–55]. The unique properties of silver nanoparticles, such as their optical properties, easy synthesis and high surface-to-volume ratio, make them suitable for treating cancer. Silver nanoparticles can also be conjugated to various molecules, including DNA and RNA, to target different cells and antibodies or polymers. These important factors are important for increasing the half life for circulating in vivo, which is very important in drug and gene delivery applications. In addition, silver nanoparticles are used as a cancer cell erosion tool because of their ability to convert radio frequency into heat [Citation53–57].
Silver nanoparticles induce changes in cell morphology, decrease cell metabolic activity, increase oxidative stress leading to mitochondrial damage, and ultimately damage DNA by producing reactive oxygen species (ROS). Adsorption of silver nanoparticles occurs through endocytosis [Citation55–58]. Examination of cancer morphology shows that synthesising silver nanoparticles can significantly increase cell death. Now, if chitosan is combined with silver nanoparticles, it increases the rate of cell death. The mechanism of anticancer activity of silver nanoparticles is that silver nanoparticles induce apoptosis in cancer cells [Citation54–57]. Silver nanoparticles are capable of altering the regulation of more than 1,000 genes. Among these genes are metallothianonine, chaperones, and histones. Autophagy is one of the anticancer mechanisms of silver nanoparticles, which induces cell death [Citation56–58]. Nanoparticle-induced autophagy is an important cell degradation process, and increased autophagy can also increase cell death. Recent studies show that silver nanoparticles can induce autophagy through the accumulation of autophagolysosomes in ovarian cancer cells. Therefore, autophagy can affect performance; at low levels it can increase cell survival and at high levels it can cause cell death. Another mechanism of silver nanoparticles is the increase in the level of internal ion concentration, which increases the production of ROS. On the other hand, uncontrolled production of ROS can lead to serious cellular damage, including DNA and mitochondrial damage, leading to programmed cell death (apoptosis) [Citation58–61]. The cytotoxic activity of silver nanoparticles in breast cancer cells is through activation of caspase 3, p53, pErk1/2 and lack of Bcl2 expression. In particular, silver nanoparticles induce cell death through various processes including ROS production and increased lactate dehydrogenase secretion, induction of apoptosis, induction of autophagic genes, mitochondrial dysfunction and activation of caspase and DNA damage [Citation51–54]. Silver nanoparticles, like other biomaterials, can cause toxic effects in living organisms. The toxicity created by these particles depends on their properties and route of entry. The toxicity induced by silver nanoparticles is due to oxidative stress, which accumulates in the cytoplasm and cell nucleus, leading to the production of free radicals [Citation56,Citation57]. Silver nanoparticles are initially separate particles that have the highest toxicity due to their high contact surface, but their toxicity decreases over time and joins together [Citation58–63].
In this experiment, the treated cells with different concentrations of the present Ag NPs were assessed by MTT assay for 48 h about the cytotoxicity properties on normal (HUVEC) and alveolar cancer A549 cell lines.
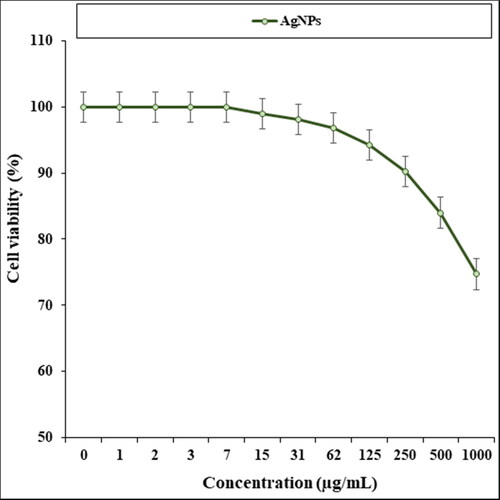
The absorbance rate was evaluated at 570 nm, which represented viability on normal cell line (HUVEC) even up to 1000 μg/mL for AgNPs ().
Figure 5. The cytotoxicity properties (cell viability (%)) of AgNPs (concentrations of 0–1000 µg/mL) against human normal (HUVEC) cell line.
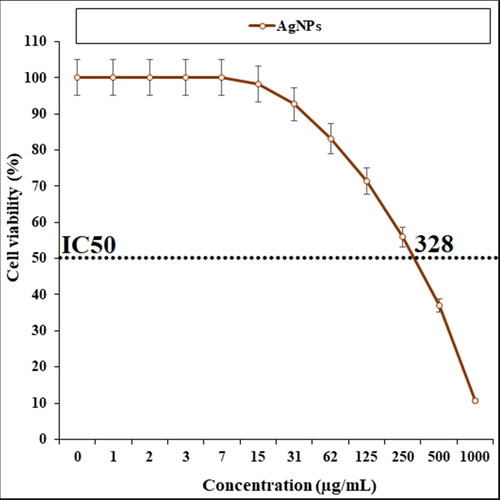
The viability of malignant alveolar cell line reduced dose-dependently in the presence of AgNPs. The IC50 of AgNPs was 328 µg/mL against A549 cell line ().
Figure 6. The anti-alveolar cancer properties (cell viability (%)) of AgNPs (concentrations of 0–1000 µg/mL).
It seems that the anti-alveolar cancer effect of recent nanoparticles is due to their antioxidant effects. Because tumor progression is so closely linked to inflammation and oxidative stress, a compound with anti-inflammatory or antioxidant properties can be an anticarcinogenic agent [Citation4,Citation5,Citation64]. Many nanoparticles have pharmacological and biochemical properties, including antioxidant and anti-inflammatory properties, which appear to be involved in anticarcinogenic and antimutagenic activities [Citation65,Citation66]. Today, nanoparticles synthesised by biological methods play a vital role in treating many diseases, including cancer [Citation67–69].
3.3. Antioxidant properties of AgNPs
In this study, we assessed the antioxidant properties of AgNPs by the DPPH test as a common free radical. Free radicals are molecules with a free electron ready to react, and oxygen is produced with some molecules. If many of them are suddenly produced in the body, they react with some parts of the cell, such as DNA and cell membranes, and cause cell damage or even death [Citation65,Citation66]. Normally, the body's defense system neutralises these harmless free radicals. Antioxidants prevent the spread of oxidation chain reactions. Thus, the strength of an antioxidant formed by the contact of an H atom with a free radical is due to the effect of an antioxidant on the ease with which this H atom separates from it. Thus, antioxidants can protect cell membranes and various living compounds against oxidants in small amounts [Citation67,Citation68]. Numerous biochemical and physiological processes may cause the production of free radicals. Reactive oxygen species (ROS) include free radicals and radical-free forms. Free radicals include hydrogen peroxide (H2O2), hydroxyl radical (.OH), and superoxide anion radical (O2). When the concentration of ROS increases, it can oxidise macromolecules such as proteins, nucleic acids, and membrane lipids, resulting in cell damage and possibly ‘cell and tissue destruction’ [Citation65–68]. Natural compounds and molecules have two main mechanisms for reducing the concentration of ROS, in other words, natural compounds and molecules reduce the concentration of ROS by producing antioxidants and thus prevent cell damage [Citation68]. Recently, many researchers have paid close attention to natural compounds and molecules and their relationship to their antioxidant properties, and many natural compounds and molecules have been studied for their antioxidant activity [Citation65–68].
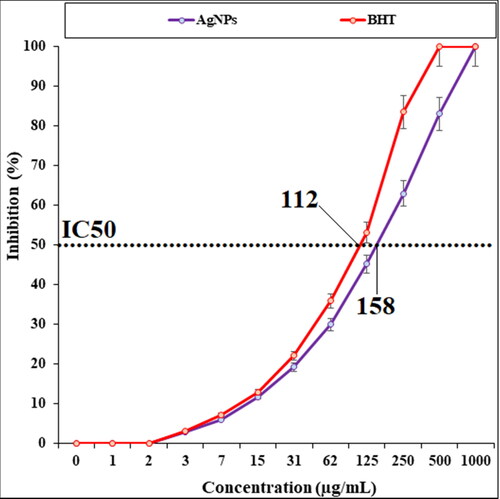
The scavenging capacity of AgNPs and BHT at different concentrations expressed as percentage inhibition has been indicated in . In the antioxidant test, the IC50 of AgNPs and BHT against DPPH free radicals were 158 and 112 µg/mL, respectively.
4. Conclusion
In our research, the silver nanoparticles were successfully obtained from the bioreduction of AgNO3 solutions using an aqueous extract of Leucus aspera leaf. The silver nanoparticles have been appropriately characterised and confirmed using FE-SEM, TEM, FT-IR, and UV–Vis. In the FT-IR test, the presence of many antioxidant compounds with related bonds caused the excellent condition for reducing of silver in the silver nanoparticles, so that the antioxidant properties of silver nanoparticles were the better than the butylated hydroxytoluene as the positive control. The silver nanoparticles indicated suitable antioxidant and anti-alveolar cancer activities without any cytotoxicity effect on the normal cell line. Seemingly, the silver nanoparticles synthesised using Leucus aspera leaf aqueous extract can be used for the treatment of human alveolar cancer in human after confirming in in vivo and clinical trial experiments.
References
- Fazaeli R, Aliyan H, Fazaeli N. Heteropoly acid in ionic liquid – an efficient catalyst for the preparation of 2H-indazolo[2,1-b]phthalazine-triones. TOCATJ. 2010;3(1):14–18.
- Liao SH, Liu CH, Bastakoti BP, et al. Int J Nanomed. 2015;10:3315–3327.
- Radini IA, Hasan N, Malik MA, et al. Biosynthesis of iron nanoparticles using Trigonella foenum-graecum seed extract for photocatalytic methyl orange dye degradation and antibacterial applications. J Photochem Photobiol B. 2018;183:154–163.
- Mao B-H, Tsai J-C, Chen C-W, et al. Mechanisms of silver nanoparticle-induced toxicity and important role of autophagy. Nanotoxicology. 2016;10(8):1021–1040.
- You C, Han C, Wang X, et al. The progress of silver nanoparticles in the antibacterial mechanism, clinical application and cytotoxicity. Mol Biol Rep. 2012;39(9):9193–9201.
- Arunachalam KD, et al. One-step green synthesis and characterization of leaf extract-mediated biocompatible silver and gold nanoparticles from memecylon umbellatum. Int J Nanomed. 2003;8:1307–1315.
- Abdel-Fattah WI, Ali GW. On the anti-cancer activities of silver nanoparticles. J Appl Biotechnol Bioeng. 2018;5:00116.
- Wolach O, Stone RM. R. M. How I treat mixed-phenotype acute leukemia. Blood. 2015;125(16):2477–2485.
- GBD. 2015 Disease and injury incidence and prevalence, collaborators. "global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1545–1602.
- Mazaahir K, Ritika C, Anwar J. Efficient CAN catalyzed synthesis of 1H-indazolo[1,2-b] phthalazine-1,6,11-triones: an eco-friendly protocol. Chin Sci Bull. 2012;57(18):2273–2279.
- Kiasat RA, Mouradezadegun A, Saghanezhad JS. Phospho sulfonic acid: a novel and efficient solid acid catalyst for the one-pot preparation of 2H-indazolo[2,1-b]-phthalazine-triones. J Serb Chem Soc. 2013;78(4):469–476.
- Celardo I, Pedersen JZ, Traversa E, et al. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420.
- De Jong WH, Borm PJ. Drug delivery and nanoparticles: applications and hazards. Int J Nanomed. 2008;3(2):133–149.
- Borm PJA, Robbins D, Haubold S, et al. The potential risks of nanomaterials: a review carried out for ECETOC. Part Fibre Toxicol. 2006;3(1):11.
- Stapleton PA, Nurkiewicz TR. Vascular distribution of nanomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2014;6(4):338–348.
- Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnol. 2018;16(1):71.
- Itani R, Al Faraj SA. siRNA conjugated nanoparticles: a next generation strategy to treat lung cancer. IJMS. 2019;20(23):6088.
- Trojer MA, Li Y, Wallin M, et al. Charged microcapsules for controlled release of hydrophobic actives part II: surface modification by lbl adsorption and lipid bilayer formation on properly anchored dispersant layers. J Colloid Interface Sci. 2013; 409:8–17.
- Liu D, Chen L, Jiang S, et al. Formulation and characterization of hydrophilic drug diclofenac sodium-loaded solid lipid nanoparticles based on phospholipid complexes technology. J Liposome Res. 2014;24(1):17–26.
- Cheng M, Cao W, Gao Y, et al. Studies on nerve cell affinity of biodegradable modified chitosan films. J Biomater Sci Polym Ed. 2003;14(10):1155–1167.
- Li Z, Ramay HR, Hauch KD, et al. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials. 2005;26(18):3919–3928.
- Fukuda J, Khademhosseini A, Yeo Y, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006;27(30):5259–5267.
- Wang G, Lu G, Ao Q, et al. Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnol Lett. 2010;32(1):59–66.
- Gray CJ, Dowsett J. Retention of insulin in alginate gel beads. Biotechnol Bioeng. 1988;31(6):607–612.
- Gutowska A, Jeong B, Jasionowski M. Injectable gels for tissue engineering. Anat Rec. 2001;263(4):342–349.
- Konda SR, Reguri BR, Kagga M. Synthesis of 3,4-dihydro-3,3-dimethyl-13-aryl-2H-indazolo [1,2-b]pthalazine-1,6,11(13H)-triones using tungstated zirconia (WO3/ZrO2. Der Pharma Chem. 2014;6:228–233.
- Becker TA, Kipke DR, Brandon T. Calcium alginate gel: a biocompatible and mechanically stable polymer for endovascular embolization. J Biomed Mater Res. 2001;54(1):76–86.
- Lu G, Sheng B, Wang G, et al. Controlling the degradation of covalently cross-linked carboxymethyl chitosan utilizing bimodal molecular weight distribution. J Biomater Appl. 2009;23(5):435–451.
- Wang A, Ao Q, Wei Y, et al. Physical properties and biocompatibility of a porous chitosan-based fiber-reinforced conduit for nerve regeneration. Biotechnol Lett. 2007;29(11):1697–1702.
- Li X, Yang Z, Zhang A, et al. Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials. 2009;30(6):1121–1132.
- Itoh S, Yamaguchi I, Suzuki M, et al. Hydroxyapatite-coated tendon chitosan tubes with adsorbed laminin peptides facilitate nerve regeneration in vivo. Brain Res. 2003;993(1–2):111–123.
- Lu Y, Wan X, Li L, et al. Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4-nitrophenol and performance as anti-colorectal carcinoma. J Mater Res Technol. 2021;12:1832–1843.
- Shaneza A, et al. Herbal treatment for the ovarian cancer. SGVU J Pharm Res Educ. 2018;3(2):325–329.
- Gao J, Wang Z, Liu H, et al. Liposome encapsulated of temozolomide for the treatment of glioma tumor: preparation, characterization and evaluation. Drug Discov Ther. 2015;9(3):205–212.
- Mohammed MI, Makky AM, Teaima MH, et al. Transdermal delivery of vancomycin hydrochloride using combination of nano-ethosomes and iontophoresis: in vitro and in vivo study. Drug Deliv. 2016;23(5):1558–1564.
- Li YN, GF. Recent progress in doxorubicin nano-drug delivery systems for reserving multidrug resistance. Drug Deliv. 2014;11(3):177–181.
- Yang F, Jin C, Jiang Y, et al. Liposome based delivery systems in pancreatic cancer treatment: from bench to bedside. Cancer Treat Rev. 2011;37(8):633–642.
- Xinli DHZS. Applications of nanocarriers with tumor molecular targeted in chemotherapy. Chemistry. 2012;75(7):621–627.
- Allen TM. Ligand-targeted therapeutics in anticancer therapy. Nat Rev Cancer. 2002;2(10):750–763.
- Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Del Rev. 2008;60(15):1615–1626.
- Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. Aaps J. 2007;9(2):E128–E147.
- Deshpande PP, Biswas S, Torchilin VP. Current trends in the use of liposomes for tumor targeting. Nanomedicine (Lond)). 2013;8(9):1509–1528.
- Zhang Y, Huang Y, Li S. Polymeric micelles: nanocarriers for cancer-targeted drug delivery. AAPS Pharm Sci Tech. 2014;15(4):862–871.
- Matsumura Y, Hamaguchi T, Ura T, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer. 2004;91(10):1775–1781.
- Nie S, Xing Y, Kim GJ, et al. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288.
- Gao Z, Lukyanov AN, Singhal A, et al. Diacyllipid–polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2(9):979–982.
- Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782.
- Beyene HD, Werkneh AA, Bezabh HK, et al. Synthesis paradigm and applications of silver nanoparticles (Ag NPs), a review. Sustain Mater Technol. 2017;13:18–23.
- Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12.
- Alexander JW. History of the medical use of silver. Surg Infect (Larchmt)). 2009;10(3):289–292.
- Bhattacharya S, Zhang Q, Carmichael PL, et al. Toxicity testing in the 21 century: defining new risk assessment approaches based on perturbation of intracellular toxicity pathways. PLoS One. 2011;6(6):e20887.
- Huang Y, Fan CQ, Dong H, et al. Current applications and future prospects of nanomaterials in tumor therapy. Int J Nanomed. 2017;12:1815–1825.
- Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2012;2012:751075.
- Rai M, Kon K, Ingle A, et al. Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol. 2014;98(5):1951–1961.
- Jo DH, Kim JH, Lee TG, et al. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomed Nanotechnol Biol Med. 2015;11(7):1603–1611.
- Riehemann K, Schneider SW, Luger TA, et al. Nanomedicine-challenge and perspectives. Angew Chem Int Ed Engl. 2009;48(5):872–897.
- Bhattacharyya S, Kudgus RA, Bhattacharya R, et al. Inorganic nanoparticles in cancer therapy. Pharm Res. 2011;28(2):237–259.
- Day ES, Morton JG, West JL. Nanoparticles for thermal cancer therapy. J Biomech Eng. 2009;131(7):074001.
- Pelaz B, del Pino P, Maffre P, et al. Surface functionalization of nanoparticles with polyethylene glycol: effects on protein adsorption and cellular uptake. ACS Nano. 2015;9(7):6996–7008.
- Sperling RA, Parak WJ. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos Trans Ser A Math Phys Eng Sci. 2010;368:1333–1383.
- Sau TK, Rogach AL, Jackel F, et al. Properties and applications of colloidal nonspherical noble metal nanoparticles. Adv Mater. 2010;22(16):1805–1825.
- Cruz LJ, Tacken PJ, Rueda F, et al. G targeting nanoparticles to dendritic cells for immunotherapy. Methods Enzymol. 2012;509:143–163.
- Andersson HA, Kim Y-S, O'Neill BE, et al. HSP70 promoterdriven activation of gene expression for immunotherapy using gold nanorods and near infrared light. Vaccines. 2014;2:216–227.
- Namvar F, Rahman HS, Mohamad R, et al. Cytotoxic effect of magnetic iron oxide nanoparticles synthesized via seaweed aqueous extract. Int J Nanomed. 2014;9:2479–2488.
- Sankar R, Maheswari R, Karthik S, et al. Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;44:234–239.
- Katata-Seru L, Moremedi T, Aremu OS, et al. Green synthesis of iron nanoparticles using Moringa oleifera extracts and their applications: removal of nitrate from water and antibacterial activity against Escherichia coli. J Mol Liq. 2018;256:296–304.
- Sangami S, Manu M. Synthesis of green iron nanoparticles using laterite and their application as a Fenton-like catalyst for the degradation of herbicide ametryn in water. Environ Technol Innov. 2017;8:150–163.
- Beheshtkhoo N, Kouhbanani MAJ, Savardashtaki A, et al. Green synthesis of iron oxide nanoparticles by aqueous leaf extract of Daphne mezereum as a novel dye removing material. Appl Phys A. 2018;124:363–369.