?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
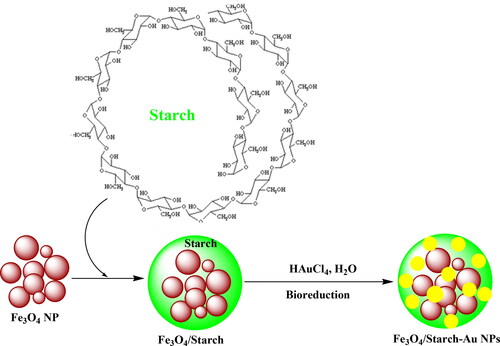
In recent days, the green synthesized nanomagnetic biocomposites have been evolved with tremendous potential as the future catalysts. This has encouraged us to design and synthesis of a novel Au NP fabricated starch functionalized core-shell type nanomaterial (Fe3O4/Starch-Au nanocomposite). It was meticulously characterized using advanced analytical techniques like FT-IR, FESEM, TEM, EDX, VSM, XRD and ICP-OES. MTT assay was used on common normal cell line i.e. Human umbilical vein endothelial cells (HUVEC) to survey the cytotoxicity effects of Fe3O4/Starch-Au nanocomposite. In the part of cutaneous wound healing, use of Fe3O4/Starch-Au nanocomposite ointment, significantly (p ≤ 0.01) raised the wound contracture, vessel, hydroxyl proline, hexuronic acid, fibrocyte, fibroblast, and fibrocytes/fibroblast rate and significantly (p ≤ 0.01) decreased the wound area, total cells, neutrophil, and lymphocyte compared to other groups in rats. Finally, the results showed the useful cutaneous wound healing properties of Fe3O4/Starch-Au nanocomposite. Maybe significant cutaneous wound healing potentials of Fe3O4/Starch-Au nanocomposite are related to their antioxidant activities. For investigating the antioxidant activities of Fe3O4/Starch-Au nanocomposite, the DPPH test was used in the presence of butylated hydroxytoluene as the positive control. The Fe3O4/Starch-Au nanocomposite inhibited half of the DPPH molecules in the concentration of 156 µg/mL.
1. Introduction
Wounds are damages resulted from the physical injury that leads to opening or breaking of skin, which includes punctured, scratches, scrapes, and cuts skin. Wound are likely to become chronic when there is a failure in the sequential phases of healing [Citation1]. The human body generally equipped with a self-healing defense mechanism, but under some critical conditions such as microbial infection, the wound healing process can be delayed. In some cases, the complexity of wound leads to lots of pain and cost in treatment [Citation2].
Wound dressing plays an important role in the healing if properly covered it reduces the time for healing and decrease the chances of scar formation. The main objective of the wound dressing is to accelerate the wound healing process by preventing microbial growth, absorbing wound fluid and by maintaining a wet environment around the wound [Citation3–5]. The metallic nanoparticles potentially have some antimicrobial activity that can reduce the growth of microorganisms, when exposed to skin, the coated medicinal herbs are absorbed by the body and provide treatment for a wide range of diseases including hypertension, arthritis, asthma, skin infections, and diabetes [Citation5–7].
The field of synthetic nanotechnology has developed the self-sufficiency of materials with specific sizes and shapes at the nanometer scale environmentally friendly. Nanomaterials can be created from the engineering of multiple or single molecules which are engineered and have several functional groups. Biocompatible nanocarriers include peptides, engineered antibodies, micelles, polymers, and liposomes [Citation7–9]. Nanomaterials can be designed to gain the desired chemical and physical characteristics and purposefully direct drugs to dynamic tumor environment with high therapeutic effects and low toxicity [Citation9–11]. Features to be controlled when designing nanoparticles are surface to volume ratio, shape, size, molecular charge, and drug release. For example, nanoparticles, after binding to functional chemical components such as antibodies and ligands, bind to the surface receptors of the abnormal cell and prevent its clearance by the blood [Citation7–9].
The most important feature of nanoparticles is particle size and particle distribution. Particle size and drug particle distribution play important roles in the biological fate, toxicity, and targeting of drug delivery systems. They affect the loading, release and stability of particle tattoos. Many studies have shown the advantages of nanoparticles compared to microparticles [Citation8, Citation9]. One of the advantages of nanoparticles over micro-particles is their high cellular uptake. Due to their small size, the particles enter various cells and intracellular space. Nanoparticles cross the blood-brain barrier following the opening of hard endothelial junctions by mannitol, which provides stable drug release systems to treat severe brain diseases [Citation10–12]. Studies have shown that Tween 80-coated nanocomposites are able to cross the barrier of blood-brain [Citation10–13]. Particles smaller than a micron are removed by a large number of cells. The cellular uptake of 100 nm nanoparticles is 2.5 times that of 1 μm particles, and the cellular uptake of 100 nm nanoparticles is 6 times that of 10 μm particles by 2-caco cells [Citation9–11]. Drug release is also controlled by particle size. Smaller particles have a lower volume-to-surface ratio. Therefore, if the drugs are placed on or near the surface of the nanoparticles, it will lead to the rapid release of the drug. However, larger particles are annealed at the center of the nanoparticles, which are released more slowly [Citation13–17]. Therefore, particle size control is effective in determining drug release. Smaller particles may form during transport, disrupting their distribution. The decomposition of polymers is affected by particle size. For example, as the particle size of polylactic-co-glycolic acid polymer increases, its decomposition rate increases. Therefore, it is hypothesized that larger particles cause the polymer to decompose faster and the drug to be released faster [Citation14–16].
Au nanoparticles has been an enchanting research area due to its unique fascinating characteristics. Au NPs are found to exhibit chemotherapeutic activities in both in vitro and in vivo experimental studies [Citation9–17].
Hence, we feel immense pleasure to introduce a green synthesized bio-nanomagnetic hybrid material (Fe3O4/Starch-Au) as novel nanocomposite with biological performance (Scheme 1). We investigated the nanocomposite in the cytotoxicity studies against common normal cell line, in-vitro. Interestingly, in the part of cutaneous wound healing, use of Fe3O4/Starch-Au nanocomposite ointment, significantly, (p ≤ 0.01) raised the wound contracture, vessel, hydroxyl proline, hexuronic acid, fibrocyte, fibroblast, and fibrocytes/fibroblast rate and significantly (p ≤ 0.01) decreased the wound area, total cells, neutrophil, and lymphocyte compared to other groups in rats.
2. Experimental section
2.1. Material
Bovine serum, antimycotic antibiotic solution, 2,2-diphenyl-1- pikrilhydrazil (DPPH), dimethyl sulfoxide (DMSO), decamplmaneh fetal, 4- (Dimethylamino) benzaldehyde, hydrolyzate, Ehrlich solution, and borax-sulfuric acid mixture, DMED, all were afforded from the US Sigma-Aldrich company.
2.2. Synthesis of Fe3O4/starch
A typical co-precipitation method was followed in ammoniacal medium to synthesize the Fe3O4 NPs as reported earlier [Citation18]. 0.5 g of Fe3O4 was dispersed in water (100 mL) by sonication for 20 min. In a separate container, 0.5 g starch was dissolved in 100 mL water at 70 °C. This was added dropwise to the ferrite suspension, sonicated for 20 min followed by stirring for 5 h at 70 °C. The composite was isolated using a magnet and rinsed successively with water and ethanol. The material was dried at 60 °C under vacuum.
2.3. Synthesis of Fe3O4/Starch-Au
0.5 g of the Fe3O4/Starch was suspended in H2O (50 mL) and sonicated for 20 min. Then 30 mg HAuCl4 was dissolved in 20 mL H2O and added to the previous suspension. The mixture was refluxed at 100 °C for 2 h. The Au3+ ions were reduced to Au NP by the oxy-functional groups of starch. The resulting solid (Fe3O4/Starch-Au) was separated by an external magnet. It was washed thoroughly with aqueous acetone and then dried in air. According to ICP-OES analysis the gold content was 0.015 mmol/g.
2.4. Determination of the antioxidant activities of Fe3O4/Starch-Au nanocomposite
In this method, DPPH was used as the standard as a stable radical compound. Thus, 50 µl of different concentrations of 1-1000 μg/ml of nanoparticle was added to 5 ml of 0.004% DPPH solution in methanol. After 30 min of incubation at room temperature, the samples were read against Blank at 517 nm. The percentage of inhibition of DPPH free radicals was calculated using the following formula [Citation19–22]:
In this test, the ability of hydrogen or electron atoms to be given by nanoparticle is measured by the amount of decolorization of purple solutions 2 and 2-diphenyl-1-picryl hydrazyl or DPPH in methanol. Then the concentration of the nanoparticle with a radical inhibition percentage of 50 was calculated by the graph. Obviously, the smaller this number, the greater the antioxidant power or inhibition of free radicals. In this experiment, the synthetic antioxidant butylated hydroxytoluene was used as a positive control and all experiments were repeated three times [Citation23–25].
2.5. Evaluation of cytotoxicity properties of Fe3O4/Starch-Au nanocomposite
The human normal cell line (Human umbilical vein endothelial cells (HUVEC)) in the MTT assay were used as follows.
In the recent study, the cells were cultured in medium (RPMI1640 = Roswell Park MemoryL Institute1640) with 10% FBS combined with penicillin and streptomycin antibiotics in an incubator containing 5% CO2 in a flask (T25). After three passages for purification, the cells were used to perform the next steps. Cell count and the number of viable cells were performed with a homocytometer slide using trypan blue. Evaluation of the cytotoxic effect of the Fe3O4/Starch-Au nanocomposite was performed by the modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric test. In this method, MTT, which is yellow, is converted to insoluble and formazan purple dye by the dehydrogenase enzymes in the mitochondria of active cells. The adsorption of this compound can be measured after dissolving at 570-540 nm. After two days and covering the flask bottom with cell, the cell layer adhering to the flask bottom was isolated enzymatically using trypsin-EDTA (5%) (Tetraacetic acid ethylenediamine), after transfer to sterile test tubes, it was centrifuged at 2000 rpm for 10 min. The cells were then resuspended in a fresh culture medium with the help of a pasteur pipette and cell suspension (106 ml/μg) was prepared from them. 40 μl of this cell suspension (equivalent to 104×4 cells) was poured into 96-well plate flat-bottomed wells (for cell culture). Then the final volume of each well with 10% FBS medium reached 200 μl. The first-row containing cell suspension was considered as negative control (control). After incubation for 18-24 h to remove cells from the stress caused by trypsinization, the supernatant was removed slowly and carefully, A new medium was added to all rows with different concentrations of the Fe3O4/Starch-Au nanocomposite (only a new medium was added to the negative and positive control rows), so that the diluted Fe3O4/Starch-Au nanocomposite with concentrations of 1-1000 μg/ml was added to the third to sixth rows, respectively, the plate was incubated in CO2 for 48, 24 and 72 h. After the incubation time, the plate was taken out of the incubator and 20 μl of MTT (Sigma) was added to all wells, and incubated for 3 h. The supernatant was then gently removed and 100 μl of DMSO was added to the wells and pipetted to dissolve the formazan crystals. The amount of light absorption (OD) according to the intensity of the blue color of formazan at 540 nm was read by Eliza reader. To convert OD to the percentage of living cells, the following formula was used and the percentage of cell life at each concentration was calculated after 48, 24 and 72 h [Citation23–25]:
The concentration of the tested compounds that reduced the percentage of cell life by half was considered as IC50 (The half maximal inhibitory concentration).
2.6. Cutaneous wound healing design
Sixty male, ten-week-old Sprague Dawley rats weighing 210-220 g were used. The rats were housed separately in transparent plastic cages in a quiet room with dim lighting in a light/dark cycle of 12 h at an ambient temperature of 21 °C and humidity of 50- 60%. They were provided ad libitum access to standard water and food pellets throughout the experiment. After induction of anesthesia by 40 mg/kg ketamine, a wound (2 × 2cm) was made by a scalpel, which involved the removal of all cutaneous layers. The depth of the wound included dermis and hypodermis. After creating the cutaneous wound, the rats were randomly divided into six groups: treatment with 0.2% Fe3O4/Starch-Au NPs ointment, treatment with 0.2% Fe3O4 ointment, treatment with 0.2% Fe3O4/Starch NPs ointment, treatment with 3% tetracycline ointment, treatment with Eucerin basal ointment, and untreated control. The ointment was applied to the wound bed for 10 consequent days. On day 10 after complete anesthesia by 40 mg/kg ketamine, a sample was taken from the wound in each group. Histological sections were equally divided into half, half of which was sent to the laboratory in 10% formalin. In the histopathological study, the number of total cells, blood vessel, fibrocyte, fibroblast, neutrophil, lymphocyte, and macrophage and ratio of fibrocyte to fibroblast were measured by an optic microscope. Biochemical studies by determining of hydroxyl proline, hexosamine, and hexuronic acid concentrations were performed on another half of the samples [Citation21–25].
2.7. Qualitative measurement
The obtained results were analyzed by SPSS (version 20) software using one-way ANOVA, followed by Duncan post-hoc test (p ≤ 0.01).
3. Results and discussion
3.1. Catalyst characterization data analysis
The Fe3O4/Starch-Au magnetic material was synthesized following a stepwise post-functionalization approach (Scheme 1). The surface functional groups of starch provided marked stability to the synthesized Au NPs. It was then physico-chemically characterized by advanced analytical techniques like FT-IR, FESEM, TEM, EDX, VSM, XRD and ICP-OES.
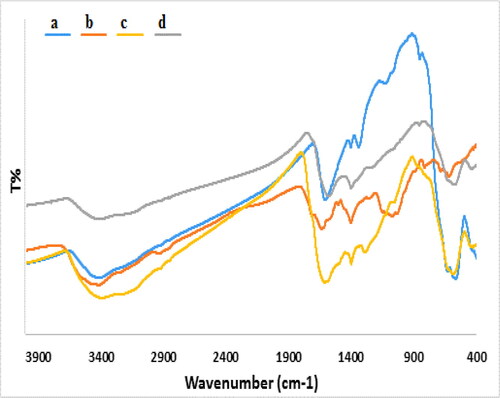
The sequential synthesis of Fe3O4/Starch-Au nanocomposite was rationalized by comparing its FT-IR spectra with all the corresponding precursors being demonstrated in . The Bare unmodified Fe3O4 NPs shows strong absorption peaks in the frequency range 585-637 cm−1 due to Fe-O-Fe stretching (). The broad absorption at around 3400 cm−1 is attributed to ferrite surface hydroxyl groups. The characteristic absorption peaks of starch () were observed at 1656 cm−1 (O-H bending), 1386 cm−1 (C–O stretching), 1158 cm−1 (asymmetric C–O–C stretching) and 1087 cm−1 (C–C stretching) respectively attributed to its functional groups. In the spectrum of Fe3O4/Starch composite, the featured peak due to Fe–O–Fe was slightly moved to 582 cm−1 () although the other peaks corresponding to starch remained unchanged indicating fruitful attachment of starch over Fe3O4 NPs. Noticeably, the peak intensity at ∼3300-3400 cm−1 was reduced in the spectrum of Fe3O4/Starch-Au () due to strong complexation between the nano-organic composite and Au NPs.
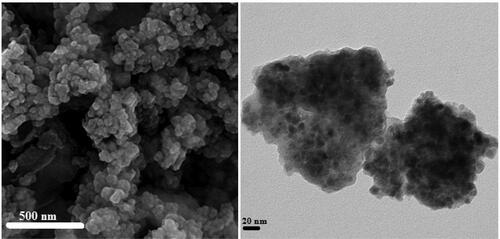
The detailed morphological structure, shape and size of the Fe3O4/Starch-Au nanocomposite was ascertained by SEM and TEM studies. The globular shape nanoparticles can be observed from SEM image having mean diameter of ∼20 nm (). The surface modification over Fe3O4 NPs by starch molecule can be adjudged from its appearances. There occurs a homogeneous growth of starch over it. The agglomeration of nanocomposite particles can be understood due to manual sample preparation. The inherent structural features are demonstrated via TEM study of the nanocomposite. On careful observation, the thin layer of starch surrounding the black colored Fe3O4 NPs can be visible (). Size of the composite particles were in accordance with SEM study and are around 10-20 nm.
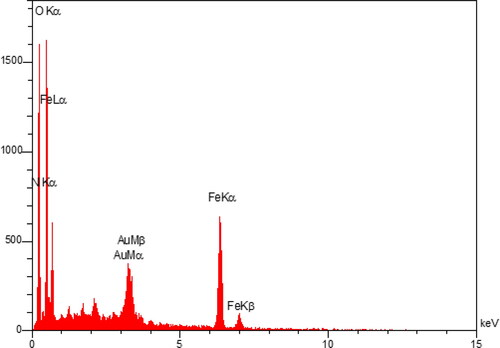
In order to have the information about the elemental composition of the nanocomposite EDX analysis was carried out. The corresponding profile exhibits the presence of Fe, O, C and Au atoms ().
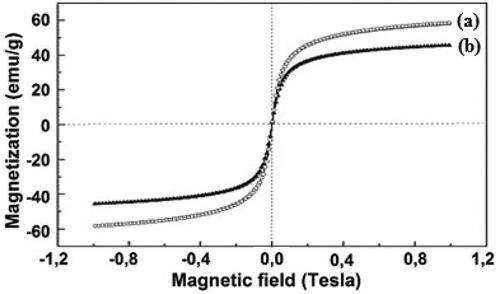
Magnetic properties of the naked Fe3O4 NP and Fe3O4/Starch-Au nanocomposite were evaluated by VSM study at 300 K. Against a variable applied magnetic field (-9000 to +9000 Oe), the hysteresis curves obtained for the two materials are depicted in . The patterns clearly specify that they are superparamagnetic in nature and their corresponding magnetization saturation (Ms) values were 59.5 and 43.8 emu/g respectively. The lower magnetization value for the nanocomposite can be accounted for the incorporation of non-magnetic starch and Au NPs over Fe3O4 surface. Still, the latter stands up with adequate magnetism and can be easily attracted by external magnets (inset ).
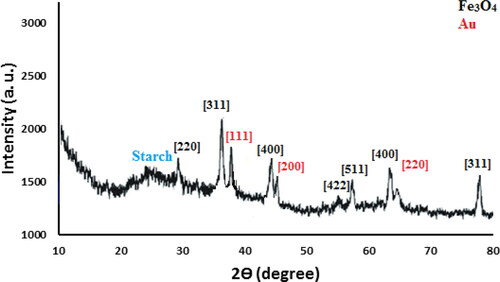
The crystalline phases of Fe3O4/Starch-Au nanocomposite was determined by XRD which has been shown in . As can be seen, the XRD profile reflects the characteristic peaks of standard cubic spinel Fe3O4 NPs appeared at 2θ = 30.4°, 35.9°, 43.2°, 54. 1°, 57.2° and 63.1°. These correspond to the (220), (311), (400), (422), (511) and (440) diffraction planes ((JCPDS No. 19-0629). The pattern signifies that the core structure left unaffected even after post-functionalizations. However, the additional peaks observed at 2θ = 38.6°, 44.3°, 64.6° and 77.6° are assigned to the (111), (200), (220) and (311) diffraction planes of Au fcc crystalline phases.
3.2. Study of the antioxidant and cytotoxicity properties of Fe3O4/Starch-Au
Free radicals are molecules with a free electron ready to react, and oxygen is produced with some molecules. If many of them are suddenly produced in the body, they react with some parts of the cell, such as DNA and cell membranes, and cause cell damage or even death. Normally, the body's defense system neutralizes these harmless free radicals [Citation19–22]. Antioxidants prevent the spread of oxidation chain reactions. Thus, the strength of an antioxidant formed by the contact of an H atom with a free radical is due to the effect of an antioxidant on the ease with which this H atom separates from it [Citation20–23]. Thus, antioxidants can protect cell membranes and various living compounds against oxidants in small amounts. Numerous biochemical and physiological processes may cause the production of free radicals. Reactive oxygen species (ROS) include free radicals and radical-free forms [Citation22–25]. Free radicals include hydrogen peroxide (H2O2), hydroxyl radical (.OH), and superoxide anion radical (O2). When the concentration of ROS increases, it can oxidize macromolecules such as proteins, nucleic acids, and membrane lipids, resulting in cell damage and possibly "cell and tissue destruction". Natural compounds and molecules have two main mechanisms for reducing the concentration of ROS, in other words, natural compounds and molecules reduce the concentration of ROS by producing antioxidants and thus prevent cell damage [Citation21–24]. Recently, many researchers have paid close attention to natural compounds and molecules and their relationship to their antioxidant properties, and many natural compounds and molecules have been studied for their antioxidant activity [Citation23–25].
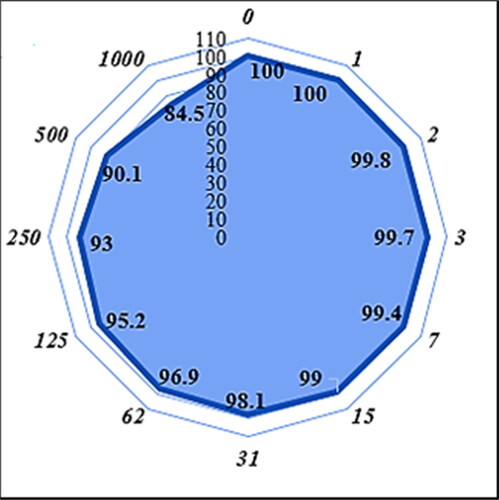
In the recent study, the concentration of 1000 µg/mL or high concentration showed the best result. Also, the antioxidant properties increased as dose dependent (). Fe3O4/Starch-Au nanoparticles revealed the most remarkable inhibition activities against free radicals. BHT revealed similar antioxidant activities compared to the Fe3O4/Starch-Au. The exact IC50 of butylated hydroxytoluene and Fe3O4/Starch-Au nanocomposite were 203 and 156 µg/mL, respectively ().
Figure 6. The antioxidant properties of nanocatalyst (A) and BHT (B) against DPPH.
The numbers indicate the percents of free radical (DPPH) inhibition in the concentrations of 0-1000 μg/mL of nanocatalyst (A) and BHT (B).
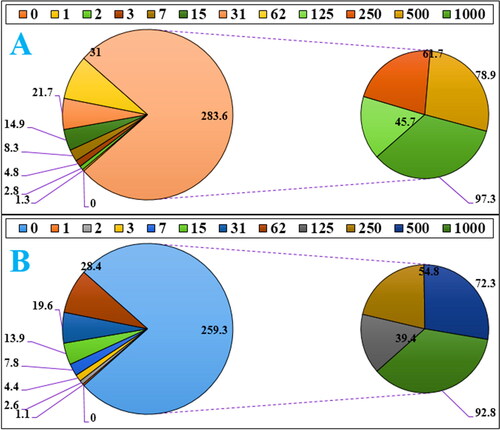
Table 1. The IC50 of nanocatalyst and BHT in the DPPH assay.
Free radicals are destructive compounds that are produced as a by-product by the body's chemical reactions and are destroyed by the body's defense system and enzyme system and antioxidants [Citation26–29]. However, in cases where the body's metabolic disorders and the production of free radicals are high and they are not destroyed by the neutralizing system, due to their instability, these compounds have a strong tendency to react with a variety of molecules in the body. It is estimated that each cell in the human body is exposed to free radicals 10,000 times a day and DNA strands 5,000 times a day [Citation29–34]. Damage to cell components includes proteins (genetic disorder), fats (lipid oxidation), and cell membranes (permeability disorder) that if the damage is not repaired, it leads to disruption of the chemical reaction and normal proteinization of the cell and the formation of harmful compounds and sometimes cancer cells in the body [Citation31–33]. It is reported that thousands of cancer cells are produced daily in the human body that are killed by the body's defense system. In some cases, due to dysfunction of the above systems, cancer cells proliferate and conditions for cancer development in different tissues. According to the above, antioxidants play a vital role in preventing disorders caused by the effects of free radicals and thus the prevention and treatment of cancer [Citation30–34]. Antioxidants are a wide range of molecular compounds with complex properties that combine with and neutralize free radicals. The results show that more than 60,000 types of molecular antioxidants have been identified so far. Antioxidants can be effective in three known ways to prevent and treat cancer; 1. Destruction of free radicals 2. Strengthen the immune system to destroy cancer cells. Prevent the adhesion of cancer cells to other cells and prevent their proliferation [Citation28–34].
In our research, the treated cell lines with Fe3O4/Starch-Au nanocomposite were tested by a well-known cytotoxicity test, i.e. MTT test for 72 h regarding the cytotoxicity activities on normal (Human umbilical vein endothelial cells (HUVEC)) cell line (). Fe3O4/Starch-Au nanocomposite didn’t show any cytotoxicity against HUVEC cells in the MTT assay.
3.4. Cutaneous wound healing potential of Fe3O4/Starch-Au nanocomposite
The results of macroscopic, microscopic, and biochemical studies showed that Fe3O4/Starch-Au nanocomposite ointment significantly decreased the percent of wound area, number of total cells, neutrophils, and lymphocytes and significantly increased the percent of wound contracture, number of blood vessels, fibrocyte, fibroblasts, fibroblast/fibrocyte ratio, and concentration of hydroxyproline, hexosamine, and hexuronic acid compared to other studied groups (). Studies have shown that the amount of angiogenesis at the wound site increases with the incidence of the wound, which causes more migration of fibroblasts to this site [Citation35, Citation36]. Then, the fibroblasts induce the production of collagen (proline, hydroxyl proline, and glycine) and extracellular matrix compounds like hexosamine and hexuronic acid [Citation37, Citation38]. After a while, the fibroblast is changed into fibrocyte. The ability of fibrocyte in wound healing is by far higher than that of fibroblast [Citation39]. Further, inflammatory cells, i.e. macrophages, neutrophils, and lymphocytes migrate to the wound site after angiogenesis [Citation40]. High activities of inflammatory cells increase the presence of pus in the region of wounds. The antioxidant compounds such as metallic nanoparticles reduce the extra activities and accumulation of the metallic nanoparticles in the region of the wounds [Citation41].
Table 2. The level of macroscopic parameters in experimental groups (p ≤ 0.01).
Table 3. The level of microscopic parameters in experimental groups (p ≤ 0.01).
Table 4. The level of biochemical parameters in experimental groups (p ≤ 0.01).
4. Conclusion
Finally, we conclude to introduce an Au NP impregnated and pectin covered magnetic core-shell nanomaterial (Fe3O4/Starch-Au). Over the nanoferrite core stepwise post-functionalization technique was used in the methodology. The starch shell was utilized as a protective shell to the Fe3O4 NPs as well as a green reductant for the in situ synthesis of Au NPs. In addition, the polar environment fashioned by the biomolecule help to disperse the surface Au NPs thus inhibiting their self-aggregation. Structural features were analyzed through a wide range of analytical techniques. The Fe3O4/Starch-Au nanocatalyst showed the best antioxidant activities against DPPH. Use of Fe3O4/Starch-Au nanocomposite ointment, significantly, (p ≤ 0.01) raised the wound contracture, vessel, hydroxyl proline, hexuronic acid, fibrocyte, fibroblast, and fibrocytes/fibroblast rate and significantly (p ≤ 0.01) decreased the wound area, total cells, neutrophil, and lymphocyte compared to other groups in rats. After clinical study Fe3O4/Starch-Au nanocatalyst can be utilized as an efficient drug for cutaneous wound healing in humans.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Oliveira Mussel RL, Sá Silva E, Costa AMA, et al. Mast cells in tissue response to dentistry materials: an adhesive resin, a calcium hydroxide and a glass ionomer cement. J Cellular Mol Med. 2003;7(2):171–173.
- Kumar B, Vijaykumar M, Govindarajan R, et al. J Ethnopharmacol. 2008; 12:103–110.
- Guo, L. A. Dipietro S. CROBM Online. 2010; 89:219–229.
- Souba WW, Wilmore D. Diet and nutrition in case of the patient with surgery. 9th ed, Baltimore, MD: Williams and Wilkins Press. 1999. 1589–1618.
- Hajialyani M, Tewari D, Sobarzo-Sánchez E, et al. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. IJN. 2018;Volume 13:5023–5043.
- Sivaraj R, Pattanathu K, Rajiv P, et al. Biogenic copper oxide nanoparticles synthesis using tabernaemontana divaricate leaf extract and its antibacterial activity against urinary tract pathogen. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2014;133:178–181.
- Mahdavi B, Saneei S, Qorbani M, et al. Appl Organometal Chem. 2019:e5164.
- (a) Veisi H, Hemmati S, Safarimehr P, In situ immobilized palladium nanoparticles on surface of poly-methyldopa coated-magnetic nanoparticles (Fe3O4@PMDA/Pd): a magnetically recyclable nanocatalyst for cyanation of aryl halides with K4[Fe(CN)6]. J. Catal. 2018;365:204–212. (b) H, Veisi S, Razeghi P, Mohammadi S, Hemmati Mater. Sci. Eng. C. 2019; 97624:–631. (c) H, Veisi SB, Moradi A, Saljooqi P, Safarimehr Mater. Sci. Eng. C. 2019; 100445:–452. (d) Nodehi M, Baghayeri M, Ansari R, & Veisi H, Mater. Chem. Phys. 244122687. 2020; e) Veisi H, Mohammadi L, Hemmati S, Tamoradi T, & Mohammadi P, ACS Omega. 413991:–14003. 2019; f) Taheri S, Veisi H, & Hekmati M, New J. Chem. 415075:–5081. 2017; g) Veisi H, Hemmati S, & Safarimehr P, J. Catal. 365204:–212. 2018; h) Tamoradi T, Veisi H, Karmakar B, Gholami J. Mater. Sci. Eng. C. 107110260:–110270. 2020.
- Oueslati MH, Tahar LB, Harrath AH. Catalytic, antioxidant and anticancer activities of gold nanoparticles synthesized by kaempferol glucoside from lotus leguminosae. Arab. J. Chem. 2020;13(1):3112–3122.
- Sun B, Hu N, Han L, et al. Anticancer activity of green synthesised gold nanoparticles from marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):4012–4019.
- Wu T, Duan X, Hu C, et al. Synthesis and characterization of gold nanoparticles from abies spectabilis extract and its anticancer activity on bladder cancer T24 cells. Artif Cells Nanomed Biotechnol. 2019;47(1):512–523.
- Sarina S, Waclawik ER, Zhu H. Photocatalysis on supported gold and silver nanoparticles under ultraviolet and visible light irradiation. Green Chem. 2013;15(7):1814–1833.
- Wang DM, Duan HC, Lü JH, et al. Fabrication of thermo-responsive polymer functionalized reduced graphene oxide@Fe 3 O 4 @Au magnetic nanocomposites for enhanced catalytic applications. J Mater Chem A. 2017;5(10):5088–5097.
- Pardo IR, Pons MR, Heredia AA, et al. Fe3O4@Au@mSiO2 as an enhancing nanoplatform for rose bengal photodynamic activity. Nanoscale. 2017;9(29):10388–10396.
- Suchomel P, Kvitek L, Prucek R, et al. Sci. Rep. 2018;8:1–11.
- Amirmahani N, Rashidi M, Mahmoodi NO. Appl. Organometal. Chem. 2020:e5625.
- Gutierrez L-F, Hamoudi S, Belkacemi K. Synthesis of gold catalysts supported on mesoporous silica materials: recent developments. Catalysts. 2011;1(1):97–154.
- Veisi H, Ghorbani M, Hemmati S. Sonochemical in situ immobilization of Pd nanoparticles on green tea extract coated Fe3O4 nanoparticles: an efficient and magnetically recyclable nanocatalyst for synthesis of biphenyl compounds under ultrasound irradiations. Mater Sci Eng C Mater Biol Appl. 2019;98:584–593.
- Zangeneh M, Bovandi MS, Gharehyakheh S, et al. Appl. Organometal. Chem. 2019;33:e4961.
- Zangeneh MM, Joshani Z, Zangeneh A, et al. Appl. Organometal. Chem. 2019;33:e5016.
- Zangeneh A, Zangeneh MM, Moradi R. Appl. Organometal. Chem. 2020;34:e5247.
- Zangeneh M, Zangeneh M, Pirabbasi AE, et al. M. Almasi. Appl. Organometal. Chem. 2019;33:e5246.
- Mahdavi B, Paydarfard S, Zangeneh MM, et al. Appl. Organometal. Chem. 2019;33:e5248.
- Zangeneh MM, Pooyanmehr M, Zangeneh A. Biochemical, histopathological, and pharmacological evaluations of cutaneous wound healing properties of quercus brantii ethanolic extract ointment in male rats. Comp Clin Pathol. 2019;28(5):1483–1493.
- Jalalvand AR, Zhaleh M, Goorani S, et al. Chemical characterization and antioxidant, cytotoxic, antibacterial, and antifungal properties of ethanolic extract of allium saralicum R.M. Fritsch leaves rich in linolenic acid, methyl ester. J Photochem Photobiol B. 2019;192:103–112.
- Zangeneh A, Zangeneh MM. Appl. Organometal. Chem. 2020;34:e5290.
- Zangeneh MM, Zangeneh A. Appl. Organometal. Chem. 2020;34:e5271.
- Hemmati S, Joshani Z, Zangeneh A, et al. Appl. Organometal. Chem. 2020;34:e5267.
- Zhaleh M, Zangeneh A, Goorani S, et al. In vitro and in vivo evaluation of cytotoxicity, antioxidant, antibacterial, antifungal, and cutaneous wound healing properties of gold nanoparticles produced via a green chemistry synthesis using gundelia tournefortii L. as a capping and reducing agent green synthesis of gold nanoparticles using gundelia tournefortii L. Appl Organometal Chem. 2019;33:e5015.
- Shahriari M, Hemmati S, Zangeneh A, et al. Appl. Organometal. Chem. 2019;33:e5189.
- Zangeneh MM, Saneei S, Zangeneh A, et al. Appl. Organometal. Chem. 2019;33:e5216.
- Zangeneh MM. Appl. Organometal. Chem. 2019;33:e4963.
- Ahmeda A, Zangeneh A, Zangeneh MM. Appl. Organometal. Chem. 2020;34:e5378.
- Zangeneh MM. Appl. Organometal. Chem. 2020;34:e5295.
- Phillips GD, Whitehead RA, Knighton DR. Initiation and pattern of angiogenesis in wound healing in the rat. Am J Anat. 1991;192(3):257–262.
- Lazarus GS, Cooper DM, Knighton DR, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–493.
- Azhdari-Zarmehri H, Nazemi S, Ghasemi E, et al. JBUMS. 2014; 16:42–48.
- Caetano GF, Fronza M, Leite MN, et al. Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm Biol. 2016;54(11):2555–2559.
- Dwivedi D, Dwivedi M, Malviya S, et al. Evaluation of wound healing, anti-microbial and antioxidant potential of pongamia pinnata in wistar rats. J Tradit Complement Med. 2017;7(1):79–85.
- Nayak BS, Isitor G, Davis EM, et al. The evidence based wound healing activity ofLawsonia inermis linn. Phytother Res. 2007;21(9):827–831.
- Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23.