Abstract
Silver nanoparticles have been synthesised by chemical reduction of silver nitrate solution by ethanol. The samples have been characterised by X-ray diffraction study. Transmission electron microscopy reveals the nanostructure of the particles produced. This study infers that the particles are mostly spherical in shape and the sizes are within 18 nm. The surface plasmon resonance peak in the UV-Vis absorption spectra shows maximum absorption at 422 nm. Fluorescence spectra of silver nanoparticles, which show an emission peak at 470 nm have also been studied.
1. Introduction
Nanoparticle syntheses are of fundamental importance in the advancement of recent research Citation1–5. The synthesis of metal nanoparticles has received much attention because of their optical, electronic, magnetic and catalytic properties, which depend on their size and shape Citation2,Citation3. Moreover, noble metal nanoparticles show surface plasmon resonance (SPR) in the visible range as well as in the infrared range. The SPR phenomenon is due to the collective oscillation of free electrons of the metal nanoparticles in resonance with the frequency of the lightwave interacting with the metal nanoparticles. Basically, SPR absorption peak occurs in metal nanoparticle only. Hence, the existence of SPR peak is the primary signature of metal nanoparticle formation. In nanoparticle synthesis, it is very important to control not only the particle size, but also the particle shape and morphology as well. In the present investigation, we discuss the synthesis of silver nanoparticles by chemical reduction method Citation4, which is a simple and convenient route for preparing silver nanoparticles in nanometre range. The colloidal solution of silver nanoparticles has been examined under X-ray diffraction (XRD), transmission electron microscopy (TEM), UV-Vis absorption spectroscopy and photoluminescence spectroscopy (PL). It has been revealed that the prepared nanoparticles are within 18 nm, having months-long stability.
2. Synthesis of silver nanoparticles
Several approaches have been developed for the preparation of silver nanoparticles. Among them, the chemical approach is an easy process. Fine and uniform silver nanoparticles can be produced through the reduction of silver nitrate solution by ethanol at a temperature of 70–90°C under atmospheric conditions Citation4. In this synthesis process, 10 mL of aqueous solution containing silver nitrate (0.3 g of AgNO3), 1 g sodium linoleate (C18H32ONa), 5 mL ethanol and 1 mL linoleic acid (C18H32O2) are added in an autoclave tube under stirring. The system has been sealed and treated at the temperature between 70°C and 90°C for 5 h. In the aqueous solution of silver nitrate, sodium linoleate and the mixture of linoleic acid and ethanol is added in order. A solid phase of sodium linoleate, a liquid phase of ethanol and linoleic acid and water ethanol solution phase containing silver ions formed in the system. Ethanol in the liquid and solution phases reduced the silver ions. Along with the reduction process, linoleic acid is absorbed on the surface of the silver nanoparticles with the alkyl chains on the outside through which the produced silver nanoparticles gain hydrophobic surfaces. After cooling to room temperature, the produced silver nanoparticles have been dispersed in chloroform to form a homogenous colloidal solution. The colour of the colloidal solution of silver nanoparticle is reddish brown, as shown in .
3. Results and discussion
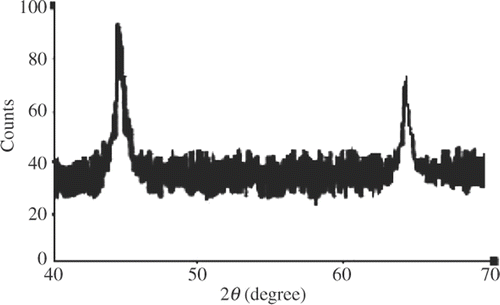
3.1. X-ray diffraction analysis
The structure of prepared silver nanoparticles has been investigated by XRD study. The XRD patterns of the sample prepared by the present reduction method are shown in . The XRD study indicates the formation of silver particles in the nanometre range. From this study, considering the peaks at 45.1 and 64.7 degrees, the average particle size is estimated by using Debye–Scherrer formula Citation6,Citation7:
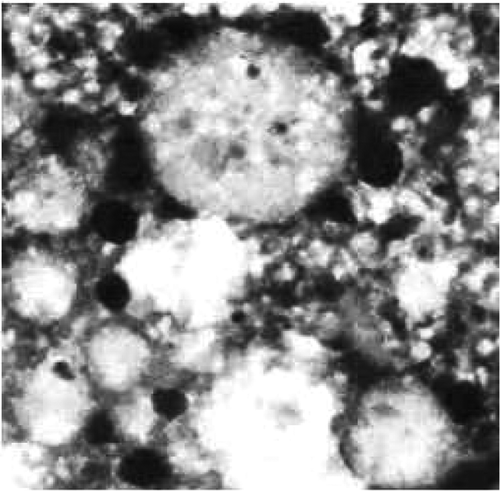
3.2. Transmission electron microscopy analysis
The TEM image of the prepared colloidal solution of silver nanoparticles is shown in . The Ag nanoparticles (black portion) are spherical in shape with smooth surface morphology. The diameter of the nanoparticles is found to be approximately 18 nm.
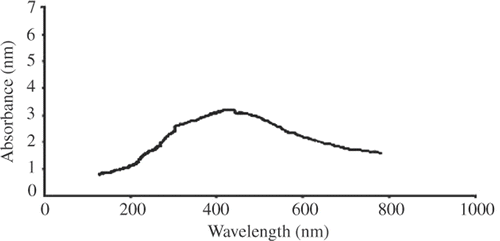
3.3. UV-Vis spectroscopy analysis
In metal nanoparticles such as in silver, the conduction band and valence band lie very close to each other in which electron moves freely. These free electrons give rise to a SPR absorption band Citation8,Citation9, occurring due to the collective oscillation of electrons of metal nanoparticles in resonance with the lightwave Citation6. This absorption strongly depends on the particle size, dielectric medium and chemical surroundings Citation9. The UV-Vis the spectrum of dilute dispersion of colloidal particles can be calculated from Mie theory Citation10. For small metal particles (diameter < 20 nm), absorption spectra significantly depend only on the dipole oscillation Citation5,Citation11. Theoretically and experimentally, it is found that when size decreases, the SPR peak shifts towards shorter wavelength side Citation5. It is also found that with the decrease in size, absorption spectra becomes weak and broad Citation5,Citation11. Similar is the case for fluorescence Citation12–16. This reduction method by ethanol produces spherical silver nanoparticles around 18 nm. The UV-Vis the absorption spectra of the silver nanoparticles dispersed in chloroform is shown in . The absorption (SPR) peak is obtained in the visible range at 422 nm, which is in good agreement with the theoretical result.
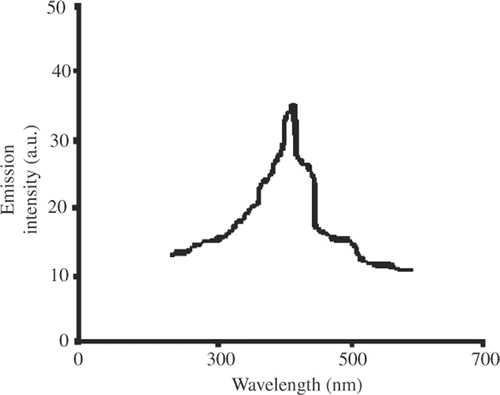
3.4. Fluorescence spectroscopy
In silver nanoparticles, the radiative recombination of electron hole pair between d-band and sp-conduction above the Fermi level produces emission Citation12–17, which occurs practically at 470 nm when excited with 350 nm of optical source. Moreover, the absorbed linoleic acid during the formation of silver nanoparticles further enhances the intensity of emission Citation12–14. Fluorescence spectra for silver nanoparticle are shown in . These spectra infer the possibility of silver nanoparticle to be used as ‘nano laser’ with optical pumping.
4. Conclusion
We have prepared silver nanoparticles through the reduction of silver ions by ethanol, which was dispersed in chloroform. XRD analysis, UV-Vis spectra, and TEM image reveal the nanonature of the prepared samples. The size estimated from above studies is within 18 nm. SPR absorption peak is observed at 422 nm, whereas fluorescence peak is obtained at 470 nm.
Acknowledgements
The authors thank Dr D.K. Avasthi (Scientist H) Material Science, IUAC, New Delhi, India, Dr B. DKHR (SO) NEHU, Shillong, India, and Dr S. Sharma (S.O.), IIT, Guwahati, Assam, India, for their suggestions and assistance during the work.
References
- Nath , SS , Chakdar , D , Gope , G and Avasthi , DK . 2008 . Characterizations of CdS and ZnS quantum dots prepared by chemical method on SBR latex . J. Nanotechnol. , 4 : 1 – 6 .
- Cao , G . 2004 . Nanostructures and Nanomaterials , London : Imperial College Press .
- Reed , MA and Lee , T (eds.) . 2003 . Molecular Nanoelectronics , Valencia, CA : American Scientific Publishers .
- Wang , X , Zhuang , J , Peng , Q and Li , Y . 2005 . A general strategy for nanocrystal synthesis . Nature , 437 : 121 – 124 .
- Alvarez , MM , Khoury , JT , Schaaff , TG , Shafigullin , MN , Vezmar , I and Whetten , RL . 1997 . Optical absorption spectra of nanocrystal gold molecules . J. Phys. Chem. B , 101 : 3706 – 3712 .
- Nath , SS , Chakdar , D and Gope , G . 2007 . Synthesis of CdS and ZnS quantum dots and their applications in electronics . Nanotrends: J. Nanotechnol. App. , 2 Available at: http://www.nstc.in/journal/default.aspx?id=02-03
- Nath , SS , Chakdar , D , Gope , G and Avasthi , DK . 2008 . Effect of 100 Mev nickel ions on silica coated ZnS quantum dot . J. Nanoelectron. Optoelectron. , 3 : 1 – 4 .
- Taleb , A , Petit , C and Pileni , MP . 1998 . Optical properties of self assembled 2D and 3D superlattices of silver nanoparticles . J. Phys. Chem. B , 102 : 2214 – 2220 .
- Noginov , MA , Zhu , G , Bahoura , M , Adegoke , J , Small , C , Ritzo , BA , Drachev , VP and Shalaev , VM . 2007 . The effect of gain and absorption on surface plasmons in metal nanoparticles . Appl. Phys. B , 86 : 458 – 460 .
- Pileni , MP . 1998 . Optical properties of nanosized particles dispersed in colloidal solutions or arranged in 2D or 3D superlattices . New J. Chem. , 22 : 693 – 702 .
- Link , S and El-Sayed , MA . 2000 . Shape and size dependence of radiative, non-radiative and photothermal properties of gold nanocrystals . Int. Rev. Phys. Chem. , 19 : 409 – 453 .
- Treguer , M , Rocco , F , Lelong , G , Nestour , AL , Cardinal , T , Maali , A and Lounis , B . 2005 . Fluorescence silver oligomeric cluster and colloidal particles . Solid State Sci. , 7 : 812 – 818 .
- Bouheiler , A , Beversluis , MR and Novonty , L . 2003 . Characterization of nano plasmonics structures by locally excited photoluminescences . Appl. Phys. Lett. , 83 : 5041 – 5044 .
- Kalele , S , Despande , AC , Singh , SB and Kulkarni , SK . 2008 . Tuning luminescences intensity of RHO6G dye using silver nano particles . Bull. Mater. Sci. , 31 : 541 – 544 .
- Drachev , VP , Khaliullin , EN , Kim , W , Alzoubi , F , Rautian , SG , Safonov , VP , Armstrong , RL and Shalaev , VM . 2004 . Quantum size effect in two-photon excited luminescence from silver nanoparticles . Phys. Rev. B: Condens. Matter , 69 : 035318 – 035323 .
- Siwach , OP and Sen , P . 2008 . Synthesis and study of fluorescence properties of Cu nanoparticles . J. Nanopart. Res. , 10 : 107 – 114 .
- Liao , H , Wen , W and Wong , GKL . 2006 . Photoluminescence from Au nanoparticles embedded in Au: Oxide composite films . J. Opt. Soc. Am. B: Opt. Phys. , 23 : 2518 – 2521 .