ABSTRACT
As with much of science, the female athlete is under researched, particularly in the area of gastrointestinal (GI) physiology. Gut function is of pivotal importance to athletes in that it supports digestion and absorption of nutrients, as well as providing a barrier between the external environment and the circulation. While sex-derived differences in GI structure and function have been well characterised at rest, there remains a paucity of data examining this during exercise. The wider impact of the GI system has begun to be realised and it is now widely acknowledged to play a role in more systemic bodily systems. In the current review, we discuss localised issues including the GI structure, function, and microbiome of male and females. We also discuss GI-related symptoms experienced by athletes, highlight the differences in incidence between males and females, and discuss contributing factors. We then move beyond the gut to discuss wider biological processes that have been shown to have both sex-related differences and that are impacted by the GI system. Some of these areas include immune function and risk of illness, sleep, hormones, bone health and the gut–brain–axis. The magnitude of such effects and relationships is currently unknown but there is enough mechanistic data for future studies to consider a more central role that the gastrointestinal tract may play in overall female athlete health.
There are both clear similarities and differences in male-female gastrointestinal structure and function.
Females typically reported a greater prevalence of gastrointestinal symptoms at rest, in particular during menstruation, but not during exercise.
The links between female microbiome, oestrogen, and systemic physiological and biological processes are yet to be fully elucidated.
Many of the male-female differences seen (e.g. in immune function) may be, at least in part, influenced by such GI related differences.
Highlights
KEYWORDS:
Introduction
The canonical role of the gastrointestinal (GI) tract is digestion and absorption. Adequate macro- and micro-nutrients must be consumed and, subsequently, digested and absorbed in order to maintain health and athletic performance. However, the GI tract has also been shown to have wider-reaching physiological effects. For example, evidence has shown that the GI microbiome contributes to our immune function, regulates systemic levels of inflammation, and it has even been suggested to affect higher cognitive functions via the gut–brain axis (Eisenstein, Citation2016). This gut microbiome can be manipulated, for better or worse, through many everyday activities including diet, exercise, and antibiotics (Dalton, Mermier, & Zuhl, Citation2019). Despite the size and multitude of functions of the GI tract, it has rarely been thought of as an athletic organ. Comparatively less research has been conducted on GI structure and function in athletes compared with other physiological systems (e.g. the musculoskeletal system) and compared with the number of studies in clinical conditions (e.g. obesity, IBS). Recently it was demonstrated that the gut microbiome of athletes differs from non-athletes at a molecular and metabolic level (Barton et al., Citation2018). However, as with many areas of athletic research, the majority of studies investigating the GI system within the areas of sport and exercise have far fewer female participants. In addition, the effects of the GI tract exhibited beyond the gut have tended not to be considered in regard to sex differences in athletes (). The aim of the current review is to evaluate our current understanding of female-specific considerations in regard to GI function and highlight more systemic areas of female athlete health that may be affected by the gut.
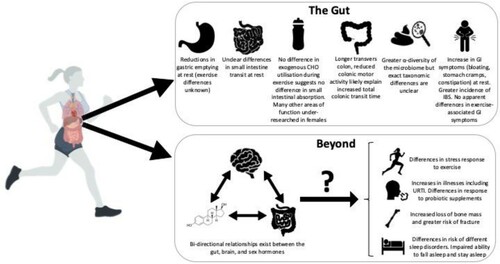
Figure 1. The gut and beyond: the central and systemic role of the gut in female athlete health. The Gut – While many aspects of GI structure and function are well characterised for females (particularly at rest), others are not (mainly during exercise). At a population level, there appear to be differences in the microbiome between males and females but data in regards to the exact taxonomic differences are inconsistent. Females generally suffer more GI symptoms at rest and there is a greater incidence of irritable bowel syndrome (IBS) in females compared to males. Beyond – there appears to be interconnected relationships between the gut, the brain, and female sex hormones. Given that there are known mechanisms by which these can affect other bodily systems, might they explain other differences between males and females in different aspects of athlete health?
Structure, function, and transit time
Differences exist between males and females in relation to transit times through the GI tract, although it should be acknowledged that there is large inter-individual variability (Graff, Brinch, & Madsen, Citation2001). Under resting conditions, gastric emptying and colonic transit times have been found to be slower in females, while differences in small bowel transit have been shown by some but not others (Graff et al., Citation2001; Southwell, Clarke, Sutcliffe, & Hutson, Citation2009). Differences in transit time may in part be due to differences in average size and length of digestive tract segments. The transverse colon is typically longer in females than males (Freire, Basit, Choudhary, Piong, & Merchant, Citation2011) and likely explains the increased colonic and total gut transit time. There may also be differences in colonic activity between males and females, with reduced motor activity in females (Rao, Sadeghi, Beaty, Kavlock, & Ackerson, Citation2001).
Studies investigating the effects of sex hormones on GI transit are inconclusive; some evidence suggests colonic transit time is prolonged during the luteal phase of the menstrual cycle, when progesterone levels are highest, whilst other studies show no difference in transit time throughout the menstrual cycle (Freire et al., Citation2011). For athletes, it is important to consider if such differences might impact nutrition guidelines and practices such as the timing and total volume of food consumed, before, during, and after training and competition. For example, there have been no differences reported in the capacity for females to absorb and utilise exogenous carbohydrates during exercise compared to males (Wallis, Dawson, Achten, Webber, & Jeukendrup, Citation2006). However, there are many other facets of sports nutrition that have predominantly been researched in males, such as protein absorption and subsequent muscle protein synthesis post exercise, ketone ingestion during exercise, and supplemental buffers (e.g. sodium bicarbonate) that require further exploration in females.
Gastrointestinal integrity
At the onset of exercise, blood is redistributed from the gastrointestinal tract to be used by more metabolically active tissues (e.g. skeletal muscle) (van Wijck et al., Citation2012). This redistribution can thus lead to GI ischemia and initiate epithelial injury, bacterial translocation, and an inflammatory cascade (reviewed in van Wijck et al., Citation2012 and Costa, Snipe, Kitic, & Gibson, Citation2017). A recent meta-analysis reported no sex-based differences on the effects of exercise on indirect markers of GI damage and permeability, although only 12% of participants from the included studies were female (Chantler et al., Citation2020). In a study investigating sex-based differences on GI integrity and subsequent inflammatory response to 2 h of running, no differences were seen between males and females (Snipe & Costa, Citation2018). In this study, however, all-female participants were in the follicular phase of the menstrual cycle. To date, data analysing exercise-associated GI integrity and menstrual cycle phase or contraceptive use are sparse.
The gut microbiome
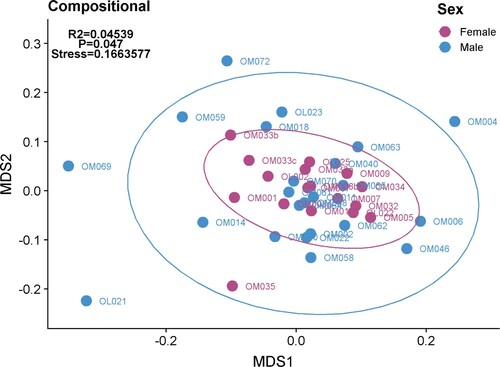
The gut microbiome plays a pivotal role in our body’s digestive, endocrine and immune functions. Recently the role of the microbiome in athletic performance has been explored, with increased microbial diversity and increases in species and metabolites associated with muscle turnover, recovery and protein breakdown being discovered (Barton et al., Citation2018; Scheiman et al., Citation2019). However, as with other areas of research the majority of these and other microbiome studies are in male-dominated studies. Research comparing the gut microbiomes of male and females in the general population are limited and inconclusive but many animal and human studies have shown sex differences in the microbiome (Kim, Unno, Kim, & Park, Citation2020). There is a general consensus that at the population level, the composition of the gut microbiota differs between sexes, where the α-diversity (i.e. Chao and Shannon) appears to be greater in females. However, exact differences in microbial taxa between sexes are inconsistent, and so it is difficult to draw conclusions. This is mirrored in athlete cohorts in that some studies show no differences (O’ Donovan, Connor, Madigan, Cotter, & O’Sullivan, Citation2020) and others show differences between genders at the diversity level (O’ Donovan et al., Citation2020). This may be due to the type of sport studied, as it has been demonstrated that different sports have varying effects on the microbiome (O’ Donovan et al., Citation2020). In these preliminary analyses of athletes though, it has been shown that when the microbiome composition of females and males are examined, there is a clustering between these groups (). That is, these data demonstrate that female athletes are more similar to each other than they are to male athletes. As can be seen in these plots though, there is large variability within the male and female groups. This highlights the difficulties and complexities of interpreting microbiome data given a large number of confounding factors that can modify the microbiome (diet, medications, living environment) and the effects of inter-study variability. Even when differences exist between males and females, there are a number of factors likely affecting sex differences in the gut microbiome. For example, gut transit time (described above) has been shown to correlate with a number of genera (Kim et al., Citation2020). Given that transit time more closely relates to body mass and size than with biological sex, other factors may have a greater impact on sex-derived differences in microbiome, such as sex hormones.
Figure 2. Overall gut microbiome composition was identified as varying between female and male cohorts. Multidimensional scaling (MDS) plot of microbial composition (species present) reveals clustering of individual samples from within the female cohort away from the male cohort. Individual samples represent those taken at a single time point from an individual. (O’Donovan et al., Citation2020)
One of the likely factors that would contribute to sex differences in the microbiome is sex hormones. An apparent bi-directional relationship between oestrogen levels and the gut microbiome has been identified (Markle et al., Citation2013) and, in mice, such associations have been shown to a greater extent in females than males (Kaliannan et al., Citation2018). Thus, there seems to be a reciprocal interaction between gut microbiota and sex hormones. It has been suggested that gut microbiota are capable of synthesising steroids, modifying oestrogen, and expressing hydroxyl-steroid dehydrogenases; enzymes responsible for regulating a balance between the active and inactive steroids (Jaggar, Rea, Spichak, Dinan, & Cryan, Citation2020). GI microbiome transfer from adult male mice to immature female mice was shown to not only alter the microbiota but also increase levels of testosterone (Markle et al., Citation2013).
GI symptoms – gender differences at rest and during exercise
It is important to consider the type and severity of GI symptoms for athletes; either those associated specifically with exercise or experienced during daily life. Such a difference is important to highlight given that the underlying aetiology, and subsequent therapeutic approaches, may be different. Although the majority of studies to date have focused on exercise-associated GI symptoms, there has been some limited data highlighting other functional GI symptoms experienced by athletes at rest. In a group of international level athletes, there was no significant difference in the prevalence of any of the GI symptoms examined between male and female athletes (O’Donovan et al., Citation2020). However, these GI symptoms were as a result of travel and only included 37 athletes (14 female). In a larger group of elite athletes (n = 249, 80 females), females reported a greater prevalence of nausea, hunger pains, bloating, and feelings of incomplete evacuation than males (Pugh, Fearn, Morton, & Close, Citation2018a). Such sex differences in GI symptom prevalence have long been established in the general population (Lovell & Ford, Citation2012). Although unexplored specifically in athletes, the potential mechanisms for such differences include differences in gut motility and transit time (as described above), visceral hypersensitivity, behavioural stress response, changes in gut microbiome, psychological factors, alterations in inflammatory and immune function, increases in GI permeability, intestinal motor and sensory functions, and hormones (Kim & Kim, Citation2018).
GI symptoms experienced by females have also been shown to increase during menstruation. Young, eumenorrheic female populations using oral contraception have reported increases in stool frequency, abdominal pain, diarrhea, and indigestion on the first day compared to any other day of the menstrual cycle (Judkins, Dennis-Wall, Sims, Colee, & Langkamp-Henken, Citation2020). A recent, large scale (n = 6812) investigation of exercising females reported that “stomach cramps” and “bloating/increased gas” was reported by over 30% of respondents during menstruation (Bruinvels et al., Citation2021). Elite female athletes referenced that GI symptoms associated with the menstrual cycle resulted in disrupted or altered training (Brown, Knight, & Forrest, Citation2021). Future work should consider the stage of the menstrual cycle when evaluating GI symptoms while more research is needed for the management of menstrual-related GI symptoms in female athletes.
Another factor to consider in athletes is the potential for undiagnosed incidences of irritable bowel syndrome (IBS). IBS is a chronic gut disorder that affects significant numbers of people in the general population. Meta-analysis data shows a prevalence of 11.2% amongst the general population, with females having a slightly higher prevalence (14.0%) compared to males (9.87%) (Lovell & Ford, Citation2012). To date, there is no data on the potential prevalence of IBS amongst elite athletes. A recent investigation found that IBS was underdiagnosed amongst competitive endurance athletes, with females having 4.9 times higher odds of IBS diagnosis (Killian & Lee, Citation2019). While differences in symptom prevalence between males and females were found at rest, there was no significant difference between symptoms experienced during or after training and competition. This distinction between those GI symptoms experienced at rest and those during exercise is of importance given the likely different aetiologies.
In regard to symptoms experienced during exercise, whilst a laboratory-based controlled study has shown a higher prevalence of GI symptoms in female athletes (Costa et al., Citation2017), field-based and observational data comparing male and female athletes is equivocal. For example, in recreational marathon runners, females have been shown to be more likely to report lower (e.g. abdominal cramps, diarrhoea, the urge to defecate) and upper GI symptoms (e.g. nausea) compared to males, (Keeffe, Lowe, Goss, & Wayne, Citation1984). More than twice as many female runners reported such symptoms negatively affected their running performance (Halvorsen, Lyng, Glomsaker, & Ritland, Citation1990). However, many of the studies that have reported differences in exercise-related GI symptoms have limitations and/or confounding factors, including a substantially lower number of female participants; with females tending to have less training experience, something that has been reported to be an independent factor associated with GI symptom prevalence (ten Haaf et al., Citation2014). In a study, where males and females both had a substantial training history (median 10 and 12 years for males [n = 70] and females [n = 75], respectively), no difference in GI symptom prevalence during training was seen between male and female runners (Wilson, Citation2017). These results are in agreement with data showing no difference in GI symptoms between male and female runners during a marathon race (Pugh, Kirk, Fearn, Morton, & Close, Citation2018b). Finally, in one of the few laboratory-controlled studies, eumenorrheic females in the follicular phase of the menstrual cycle had similar GI and systemic cytokine responses to males during a 2-hr run in the heat (Snipe & Costa, Citation2018). Therefore, while early studies appeared to show a greater prevalence of GI symptoms in female athletes compared to males, more recent investigations have shown no difference in exercise-associated GI symptoms. These studies have begun to better address the potential confounding factors of sample size proportion, training history, and menstrual cycle timing. Future studies should focus on disparities in other areas such as habitual intake during exercise as participants may self-adjust in order to prevent GI symptoms. In addition, studies to date have been limited to endurance-based athletes and so it is unknown if there are sex differences in exercise-associated GI symptoms in other forms of exercise.
A final consideration for GI-related symptoms is low energy availability (EA). Adequate EA, ensures that athletes have energy available for basic physiological functions, and it is known to positively impact health and performance (Mountjoy et al., Citation2018). The term Relative Energy Deficiency in Sports (RED-S) has been described and refers to low EA (LEA) in both male and female athletes, although it is more common in females compared to male athletes (Mountjoy et al., Citation2018). GI complaints are also commonplace in athletes where RED-S or the Female Athlete Triad is observed and is specifically included as a symptom that is assessed (Mountjoy et al., Citation2018). Specifically, delayed gastric emptying, constipation and increased intestinal transit time have all been reported (Melin et al., Citation2014). The mechanisms for such findings have not been well explored. While the research to date is limited to Anorexia Nervosa, there is growing evidence suggesting that related starvation is associated with profound alterations of the gut microbiome (Mörkl et al., Citation2017). As this data may relate to extreme LEA, changes to the gut microbiome should be examined following a more modest period of low EA, or low EA that is characterised by very high energy expenditure rather than severely restricted energy intake.
Beyond the gut – do sex differences in the GI tract impact other bodily systems?
There are many sex-related differences in a number of sport-related measures. For example, there are differences in the incidence of particular injuries and illness, differences in bone health, differences in sleep patterns, the gut–brain–axis and, more specifically for females, oestrogen. While there are clearly many factors that might explain some of the variance, the role of gut has often been unexplored. In the wider literature, there is an increasing appreciation that the GI tract has further reaching, systemic effects on human health. There are numerous mechanisms by which the GI tract likely interacts either directly or indirectly with these bodily process beyond the gut (). In the sections below, we highlight some of these areas, as well as speculate how sex-derived gut differences may play a role (of unknown magnitude) in sex-derived differences in these other bodily systems.
Oestrogen
In athletes, low oestrogen levels have been associated with reduced bone mineral density (Wolman, Citation1990). This is a result of the negative effect of low oestrogen levels has on calcium absorption from the GI tract, thereby reducing the availability of calcium for bone reabsorption, leading to continued bone loss (Nelson et al., Citation1986). Conversely, high levels are associated with reduced power and performance, through the negative effect on connective tissue stiffness, which can also result in higher injury rates (Chidi-Ogbolu & Baar, Citation2019). This is best exemplified by the higher rates of anterior cruciate ligament rupture in the pre-ovulatory and ovulatory phases of the menstrual cycle, when oestrogen levels are highest (Lefevre, Bohu, Klouche, Lecocq, & Herman, Citation2013). Oestrogen concentrations are associated with gut microbiome alpha diversity and fecal Clostrdia taxa (Flores et al., Citation2012). A reduction in the diversity of the gut microbiome negatively impacts β-glucuronidase activity and subsequent levels of oestrogen (Baker, Al-Nakkash, & Herbst-Kralovetz, Citation2017). The resultant hypo-oestrogenic pathologies may include metabolic syndrome, obesity, cardiovascular disease, and cognitive decline. Changes in oestrogen levels can also modulate synaptic plasticity and neuronal circuits that can ultimately influence stress and behaviour (Jaggar et al., Citation2020). The short-chain fatty acid (SCFA) producing genera Prevotella, Ruminococcus and Roseburia are reported to depend on sex and hormonal status (Jaggar et al., Citation2020). Studies to date though have focused on animal models, clinical populations and menopausal females. Future studies may then consider the bidirectional relationship between the microbiome and oestrogen levels in female athletes and the role this may play in aspects of physical and psychological wellbeing and, subsequently, performance.
The gut–brain–axis
Activation of the sympathetic and parasympathetic divisions of the autonomic nervous system (ANS) result in an increase in noradrenaline and other neurotransmitters which flood the peripheral tissues, including the GI tract. This link with the GI tract is known as the gut–brain–axis, which is the term given to link between the central (CNS) and enteric nervous systems (ENS), which further incorporate the ANS and the immune and endocrine systems to facilitate communication. Alterations in the gut microbiome can be communicated to the CNS directly via the vagus nerve, resulting in an appropriate CNS response (Dalton et al., Citation2019), or via other pathways, including neurotransmitters. While data in female athletes is lacking, the bi-directional relationship between some neurotransmitters and the GI tract could in part explain differences in GI function and symptom prevalence between male and female athletes. For example, gamma-aminobutyric acid (GABA) is the predominant inhibitory neurotransmitter in the CNS and can be produced by commensal gut bacteria (Clarke et al., Citation2013). In the GI tract, it affects motility, oesophageal sphincter relaxation and transit time (Hyland & Cryan, Citation2010). Jiang and colleagues (Citation2019), reported that, in mice, higher circulating oestrogen resulted in reductions in gastric motility via differences in the modulation of GABAergic modulation of vagal output to the stomach.
Beyond GI symptoms, the gut–brain–axis may have further-reaching impact. The demands of exercise can evoke a large stress response, which activates the adrenomedullary and hypothalamus–pituitary–adrenal (HPA) axes (Dalton et al., Citation2019). The downstream effects include a release of catabolic and stress hormones and inflammatory cytokines. The HPA axis is activated to a greater extent in females compared to males, believed to be, in part, due to the effect of oestrogen, with ovariectomy and oestradiol substitution showing attenuated and stimulatory effects of the HPA, respectively (Verma, Balhara, & Gupta, Citation2011). There are also sex-related differences in concentrations and effects of a number of gut-derived neurotransmitters including GABA, dopamine, and serotonin (Clarke et al., Citation2013), and such differences have begun to be linked to disease risk and resilience throughout the lifespan (Jašarević, Morrison, & Bale, Citation2016). While speculative, this may play a role in the differences between males and females regarding illnesses, recovery or the stress response evoked by exercise.
Immunity
There has been increasing interest in the role of the gut microbiome and the potential role that host microbes play in a healthy immune system. There is growing evidence showing that the microbiota plays important roles in the maturation of the immune system and protection against some infectious agents (Hooper, Littman, & Macpherson, Citation2012) and from an immunology perspective, compromise of the microbiome and subsequent immunopathology has been associated with atopic and allergic conditions such as asthma (Rankin, O’Donavon, Madigan, O’Sullivan, & Cotter, Citation2017). These allergic reactions can increase the risk of upper respiratory symptoms (URS), which are some of the most common medical complaints affecting athletes. Recurrent or persistent respiratory illness can have a negative impact on the health and performance of athletes undertaking high levels of strenuous exercise (Gleeson & Pyne, Citation2016. It has also been noted that elite female athletes are more likely to suffer with illness at competition than their male counterparts (Soligard et al., Citation2017). Gender differences in immune response have been shown, in animal models, to be connected to the interaction of sex hormones and the microbiome (Rizzetto, Fava, Tuohy, & Selmi, Citation2018), although translational investigations in humans are lacking.
The link between the gut microbiome specifically and the potential for reducing respiratory illness has been a driver for the use of gut health foods and supplements such as pre- and pro-biotics. Probiotics represent a potential means for beneficially influencing the gut microbiota composition/function and can also impact the overall health of the host including reductions in GI symptoms, upper respiratory tract infections, and recovery from exercise. Of note, in a recent position stand, from 22 studies investigating the effects of probiotics on immune function in athletes, 14 showed positive outcomes (Jäger et al., Citation2019). However, one study has shown sex differences on the effects of probiotic supplementation on URS symptoms which did not favour females. Competitive cyclists (n = 64 male; n = 35 female) were given Lactobacillus fermentum for 11 weeks. Respiratory symptoms were reduced in male compared to female athletes and, in fact, increased in the female group (West et al., Citation2011). The authors were unable to give any explanation for the higher number and duration of self-reported symptoms of lower respiratory illness in females supplementing with the probiotics, but do indicate that further work is required to clarify this apparent discrepancy between the sexes in physiological and clinical responses to probiotic supplementation. It has been shown that the response to probiotic supplementation may be related to an individual’s indigenous microbiome, although the exact compositional nature is yet to be elucidated (Zmora et al., Citation2018). As with the aforementioned differences in immune function between males and females, any sex-derived differences in response to probiotic supplementation is likely due to the inherent differences in the microbiome. Information as to the exact genera, species, strains, or global taxonomy that might explain these differences, or promote increases in immune function is still lacking.
Bone health
The skeletal system is oftentimes the subject of a variety of stresses, which can impact the structural integrity of the bones. To minimise the impact of these strains, the body continually remodels bones. The homeostasis of this physiological process can be regulated by gut microbiota, potentially via effects on nutrient absorption (Ibañez, Rouleau, Wakkach, & Blin-Wakkach, Citation2019). For instance, Lactobacillus and Bifidobacteria in the GI tract can increase the absorption of calcium, magnesium, and phosphorus resulting in an increase in bone mineral density (BMD) (Rodrigues et al., Citation2012). Gut microbes can also alter the pH of the GI tract, assist with the synthesis of B vitamins, vitamin K and the metabolism of bile salts, thereby affecting nutrient absorption, bone health and calcium absorption, respectively (Quigley, Citation2013). SCFAs, produced in the GI tract, have been shown to regulate osteoclast production, thereby reducing bone resorption, resulting in improved bone health in lab mice (Ding, Hua, & Ding, Citation2020).
Female athletes are at greater risk of stress fractures compared to males, attributed in part to lower BMD, poor energy availability, and reduced calcium and vitamin D intake (Taunton, Ryan, Mackenzie, Lloyd-Smith, & Zumbo, Citation2002). In the athletic population, the incidence of osteoporosis in females is believed to be greater due to reduced bone accretion, resulting in a lower peak bone mass which can be exacerbated if RED-S is also present (Warren & Stiehl, Citation1999). Given the ways in which the GI tract can affect bone health, this is an under-researched area for female athletes. Differences in the gut microbiome could, in part, explain some of the variance seen between males and females, and indeed between females, in their susceptibility to skeletal injury.
Sleep
Sleep is not only vital for exercise recovery, but adequate quantity and improved quality have been shown to beneficially affect sports performance (Watson, Citation2017). Recent research has shown that the gut microbiome can affect sleep. Smith and colleagues (Citation2019) reporting a positive correlation between microbiome diversity and sleep time in males. In addition, the Bacteroidetes and Firmicutes phyla were positively associated with sleep efficiency. On the other hand, sleep deprivation negatively correlates with microbiome diversity (Benedict et al., Citation2016). Another way in which the GI microbiome could affect sleep is via the neurotransmitter serotonin. Over 95% of the body’s serotonin is produced in the GI tract by commensal bacteria, where it acts to regulate a host of physiological responses (Eisenstein, Citation2016). Elevated levels of serotonin are associated with sleep disturbance and fatigue, which can hamper physical performance (Best, Nijhout, & Reed, Citation2010). Differences in sleep between males and females is recognised to be influenced by sex hormones (Orff, Meliska, Martinez, & Parry, Citation2014). While males and females have similar sleep needs, the risk and type of sleep disorders may vary with females tending to have comparatively impaired ability to fall asleep and stay asleep. Given the acknowledgement of differences in the microbiome, and gut-derived neurotransmitters, these may play some part in potential sex-derived differences in sleep quality.
Conclusion and future recommendations
We have described clear similarities and differences between male–female GI tract structure and function. Motility through the stomach and large intestine have been shown to be slower in females. This likely in part explains the higher incidence of GI symptoms such as bloating, stomach cramps and constipation reported by females at rest, although no difference is seen during exercise. Symptoms appear to also be linked to menstruation and so this suggests a role of sex hormones. With the exception of carbohydrate intake, differences in GI motility and absorption during exercise has received little research in females and so many nutritional recommendations still rely on data derived from male participants. In regards to the GI microbiome, there does appear to be a difference between males and females, which may be due to differences in oestrogen concentrations. Given the multitude of factors that can impact the microbiome, it is difficult to quantify this difference. However, that such a difference exists could give the GI tract a more central role in female athlete health and explain sex-derived differences in other physiological and biological processes. We have highlighted here a number of these processes and have shown the potential mechanisms by which the GI tract might explain differences seen between males and females (in immune function and incidence of illness for example). It is important that researchers consider the effects of multiple factors (e.g. hormonal, psychological, dietary) which may have an impact on the future research results with female athletes. While evidence suggests that some sports nutrition recommendations appear universal for males and females from a GI tract perspective (e.g. CHO feeding during exercise), there is a lack of data for such recommendations for many other facets of dietary intake, particularly in relation to the wider aspects of female health. Future research may result in personalised interventions for females based upon, amongst other things, hormonal and microbiome-related measures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Baker, J. M., Al-Nakkash, L., & Herbst-Kralovetz, M. M. (2017). Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas, 103, 45–53.
- Barton, W., Penney, N. C., Cronin, O., Garcia-Perez, I., Molloy, M. G., Holmes, E., … O’Sullivan, O. (2018). The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut, 67, 625–633.
- Benedict, C., Vogel, H., Jonas, W., Woting, A., Blaut, M., Schürmann, A., & Cedernaes, J. (2016). Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism, 5(12), 1175–1186.
- Best, J., Nijhout, H. F., & Reed, M. (2010). Serotonin synthesis, release and reuptake in terminals: A mathematical model. Theoretical Biology and Medical Modelling, 7(1), 1–26.
- Brown, N., Knight, C. J., & Forrest, L. J. (2021). Elite female athletes’ experiences and perceptions of the menstrual cycle on training and sport performance. Scandinavian Journal of Medicine & Science in Sports 31(1), 52–69.
- Bruinvels, G., Goldsmith, E., Blagrove, R., Simpkin, A., Lewis, N., Morton, K., … Pedlar, C. (2021). Prevalence and frequency of menstrual cycle symptoms are associated with availability to train and compete: A study of 6812 exercising women recruited using the strava exercise app. British Journal of Sports Medicine, 55(8), 438–443.
- Chantler, S., Griffiths, A., Matu, J., Davison, G., Jones, B., & Deighton, K. (2020). The effects of exercise on indirect markers of gut damage and permeability: A systematic review and meta-analysis. Sports Medicine, 51, 113–124.
- Chidi-Ogbolu, N., & Baar, K. (2019). Effect of estrogen on musculoskeletal performance and injury risk. Frontiers in Physiology, 9, 1834.
- Clarke, G., Grenham, S., Scully, P., Fitzgerald, P., Moloney, R. D., Shanahan, F., … Cryan, J. F. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry, 18(6), 666–673.
- Costa, R. J. S., Snipe, R. M. J., Kitic, C. M., & Gibson, P. R. (2017). Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Alimentary Pharmacology & Therapeutics, 46(3), 246–265.
- Dalton, A., Mermier, C., & Zuhl, M. (2019). Exercise influence on the microbiome-gut-brain axis. Gut Microbes, 10(5), 555–568.
- Ding, K., Hua, F., & Ding, W. (2020). Gut microbiome and osteoporosis. Aging and Disease, 11(2), 438–447.
- Eisenstein, M. (2016). Microbiome: Bacterial broadband. Nature, 533, S104–S106.
- Flores, R., Shi, J., Fuhrman, B., Xu, X., Veenstra, T. D., Gail, M. H., … Goedert, J. J. (2012). Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. Journal of Translational Medicine, 10(1), 1–11.
- Freire, A. C., Basit, A. W., Choudhary, R., Piong, C. W., & Merchant, H. A. (2011). Does sex matter? The influence of gender on gastrointestinal physiology and drug delivery. International Journal of Pharmaceutics, 415(1-2), 15–28.
- Gleeson, M., & Pyne, D. B. (2016). Respiratory inflammation and infections in high-performance athletes. Immunology and Cell Biology, 94(2), 124–131.
- Graff, J., Brinch, K., & Madsen, J. L. (2001). Gastrointestinal mean transit times in young and middle-aged healthy subjects. Clinical Physiology, 21(2), 253–259.
- Halvorsen, F. A., Lyng, J., Glomsaker, T., & Ritland, S. (1990). Gastrointestinal disturbances in marathon runners. British Journal of Sports Medicine, 24, 266–268.
- Hooper, L. V., Littman, D. R., & Macpherson, A. J. (2012). Interactions between the microbiota and the immune system. Science, 336, 1268–1273.
- Hyland, N. P., & Cryan, J. F. (2010). A gut feeling about GABA: Focus on GABAB receptors. Frontiers in Pharmacology, 1, 124.
- Ibañez, L., Rouleau, M., Wakkach, A., & Blin-Wakkach, C. (2019). Gut microbiome and bone. Joint, Bone, Spine: Revue du Rhumatisme, 86(1), 43–47.
- Jaggar, M., Rea, K., Spichak, S., Dinan, T. G., & Cryan, J. F. (2020). You’ve got male: Sex and the microbiota-gut-brain axis across the lifespan. Frontiers in Neuroendocrinology, 56, 100815.
- Jašarević, E., Morrison, K. E., & Bale, T. L. (2016). Sex differences in the gut microbiome–brain axis across the lifespan. Philosophical Transactions of the Royal Society B: Biological Sciences, 371(1688), 20150122.
- Jäger, R., Mohr, A. E., Carpenter, K. C., Kerksick, C. M., Purpura, M., Moussa, A., … Gleeson, M. (2019). International society of sports nutrition position stand: Probiotics. Journal of the International Society of Sports Nutrition, 16(1), 1–44.
- Jiang, Y., Babic, T., & Travagli, R. A. (2019). Sex differences in GABAergic neurotransmission to rat DMV neurons. American Journal of Physiology-Gastrointestinal and Liver Physiology, 317(4), G476–G483.
- Judkins, T. C., Dennis-Wall, J. C., Sims, S. M., Colee, J., & Langkamp-Henken, B. (2020). Stool frequency and form and gastrointestinal symptoms differ by day of the menstrual cycle in healthy adult women taking oral contraceptives: A prospective observational study. BMC Women's Health, 20(1), 1–9.
- Kaliannan, K., Robertson, R. C., Murphy, K., Stanton, C., Kang, C., Wang, B., … Kang, J. X. (2018). Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome, 6(1), 1–22.
- Keeffe, E. B., Lowe, D. K., Goss, J. R., & Wayne, R. (1984). Gastrointestinal symptoms of marathon runners. Western Journal of Medicine, 141(4), 481–484.
- Killian, L. A., & Lee, S. Y. (2019). Irritable bowel syndrome is underdiagnosed and ineffectively managed among endurance athletes. Applied Physiology, Nutrition, and Metabolism, 44(12), 1329–1338.
- Kim, Y. S., & Kim, N. (2018). Sex-gender differences in irritable bowel syndrome. Journal of Neurogastroenterology and Motility, 24(4), 544–558.
- Kim, Y. S., Unno, T., Kim, B.-Y., & Park, M.-S. (2020). Sex differences in gut microbiota. The World Journal of Men's Health, 38(1), 48–60.
- Lefevre, N., Bohu, Y., Klouche, S., Lecocq, J., & Herman, S. (2013). Anterior cruciate ligament tear during the menstrual cycle in female recreational skiers. Orthopaedics and Traumatology: Surgery and Research, 99(5), 571–575.
- Lovell, R. M., & Ford, A. C. (2012). Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clinical Gastroenterology and Hepatology, 10(7), 712–721.e4.
- Markle, J. G. M., Frank, D. N., Mortin-Toth, S., Robertson, C. E., Feazel, L. M., Rolle-Kampczyk, U., … Danska, J. S. (2013). Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science, 339(6123), 1084–1088.
- Melin, A. K., Tornberg, A. B., Skouby, S., Sundgot-Borgen, J., Faber, J., Sidelmann, J. J., & Sjödin, M. A. A. (2014). The LEAF questionnaire: A screening tool for the identification of female athletes at risk for the female athlete triad. British Journal of Sports Medicine, 48, 540–545.
- Mountjoy, M., Sundgot-Borgen, J. K., Burke, L. M., Ackerman, K. E., Blauwet, C., Constantini, N., … Budgett, R. (2018). IOC consensus statement on relative energy deficiency in sport (RED-S): 2018 update. British Journal of Sports Medicine, 52(11), 687–697.
- Mörkl, S., Lackner, S., Müller, W., Gorkiewicz, G., Kashofer, K., Oberascher, A., … Holasek, S. (2017). Gut microbiota and body composition in anorexia nervosa inpatients in comparison to athletes, overweight, obese, and normal weight controls. International Journal of Eating Disorders, 50(12), 1421–1431.
- Nelson, M. E., Fisher, E., Catsos, P., Meredith, C., Turksoy, R., & Evans, W. (1986). Diet and bone status in amenorrheic runners. The American Journal of Clinical Nutrition, 43(6), 910–916.
- O’ Donovan, C. M., Connor, B., Madigan, S. M., Cotter, P. D., & O’Sullivan, O. (2020a). Instances of altered gut microbiomes among Irish cricketers over periods of travel in the lead up to the 2016 World Cup: A sequencing analysis. Travel Medicine and Infectious Disease, 35, 101553.
- O’ Donovan, C. M., Madigan, S. M., Garcia-Perez, I., Rankin, A., O’Sullivan, O., & Cotter, P. D. (2020b). Distinct microbiome composition and metabolome exists across subgroups of elite Irish athletes. Journal of Science and Medicine in Sport, 23(1), 63–68.
- Orff, H. J., Meliska, C. J., Martinez, L. F., & Parry, B. L. (2014). The influence of sex and gonadal hormones on sleep disorders. ChronoPhysiology and Therapy, 4, 15–25.
- Pugh, J. N., Fearn, R., Morton, J. P., & Close, G. L. (2018a). Gastrointestinal symptoms in elite athletes: Time to recognise the problem? British Journal of Sports Medicine, 52, 487–488.
- Pugh, J. N., Kirk, B., Fearn, R., Morton, J. P., & Close, G. L. (2018b). Prevalence, severity and potential nutritional causes of gastrointestinal symptoms during a marathon in recreational runners. Nutrients, 10(7), 811.
- Quigley, E. M. (2013). Gut bacteria in health and disease. Gastroenterology and Hepatology, 9(9), 560–569.
- Rankin, A., O’Donavon, C., Madigan, S. M., O’Sullivan, O., & Cotter, P. D. (2017). ‘Microbes in sport’ – The potential role of the gut microbiota in athlete health and performance. British Journal of Sports Medicine, 51, 698–699.
- Rao, S. S., Sadeghi, P., Beaty, J., Kavlock, R., & Ackerson, K. (2001). Ambulatory 24-h colonic manometry in healthy humans. American Journal of Physiology-Gastrointestinal and Liver Physiology, 280(4), G629–G639.
- Rizzetto, L., Fava, F., Tuohy, K. M., & Selmi, C. (2018). Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. Journal of Autoimmunity, 92, 12–34.
- Rodrigues, F. C., Castro, A. S. B., Rodrigues, V. C., Fernandes, S. A., Fontes, E. A. F., de Oliveira, T. T., … de Luces Fortes Ferreira, C. L. (2012). Yacon flour and Bifidobacterium longum modulate bone health in rats. Journal of Medicinal Food, 15(7), 664–670.
- Scheiman, J., Luber, J. M., Chavkin, T. A., MacDonald, T., Tung, A., Pham, L.-D., … Kostic, A. D. (2019). Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nature Medicine, 25, 1104–1109.
- Smith, R. P., Easson, C., Lyle, S. M., Kapoor, R., Donnelly, C. P., Davidson, E. J., Parikh, E., Lopez, J. V., & Tartar, J. L. (2019). Gut microbiome diversity is associated with sleep physiology in humans. PLoS One, 14(10), e0222394.
- Snipe, R. M., & Costa, R. J. (2018). Does biological sex impact intestinal epithelial injury, small intestine permeability, gastrointestinal symptoms and systemic cytokine profile in response to exertional-heat stress? Journal of Sports Sciences, 36(24), 2827–2835.
- Soligard, T., Steffen, K., Palmer, D., Alonso, J. M., Bahr, R., Lopes, A. D., … Engebretsen, L. (2017). Sports injury and illness incidence in the Rio de Janeiro 2016 olympic summer games: A prospective study of 11274 athletes from 207 countries. British Journal of Sports Medicine, 51, 1265–1271.
- Southwell, B. R., Clarke, M. C., Sutcliffe, J., & Hutson, J. M. (2009). Colonic transit studies: Normal values for adults and children with comparison of radiological and scintigraphic methods. Pediatric Surgery International, 25(7), 559–572.
- Taunton, J. E., Ryan, M. B., Mackenzie, D. C., Lloyd-Smith, D. R., & Zumbo, B. D. (2002). A retrospective case-control analysis of 2002 running injuries. British Journal of Sports Medicine, 36(2), 95–101.
- ten Haaf, D. S., van der Worp, M. P., Groenewoud, H. M., Leij-Halfwerk, S., Nijhuis-van der Sanden, M. W., Verbeek, A. L., & Staal, J. B. (2014). Nutritional indicators for gastrointestinal symptoms in female runners: The 'Marikenloop study'. BMJ Open, 4(8), e005780.
- van Wijck, K., Lenaerts, K., Grootjans, J., Wijnands, K. A., Poeze, M., Van Loon, L. J., C. H. C. Dejong, & Buurman, W. A. (2012). Physiology and pathophysiology of splanchnic hypoperfusion and intestinal injury during exercise: Strategies for evaluation and prevention. American Journal of Physiology-Gastrointestinal and Liver Physiology, 303(2), G155–G168.
- Verma, R., Balhara, Y. P., & Gupta, C. S. (2011). Gender differences in stress response: Role of developmental and biological determinants. Industrial Psychiatry Journal, 20(1), 4–10.
- Wallis, G. A., Dawson, R., Achten, J., Webber, J., & Jeukendrup, A. E. (2006). Metabolic response to carbohydrate ingestion during exercise in males and females. American Journal of Physiology-Endocrinology and Metabolism, 290(4), E708–E715.
- Warren, M. P., & Stiehl, A. L. (1999). Exercise and female adolescents: Effects on the reproductive and skeletal systems. Journal of the American Women’s Association, 54(3), 115–120. 138.
- Watson, A. M. (2017). Sleep and athletic performance. Current Sports Medicine Reports, 16(6), 413–418.
- West, N. P., Pyne, D. B., Cripps, A. W., Hopkins, W. G., Eskesen, D. C., Jairath, A., … Fricker, P. A. (2011). Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutrition Journal, 10(1), 1–11.
- Wilson, P. B. (2017). Frequency of chronic gastrointestinal distress in runners: Validity and reliability of a retrospective questionnaire. International Journal of Sport Nutrition and Exercise Metabolism, 27(4), 370–376.
- Wolman, R. L. (1990). Bone mineral density levels in elite female athletes. Annals of the Rheumatic Diseases, 49(12), 1013–1016.
- Zmora, N., Zilberman-Schapira, G., Suez, J., Mor, U., Dori-Bachash, M., Bashiardes, S., … Elinav, E. (2018). Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell, 174(6), 1388–1405.e21.
