 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A vegan diet is associated with reduced cardiovascular morbidity and mortality, but protein deficiencies may be detrimental to skeletal muscle structure and function. The aim of this study was to compare the vascular and skeletal muscle properties between young, healthy, recreationally active habitual vegan (VEG) and omnivorous (OMN) men. Sixteen OMN and nine VEG underwent ultrasound scans to determine brachial artery flow-mediated dilation (FMD) and carotid artery intima-media thickness (cIMT) and vastus lateralis (VL) muscle thickness and fascicle pennation angle. Knee extension maximal voluntary isometric contraction (MVIC) force was assessed on an isokinetic dynamometer, and O2max on a cycle ergometer and online gas analysis system. A three-day food diary determined habitual dietary behaviour. Bayesian analyses of independent groups provided “moderate” to “very strong” evidence for lower consumption of absolute (63±21 g/d vs. 98 ± 30 g/d; Bayes Factor (BF01) = 0.140) and relative (0.86 ± 0.29 g/kg/d vs.1.36 ± 0.52 g/kg/d; BF01 = 0.259) protein, absolute saturated fat (15.2 ± 7.9 g vs. 30.3 ± 11.8 g; BF01 = 0.089) and cholesterol (5.0 ± 6.0 mg vs. 337.9 ± 232.6 mg; BF01 = 0.019) in VEG compared to OMN, respectively. Further, there was “anecdotal” evidence to support no differences in FMD (3.37 ± 3.31% vs. 4.58 ± 5.82%; BF01 = 2.591), cIMT (0.51 ± 0.07 mm vs. 0.49 ± 0.04 mm; BF01 = 2.510), VL thickness (26.1 ± 3.7 mm vs. 27.8 ± 6.4 mm; BF01 = 2.726), fascicle pennation angle (16.6 ± 4.7° vs. 17.7 ± 3.7°; BF01 = 2.844), MVIC (627 ± 182 N vs. 551 ± 102 N; BF01 = 1.656) or
O2max (40.8 ± 9.8 ml/kg/min vs. 35.8 ± 5.2 ml/kg/min; BF01 = 1.218) between VEG and OMN, respectively. Despite marked differences in habitual nutrient intake, healthy, young vegan and omnivorous men did not differ regarding vascular and skeletal muscle structure and function, or cardiovascular fitness.
Introduction
Epidemiological studies suggest that plant-based diets, characterised by a high consumption of whole grains, fruits and vegetables, are typically associated with a reduced risk of cardiovascular disease incidence and mortality compared to omnivorous diets (Kim et al., Citation2019). However, less is known about the effect plant-based diets have on specific skeletal muscle and vascular characteristics that are associated with long-term health conditions.
Ultrasound measurements of carotid artery intima-media thickness (cIMT) and endothelial function assessed via flow-mediated dilation (FMD) are commonly used surrogate markers of atherosclerotic development and cardiovascular disease risk (Kokubo et al., Citation2018; Matsuzawa, Kwon, Lennon, Lerman, & Lerman, Citation2015). Healthy elderly vegetarian men and women have been shown to exhibit higher brachial artery FMD compared with omnivores (Lin, Fang, & Gueng, Citation2001) albeit with a suboptimal measurement approach for FMD (Thijssen et al., Citation2019). More recently, research has demonstrated lower cIMT in young healthy vegetarian men compared with their omnivorous counterparts (Acosta-Navarro et al., Citation2017). To our knowledge there is only one study that has investigated the effects of a vegan diet on the vasculature, indicating that microvascular function did not improve following just four weeks compliance to a vegan diet (Rogerson, Maçãs, Milner, Liu, & Klonizakis, Citation2018). Consequently, it is unclear whether a habitual vegan diet confers any direct benefits to vascular structure or function.
Despite the purported health benefits, there are potential risks of nutrient deficiencies associated with vegan diets. Protein intake, for example, is lower in vegan dieters compared with omnivores, and in some cases less than the minimum recommended daily protein intake (Sobiecki, Appleby, Bradbury, & Key, Citation2016). Protein consumption increases postprandial myofibrillar protein synthesis (MPS) rates (Tipton, Ferrando, Phillips, Doyle, & Wolfe, Citation1999), subsequently contributing to muscle mass accretion (Hartman et al., Citation2007). Given that muscle mass is the main determinant of maximum strength (Jones, Rutherford, & Parker, Citation1989), it is plausible that lower protein consumption in vegan diets could be associated with (smaller and therefore) weaker muscles (Sahni, Mangano, Hannan, Kiel, & McLean, Citation2015). However, previous studies have demonstrated no differences in upper and lower body one-repetition maximum (1-RM) (Boutros, Landry-Duval, Garzon, & Karelis, Citation2020), or knee extensor isokinetic peak torque between young physically active vegan/vegetarian and omnivorous women (Lynch, Wharton, & Johnston, Citation2016) Nonetheless, as participants were highly trained, it is not clear how training status and the interaction with diet may have impacted these findings. It should also be noted that no prior studies have included assessment of muscle morphology or architecture. Skeletal muscle architecture (including thickness and fascicle pennation angle) are key determinants of the maximum force of a muscle (Aagaard et al., Citation2001), therefore assessment of muscle architecture in habitual, untrained vegan and omnivorous dieters may confirm existing evidence suggesting similarities in maximal muscle strength.
Despite the potential disadvantages of plant-based diets for strength/power performance, evidence suggests no differences exist in maximal power output during an incremental cycling bout between vegans, lacto-ovovegetarians and omnivores (Nebl et al., Citation2019). Contrarily, there is some evidence that O2max is higher in female elite vegetarian vs. omnivore endurance athletes (Lynch et al., Citation2016). Further, highly physically active (150–200 min of moderate intensity physical activity/week) vegan females exhibit a greater estimated
O2max when compared with omnivores (Boutros et al., Citation2020). However, estimating rather than measuring
O2max is problematic and both studies used highly trained populations, which limits the application of the study findings. For example, chronic endurance (McPhee et al., Citation2009) and resistance (Erskine et al., Citation2011; Erskine, Jones, Williams, Stewart, & Degens, Citation2010) exercise, as well as ageing (Janssen, Heymsfield, Wang, & Ross, Citation2000) induce physiological adaptations that will likely confound any measurable effect of diet on vascular or muscle properties.
To date, no study has combined ultrasound assessments of vascular structure and function with skeletal muscle architecture, as well as maximal force and measured O2max, in habitual vegan and omnivorous dieters. The aim of this study, therefore, was to compare the vascular and skeletal muscle properties between young, healthy, untrained men who were either habitual vegan or omnivorous dieters. We hypothesised that vegan dieters would have higher FMD and
O2max, but lower cIMT values compared with omnivorous dieters, whereas omnivores would have larger and stronger knee extensor muscles.
Methods
Twenty-five young, healthy men with no history of resistance or endurance exercise training in the preceding six months were recruited for the current study, which was a single-centre, cross-sectional design. Participant characteristics are shown in . Further exclusion criteria included smokers, those with a history of lower limb injuries in the six months prior to testing and those engaging in more than two hours per week of structured exercise. Participants were established as vegans or omnivores via a series of questions regarding consumption of meat, fish/seafood, eggs and dairy products such as milk and cheese. Subsequently, vegans were defined as those who consumed no animal products in their diet for at least six months prior to testing, and omnivores were defined as those who consumed animal products (including meat) in their habitual diet. This was then confirmed following completion of a three-day food diary (see below). Sixteen omnivores (OMN) and nine vegans (VEG) provided written informed consent prior to taking part in the study, which was approved by the Research Ethics Committee of Liverpool John Moores University (approval number: U18SPS007) and complied with the Declaration of Helsinki (World Medical Association, Citation2013).
Table 1. Participant information for omnivores (OMN) and vegans (VEG) presented as mean ± SD. Evidence for differences between groups was determined using BF01.
Experimental design. All participants visited the laboratory on three occasions and were instructed to abstain from alcohol (at least 24 h before each visit) and caffeine consumption the day of each visit. Additionally, the participants underwent an overnight fast and avoided exercise for 24 h prior to the first visit. During the first visit, a brachial artery FMD and cIMT assessment was conducted by the same trained sonographer for all trials. Following the vascular assessment, participants were familiarised with the protocol for the knee extension maximum voluntary isometric contraction (MVIC) assessment in the right leg on the isokinetic dynamometer (IKD), and the O2max test on a cycle ergometer, for which they completed the warm-up and first stage only. Participants were then provided with a three-day food diary and received full instructions on how to complete the diary. During visit two, subjects underwent an assessment of vastus lateralis (VL) muscle architecture using ultrasonography and completed a quadriceps MVIC on the IKD. The final visit occurred at least two days following visit two and incorporated a
O2max test to volitional fatigue on a cycle ergometer.
Vascular Assessment. Participants arrived at the laboratory at 08.00 h. They then lay in the supine position for 20 min before extending their right arm at an approximate 80° angle from the torso. All ultrasound measures were carried out by the same trained sonographer using high-resolution ultrasonography (Acuson, Terason, t3000, Teratec, USA) with a 40 mm wide 10–12 MHz probe.
FMD was assessed in the brachial artery in the distal third of the upper arm at an angle of 60°. Ultrasound parameters remained constant throughout each assessment, with the probe held in a constant position, to optimise longitudinal two-dimensional B mode images of the near arterial wall. A 1 min baseline measurement of artery diameter was recorded prior to a rapid inflator (AG 101 Hokanson, Bellevue, USA) inflating the forearm cuff to 220 mmHg for a 5 min ischaemic stimulus. The endothelium-mediated vasodilation response to the cuff deflation was then recorded, beginning 30 s prior to deflation, for a period of 3 min, according to previous guidelines (Thijssen et al., Citation2019). Post-test analysis was performed using custom designed automated edge detection and wall tracking software. B mode images were analysed at ∼20 Hz, with a specific region of interest identified and lumen diameter then calculated by averaging the number of pixel columns in each frame. Shear rate (SR) was then calculated as the area under the curve (AUC) up to maximal post-deflation diameter. The test-retest coefficient of variation (CV) for FMD measurements previously performed in our laboratory is 11.7% (Carter et al., Citation2019), which is within the acceptable limits of 15% (Thijssen et al., Citation2019).
For cIMT assessment, participants remained in the supine position, with the head tilted 90° to the left. The common carotid artery was examined ∼1 cm proximal to the bulbus and ultrasound parameters set to optimise longitudinal two-dimensional B mode images of the luminal-wall interface. The probe and ultrasound parameters were held constant throughout each scan. Once a clear image with visible contrast between the arterial lumen, walls and IMT was established a 30 s video was recorded at approximately 40°, 90° and 150° angles. All assessments were carried out by the same researcher. Post-test analysis was performed using v3.0 custom designed edge detection software with the most distinct 6–8 s from recorded scans containing an optimal area of 1 cm including both vessel walls selected for analysis. The software produced an edge detection output from the lumen-intima interface and the media-adventitia interface at each angle to provide an average IMT and lumen diameter over the 6–8 s. The mean cIMT and diameter calculated from the 3 recorded angles is presented. The test-retest CVs for carotid artery wall thickness and diameter measurements previously recorded in our laboratory are 8.6% and 3.6%, respectively (Hopkins et al., Citation2013).
VL Muscle Architecture. Participants were seated in an IKD chair (Lido Active, Loredan, Davis, CA, USA), with a hip angle of 90° (180° = supine) and a 90° knee flexion angle (0° = full knee extension). The length of the VL was determined at rest by measuring the distance between the muscle origin and insertion, identified via B-mode ultrasonography (40 mm/10–15 MHz transducer, MyLab30, Esaote, Genoa, Italy). The medial and lateral borders of the VL were determined at 50% of the muscle length to establish the middle of the muscle belly for the assessment. Two sagittal ultrasound images were taken at this site with the probe placed perpendicular to the skin, in line with the muscle fascicles. Depth and frequency were altered to obtain the optimal image, with both deep and superficial aponeuroses and at least three fascicles clearly visible. Subsequently, images were analysed using a computer software package (NIH ImageJ, version 1.39b, National Institutes of Health, Bethesda, USA). Muscle thickness was measured using the mean value from three measurements of the distance between the superficial and deep aponeuroses, while fascicle pennation angle was measured using the mean value of three fascicles inserting into the deep aponeurosis. The test-retest CVs for VL muscle thickness and fascicle pennation angle measurements in this study were 4.9% and 5.8%, respectively.
Knee Extension MVIC. Participants remained seated in the IKD as above. They were securely strapped with inextensible straps around the waist and chest to prevent movement, and the right ankle was strapped to the force transducer (KAP, Bienfait B.V. Haarlem, The Netherlands). Force output was recorded using a data acquisition package (Acqknowledge, Biopac Systems, CA, USA). Ten submaximal contractions were completed as a warm-up with participants increasing their force output each time. Once fully prepared, participants were instructed to perform two MVICs separated by a one-minute rest period. The true MVIC value was determined by a difference of <5% between each trial, with trials being repeated if larger differences occurred. Verbal encouragement was provided by researchers and feedback was provided to participants by projecting their MVIC attempts on a screen in front of them in real time. Test-retest CV for the MVIC measurement in this study was 4.5%.
O2max. During the final visit participants completed a
O2max test to volitional fatigue on a cycle ergometer (Lode Corival, Groningen, Netherlands). Upon arrival at the laboratory participants rested for ten minutes before their resting heart rate was measured (Polar, Kempele, Finland) and their blood lactate measurement (Nova Biomedical, Massachusetts, USA) was taken from a finger prick blood sample. The participant was fitted with a facemask to collect inspired and expired air. The test began with a 3 min warm up cycling at a power output of 50 W, before the first stage started with subjects cycling at 95 W. The power output increased by 35 W every 3 min until volitional fatigue, with heart rate (HR), blood lactate and rate of perceived exertion (RPE via Borg Scale) recorded at the end of each increment. Expired air samples were measured using an online gas analysis system (Cortex, Leipzig, Germany) to determine
O2 and respiratory exchange ratio (RER). The criteria to determine whether
O2max had been reached consisted of a HR value within 10 bpm of subjects’ age-predicted HR maximum (220-minus age in years), a blood lactate value > 8.0 mmol.L−1, an RER > 1.15 and if subjects were unable to maintain the optimum cycling cadence of 80 rpm. Final HR, blood lactate, RER and RPE measurements were taken immediately following cessation of the test. Test-retest CV for
O2max assessment in this study was 5.2%.
Dietary Analysis. Participants were provided with a three-day food diary with full instructions to record all food and drink consumed over two weekdays (Thursday and Friday) and one weekend day (Saturday). A three-day food diary was deemed appropriate, as opposed to the seven-day method that has previously been suggested to decrease in validity in the later days, due to participant fatigue and foods being recorded retrospectively (Thompson & Subar, Citation2017). Time of consumption and quantity of food were also recorded. Dietary diary analysis was conducted using an online dietary analysis software (Nutritics, Research Edition (v5.5), Dublin, Ireland), which produced a full dietary report (including macro and micronutrients). In a separate questionnaire, participants were asked if they consumed any dietary supplements and, if so, what dose/frequency. Just two (both vegans) reported taking vitamin B12 (one participant) and omega-3 and vitamin B12 (one participant), although dose/frequency were not stated. Further, these participants did not record consumption of these supplements in their food diary, so they were not included in their dietary analysis.
Statistical Analysis. Differences between groups were estimated via a Bayesian analysis of independent groups, comprising a null hypothesis (H0 = there are no differences between OMN and VEG for each outcome variable measured) and an alternative hypothesis (H1 = differences exist between OMN and VEG for each outcome variable measured). Uninformative priors were assumed for all statistical analyses and statistical outputs are presented as estimated mean differences (EMD), with 95% credible intervals (CI) and Bayes factors (BF). Data for BF are presented as BF01 (null hypothesis given alternative hypothesis) and can be interpreted as evidence to support the hypotheses based on previously defined thresholds- BF01 = 1–3 and 3–10 represent anecdotal and moderate strength evidence for H0, respectively (Lee & Wagenmakers, Citation2013). Whereas a BF01 = 0.1–0.33, 0.033–0.1 and 0.01–0.033 represent moderate, strong and very strong evidence for H1, respectively (Lee & Wagenmakers, Citation2013). All statistical analyses were performed with SPSS 26 (IBM SPSS Statistics for Windows, Version 26.0. Armonk, New York, USA) and data are presented as means ± SD.
Results
Participant physical characteristics are displayed in . There was “strong” evidence to suggest VEG were older than OMN (BF01 = 0.043).
Daily nutrient intake (both in absolute terms and normalised to body mass) are presented in . Compared to OMN, there was “moderate” to “extremely strong” evidence to suggest VEG ingested less protein, saturated fat, cholesterol and vitamin B12, but more fibre, iron and vitamin C (BF01 = 0.259–0.000).
Table 2. Daily energy and macro- and micronutrient intake in omnivores (OMN) and vegans (VEG). Data are presented as mean ± SD. Evidence for differences between groups was determined using BF01.
Vascular data are presented in . Due to technical issues, cIMT data were excluded from one OMN and one VEG, and FMD data from two OMN. There was “anecdotal” evidence to suggest vascular properties did not differ between VEG and OMN (BF01 = 1.557–2.591).
Table 3. Vascular properties for omnivores (OMN) and vegans (VEG) are presented as mean ± SD. Evidence for no differences between groups was determined using BF01.
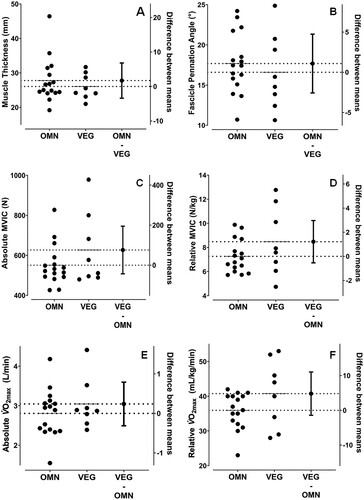
The estimated mean difference (EMD) in muscle thickness between OMN and VEG was −1.72 mm (95% CI = −6.33–2.89, BF01 = 2.726, (A)) and for fascicle pennation angle was −1.09° (95% CI = −5.52–3.35, BF01 = 2.844, (B)). Furthermore, the EMD in both absolute and relative MVIC values were 75.93 N (95% CI = −85.27–237.13, BF01 = 1.656, (C)) and 1.214 N/kg (CI = −1.262–3.689, BF01 = 1.470, (D)), respectively, indicating no differences in muscle properties between OMN and VEG. The EMD in absolute and relative O2max between OMN and VEG were 0.24 L/min (95% CI = −0.39–0.87, BF01 = 2.392, (E)) and 4.81 mL/kg/min (95% CI = −3.77–13.40, BF01 = 1.218, (F)), respectively, therefore indicating no differences between groups.
Figure 1. Estimation plots for vastus lateralis (VL) muscle thickness (A) and fascicle pennation angle (B); knee extension maximal voluntary isometric contraction (MVIC) force in absolute terms (C) and normalised to body mass (D); and values in absolute terms (E) and normalised to body mass (F) in omnivores (OMN) and vegans (VEG). Data for both groups are presented according to the primary Y-axis, while the mean difference and 95% CI between groups are presented according to the secondary Y-axis.
Discussion
This is the first study to combine ultrasound assessments of vascular and skeletal muscle properties, as well as maximal muscle force and O2max, for comparison between healthy, young, untrained habitual vegan and omnivorous men. Importantly, by assessing young, recreationally active but non-resistance/endurance trained individuals, we were able to examine the effect of a vegan diet on the vasculature and musculature independent of training status and ageing. Our novel findings demonstrate that no differences existed in vascular structure and function, or skeletal muscle properties, between habitual vegan and omnivorous dieters despite marked differences in macro- and micronutrient intake. Additionally, vegans and omnivores did not differ regarding cardiorespiratory fitness.
Prior research has demonstrated both higher FMD scores and smaller cIMT values in vegetarians compared with omnivores (Acosta-Navarro et al., Citation2017; Lin et al., Citation2001). However, in our study, we demonstrated in a young, recreationally active male cohort that there were no differences in vascular structure or function between vegans and omnivores, despite vegans consuming less saturated fat and cholesterol in their diet. One possible explanation for this discrepancy may be that in prior studies, participants were significantly older than our subjects (mean ages ranged from 32 to 56 years old compared to ∼22 years old). As ageing influences the decline in vascular structure and function (Xu et al., Citation2017) and, given that our participants were free from cardiovascular disease risk factors, it is likely that our participants’ vasculature was intact and working optimally, whereas participants in previous studies were possibly experiencing some degree of vascular decline. Taken together, these data suggest that plant-based diets confer protection from, or a slowing of, age-induced vascular degeneration, but do not upregulate an already intact vasculature, however, this hypothesis needs to be explored further. Finally, the assessment of endothelial function by Lin et al. (Citation2001) utilises an outdated methodology, which is highly likely to result in inaccuracies in the data collected. Therefore, it remains unclear whether, and in whom, plant-based diets improve vascular outcomes, but it appears that age may be an important factor to consider for future research.
Unlike vascular properties, to our knowledge, no previous study has investigated the effects of habitual vegan diet on skeletal muscle architecture. The VL muscle thickness and fascicle pennation angle values in our study were similar to those reported previously in similar populations (Erskine et al., Citation2010; Erskine et al., Citation2011; Franchi et al., Citation2018). We found no differences in VL thickness or fascicle pennation angle between vegans and omnivores, despite omnivores consuming a higher daily protein intake compared with vegans. This is surprising, given plant-based sources of protein, such as wheat and soy, fail to stimulate muscle protein synthesis to the same extent as some animal protein sources (e.g. whey protein) (Gorissen et al., Citation2016; Tang, Moore, Kujbida, Tarnopolsky, & Phillips, Citation2009). However, it has been demonstrated that gains in lean body mass and muscular strength are similar in response to supplementation with either soy or animal protein sources (Messina, Lynch, Dickinson, & Reed, Citation2018). Therefore, although the quality of protein might be important for efficient delivery of amino acids to the muscle following resistance exercise (thereby potentially maximising the muscle hypertrophic response in athletes), it may not be so important in non-resistance trained individuals. Furthermore, although vegans consumed less protein than omnivores in our study, they still consumed 0.9 g/kg/d protein, which is within the recommended range for daily protein intake (0.8–2 g/kg/d) (Thomas, Erdman, & Burke, Citation2016), thus potentially explaining the similar muscle size and architecture between our vegans and omnivores.
As the architecture of a muscle is related to the force it can produce (Aagaard et al., Citation2001) and given that no differences were observed in muscle architecture in our study, it was not surprising that no difference existed in MVIC force between vegans and omnivores either. No differences in strength or lean body mass have previously been reported in elite athlete vegetarian and omnivorous men and women (Lynch et al., Citation2016). Similarly, Boutros et al. (Citation2020) demonstrated no differences in leg or chest press 1-RM between young, physically active vegan and omnivorous women. It therefore appears that adopting a vegan diet may not be detrimental for skeletal muscle size, architecture or function in both young, healthy men and women.
Recent findings support those from our study, demonstrating no difference in endurance exercise capacity between vegans, lacto-ovovegetarians and omnivores, albeit in male and female runners (Nebl et al., Citation2019). However, contradictory findings have also recently been published, suggesting that vegans/vegetarians exhibit greater O2max values compared with omnivores (Boutros et al., Citation2020; Lynch et al., Citation2016). Intriguingly, the
O2max differences observed by Lynch et al. were only found in female and not male endurance athletes, and only when normalised to body mass. Although not reported, the ratio of lean mass to total body mass appeared to be higher in the female VEG vs. OMN, and a relatively greater skeletal muscle composition would be expected to utilise more O2 during maximal exercise, particularly when normalised to total body mass (which was higher in female OMN vs.VEG). Alternatively, differences in
O2max could have been caused by the higher weekly energy expenditure in the female VEG vs. OMN (∼20 METS, kcal·kg−1·week−1), rather than any dietary effect per se. Further, Boutros et al. used a non-validated single question for participants to self-report their physical activity level, while
O2max was estimated rather than measured directly, both likely having implications for the accuracy of the data (Boutros et al., Citation2020). It is therefore difficult to discern whether the differences in
O2max reported in the studies by Lynch et al. and Boutros et al. were due to differences in diet, physical activity, or body composition.
While we did not find any effect of diet on O2max, vascular or skeletal muscle properties, we did observe between group differences in key micronutrients, which can impact vascular and muscular health. Firstly, our vegans were deficient in vitamin B12 and consumed less than their omnivorous counterparts. However, two vegan participants reported vitamin B12 supplementation but did not specify dose/frequency and did not include any relevant information in their food diary, so it could not be included in their dietary analysis. Vitamin B12 deficiency is common amongst vegans (Sobiecki et al., Citation2016) and can lead to hyperhomocysteinaemia (Krajčovičová-Kudláčková, Blažíček, Kopčová, Bederova, & Babinska, Citation2000), a condition that is associated with atherosclerosis development (de Koning, Werstuck, Zhou, & Austin, Citation2003). However, there was no evidence of altered vasculature in our vegan population, potentially as a result of lower intake of saturated fat and cholesterol and higher vitamin C ingestion than omnivores, which is in accordance with previous findings (Sobiecki et al., Citation2016). Vitamin C has antioxidant properties and has subsequently been associated with a reduced rate of cIMT growth (Ellingsen, Seljeflot, Arnesen, & Tonstad, Citation2009) and prevention of endothelial dysfunction (May & Harrison, Citation2013). Furthermore, vitamin C is also an essential co-factor for lysyl oxidase activation in the collagen synthesis pathway (Boyera, Galey, & Bernard, Citation1998), which in turn, could be important for bone, tendon and intramuscular connective tissue health and function. Future studies should investigate whether differences between vegans and omnivores exist regarding other collagenous tissues, such as tendon properties.
Limitations and future directions
Our cohort included young, healthy, recreationally active men in order to minimise the effect of any potential confounders such as ageing (Narici, Maganaris, Reeves, & Capodaglio, Citation2003; Xu et al., Citation2017), training status (Erskine et al., Citation2010; Green & Smith, Citation2018; McPhee et al., Citation2009) or disease incidence (Li et al., Citation2015; Vrtovec, Keber, Gadzijev, Bardorfer, & Keber, Citation1999) on vascular and skeletal muscle phenotypes. We also utilised updated vascular assessment protocols (Thijssen et al., Citation2019) and included measurements of skeletal muscle architecture and isometric strength to draw more precise comparisons between vegan and omnivorous dieters, regarding vascular health and skeletal muscle structure and function. We do acknowledge that additional measures of muscle size, e.g. volume of the four individual heads of the quadriceps femoris muscle group, may have provided more support for our findings. However, we chose to study the VL, because it is the largest of the four quadriceps femoris heads (Erskine, Jones, Maganaris, & Degens, Citation2009) and VL muscle thickness (measured with ultrasound) correlates strongly with muscle volume (measured with MRI), both before and after chronic resistance training (Franchi et al., Citation2018). Thus, the fact that we found no difference in VL muscle thickness or fascicle pennation angle between VEG and OMN suggests we would not have observed a difference in quadriceps femoris muscle volume either.
Regarding the assessment of dietary behaviour, food diaries are a prospective dietary assessment tool that provide detailed descriptions of foods consumed as well as portion sizes and cooking/preparation methods. However, participants may alter both the type and amount of food they consume during the assessment (Rebro, Patterson, Kristal, & Cheney, Citation1998). We acknowledge that a longer recording period and/or additional assessments of weighed nutrient intake might have provided a more comprehensive account of habitual diet (Ortega, Pérez-Rodrigo, & López-Sobaler, Citation2015) and further reinforced our results. However, in light of potential participant fatigue (Vereecken, Rossi, Giacchi, & Maes, Citation2008) and, with the accuracy of seven-day recording periods decreasing in the later stages (Ortega et al., Citation2015), a three-day food diary was deemed most appropriate. Furthermore, we did not measure amino acid intake in the current study, which could have provided further information regarding skeletal muscle and vascular health (Churchward-Venne et al., Citation2012; Huynh & Chin-Dusting, Citation2006). For example, leucine is considered the most important amino acid for stimulating muscle protein synthesis (Churchward-Venne et al., Citation2012), and is more abundant in whey compared to soy protein (Tang et al., Citation2009). However, there are a number of vegan-suitable protein sources that contain all nine EAAs (e.g. soy, quinoa and buckwheat), but it may be that the quantity rather than quality of protein is more important for maintaining muscle health, particularly in non-athletes. Finally, we examined the impact of a vegan diet in a young, healthy cohort in order to remove ageing as a potential confounding variable, however, it is possible that no differences were observed between vegans and omnivores due to the lack of muscular or vascular degeneration that is seen with ageing (Narici et al., Citation2003; Xu et al., Citation2017).
Conclusion
With these results, we show for the first time that young, healthy, recreationally active vegan and omnivorous men do not differ regarding novel measurements of vascular and skeletal muscle structure and function, despite differences in key macro- and micronutrient intake. Our data, therefore, indicate that a healthy vegan diet is neither beneficial nor detrimental for vascular and skeletal muscle health when compared with an omnivorous diet in young, healthy, untrained men.
Ethics approval
The study was approved by the Research Ethics Committee of Liverpool John Moores University and complied with the Declaration of Helsinki.
Consent to participate
All participants provided written informed consent prior to taking part in the study.
Availability of data and material
All data are presented within the manuscript.
Code availability
Not applicable.
Authors’ contributions
Authors contributed to this manuscript as follows: conceptualisation (NDH, RME); data curation (JP, NDH, RME); formal analysis (JP, NDH, RME); investigation (JP); methodology (NDH, RME); project administration (JP, NDH, RME); supervision (NDH, RME); writing – original draft (JP); writing – review and editing (JP, NDH, RME).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aagaard, P., Andersen, J. L., Dyhre-Poulsen, P., Leffers, A.-M., Wagner, A., Magnusson, S. P., … Simonsen, E. B. (2001). A mechanism for increased contractile strength of human pennate muscle in response to strength training: Changes in muscle architecture. The Journal of Physiology, 534, 613–623.
- Acosta-Navarro, J., Antoniazzi, L., Oki, A. M., Bonfim, M. C., Hong, V., Acosta-Cardenas, P., … Salgado Filho, W. (2017). Reduced subclinical carotid vascular disease and arterial stiffness in vegetarian men: The CARVOS study. International Journal of Cardiology, 230, 562–566.
- Boutros, G. H., Landry-Duval, M.-A., Garzon, M., & Karelis, A. D. (2020). Is a vegan diet detrimental to endurance and muscle strength? European Journal of Clinical Nutrition, 74, 1–6.
- Boyera, N., Galey, I., & Bernard, B. (1998). Effect of vitamin C and its derivatives on collagen synthesis and cross-linking by normal human fibroblasts. International Journal of Cosmetic Science, 20, 151–158.
- Carter, S. E., Draijer, R., Holder, S. M., Brown, L., Thijssen, D. H. J., & Hopkins, N. D. (2019). Effect of different walking break strategies on superficial femoral artery endothelial function. Physiological Reports, 7, e14190.
- Churchward-Venne, T. A., Burd, N. A., Mitchell, C. J., West, D. W. D., Philp, A., Marcotte, G. R., … Phillips, S. M. (2012). Supplementation of a suboptimal protein dose with leucine or essential amino acids: Effects on myofibrillar protein synthesis at rest and following resistance exercise in men. The Journal of Physiology, 590, 2751–2765.
- de Koning, A. L., Werstuck, G. H., Zhou, J., & Austin, R. C. (2003). Hyperhomocysteinemia and its role in the development of atherosclerosis. Clinical Biochemistry, 36, 431–441.
- Ellingsen, I., Seljeflot, I., Arnesen, H., & Tonstad, S. (2009). Vitamin C consumption is associated with less progression in carotid intima media thickness in elderly men: A 3-year intervention study. Nutrition, Metabolism and Cardiovascular Diseases, 19, 8–14.
- Erskine, R. M., Jones, D. A., Maffulli, N., Williams, A. G., Stewart, C. E., & Degens, H. (2011). What causes in vivo muscle specific tension to increase following resistance training? Experimental Physiology, 96, 145–155.
- Erskine, R. M., Jones, D. A., Maganaris, C. N., & Degens, H. (2009). In vivo specific tension of the human quadriceps femoris muscle. European Journal of Applied Physiology, 106, 827–838.
- Erskine, R. M., Jones, D. A., Williams, A. G., Stewart, C. E., & Degens, H. (2010). Resistance training increases in vivo quadriceps femoris muscle specific tension in young men. Acta Physiologica, 199, 83–89.
- Franchi, M. V., Longo, S., Mallinson, J., Quinlan, J. I., Taylor, T., Greenhaff, P. L., & Narici, M. V. (2018). Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scandinavian Journal of Medicine & Science in Sports, 28, 846–853.
- Gorissen, S. H. M., Horstman, A. M. H., Franssen, R., Crombag, J. J. R., Langer, H., Bierau, J., … Van Loon, L. J. (2016). Ingestion of wheat protein increases in vivo muscle protein synthesis rates in healthy older men in a randomized trial. The Journal of Nutrition, 146, 1651–1659.
- Green, D. J., & Smith, K. J. (2018). Effects of exercise on vascular function, structure, and health in humans. Cold Spring Harbor Perspectives in Medicine, 8, a029819.
- Hartman, J. W., Tang, J. E., Wilkinson, S. B., Tarnopolsky, M. A., Lawrence, R. L., Fullerton, A. V., & Phillips, S. M. (2007). Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. The American Journal of Clinical Nutrition, 86, 373–381.
- Hopkins, N. D., van den Munckhof, I., Thijssen, D. H. J., Tinken, T. M., Cable, N. T., Stratton, G., & Green, D. J. (2013). Are changes in conduit artery function associated with intima-medial thickness in young subjects? European Journal of Preventive Cardiology, 20, 904–910.
- Huynh, N. N., & Chin-Dusting, J. (2006). Amino acids, arginase and nitric oxide in vascular health. Clinical and Experimental Pharmacology and Physiology, 33, 1–8.
- Janssen, I., Heymsfield, S. B., Wang, Z., & Ross, R. (2000). Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of Applied Physiology, 89, 81–88.
- Jones, D., Rutherford, O., & Parker, D. (1989). Physiological changes in skeletal muscle as a result of strength training. Quarterly Journal of Experimental Physiology: Translation and Integration, 74, 233–256.
- Kim, H., Caulfield, L. E., Garcia-Larsen, V., Steffen, L. M., Coresh, J., & Rebholz, C. M. (2019). Plant-based diets are associated with a lower risk of incident cardiovascular disease, cardiovascular disease mortality, and All-cause mortality in a general population of middle-aged adults. Journal of the American Heart Association, 8, e012865.
- Kokubo, Y., Watanabe, M., Higashiyama, A., Nakao, Y. M., Nakamura, F., & Miyamoto, Y. (2018). Impact of intima–media thickness progression in the common carotid arteries on the risk of incident cardiovascular disease in the suita study. Journal of the American Heart Association, 7, e007720.
- Krajčovičová-Kudláčková, M., Blažíček, P., Kopčová, J., Bederova, A., & Babinska, K. (2000). Homocysteine levels in vegetarians versus omnivores. Annals of Nutrition and Metabolism, 44, 135–138.
- Lee, M., & Wagenmakers, E.-J. (2013). Bayesian data analysis for cognitive science: A practical course. New York, NY: Cambridge University Press.
- Li, C.-I., Li, T.-C., Lin, W.-Y., Liu, C.-S., Hsu, C.-C., Hsiung, C. A., … Wang, C.-Y. (2015). Combined association of chronic disease and low skeletal muscle mass with physical performance in older adults in the Sarcopenia and translational aging research in Taiwan (START) study. BMC Geriatrics, 15, 11.
- Lin, C.-L., Fang, T.-C., & Gueng, M.-K. (2001). Vascular dilatory functions of ovo-lactovegetarians compared with omnivores. Atherosclerosis, 158, 247–251.
- Lynch, H. M., Wharton, C. M., & Johnston, C. S. (2016). Cardiorespiratory fitness and peak torque differences between vegetarian and omnivore endurance athletes: A cross-sectional study. Nutrients, 8, 726.
- Matsuzawa, Y., Kwon, T. G., Lennon, R. J., Lerman, L. O., & Lerman, A. (2015). Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: A systematic review and meta-analysis. Journal of the American Heart Association, 4, e002270.
- May, J. M., & Harrison, F. E. (2013). Role of vitamin C in the function of the vascular endothelium. Antioxidants & Redox Signaling, 19, 2068–2083.
- McPhee, J. S., Williams, A. G., Stewart, C., Baar, K., Schindler, J. P., Aldred, S., … Jones, D. A. (2009). The training stimulus experienced by the leg muscles during cycling in humans. Experimental Physiology, 94, 684–694.
- Messina, M., Lynch, H., Dickinson, J. M., & Reed, K. E. (2018). No difference between the effects of supplementing with soy protein versus animal protein on gains in muscle mass and strength in response to resistance exercise. International Journal of Sport Nutrition and Exercise Metabolism, 28, 674–685.
- Narici, M. V., Maganaris, C. N., Reeves, N. D., & Capodaglio, P. (2003). Effect of aging on human muscle architecture. Journal of Applied Physiology, 95, 2229–2234.
- Nebl, J., Haufe, S., Eigendorf, J., Wasserfurth, P., Tegtbur, U., & Hahn, A. (2019). Exercise capacity of vegan, lacto-ovo-vegetarian and omnivorous recreational runners. Journal of the International Society of Sports Nutrition, 16, 23.
- Ortega, R. M., Pérez-Rodrigo, C., & López-Sobaler, A. M. (2015). Dietary assessment methods: Dietary records. Nutricion Hospitalaria, 31, 38–45.
- Rebro, S. M., Patterson, R. E., Kristal, A. R., & Cheney, C. L. (1998). The effect of keeping food records on eating patterns. Journal of the Academy of Nutrition and Dietetics, 98, 1163.
- Rogerson, D., Maçãs, D., Milner, M., Liu, Y., & Klonizakis, M. (2018). Contrasting effects of short-term Mediterranean and vegan diets on microvascular function and cholesterol in younger adults: A comparative pilot study. Nutrients, 10, 1897.
- Sahni, S., Mangano, K. M., Hannan, M. T., Kiel, D. P., & McLean, R. R. (2015). Higher protein intake is associated with higher lean mass and quadriceps muscle strength in adult men and women. The Journal of Nutrition, 145, 1569–1575.
- Sobiecki, J. G., Appleby, P. N., Bradbury, K. E., & Key, T. J. (2016). High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European prospective investigation into cancer and nutrition–Oxford study. Nutrition Research, 36, 464–477.
- Tang, J. E., Moore, D. R., Kujbida, G. W., Tarnopolsky, M. A., & Phillips, S. M. (2009). Ingestion of whey hydrolysate, casein, or soy protein isolate: Effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. Journal of Applied Physiology, 107, 987–992.
- Thijssen, D. H., Bruno, R. M., van Mil, A. C., Holder, S. M., Faita, F., Greyling, A., … Luscher, T. (2019). Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. European Heart Journal, 40, 2534–2547.
- Thomas, D. T., Erdman, K. A., & Burke, L. M. (2016). Position of the academy of nutrition and dietetics, dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. Journal of the Academy of Nutrition and Dietetics, 116, 501–528.
- Thompson, F. E., & Subar, A. F. (2017). Dietary assessment methodology. In A.M. Coulston, C.J. Boushey, M.G. Ferruzzi, & L.M. Delahanty (Eds.), Nutrition in the prevention and treatment of disease (pp. 5–48). Amsterdam, The Netherlands: Elsevier.
- Tipton, K. D., Ferrando, A. A., Phillips, S. M., Doyle, D., Jr., & Wolfe, R. R. (1999). Postexercise net protein synthesis in human muscle from orally administered amino acids. American Journal of Physiology-Endocrinology and Metabolism, 276, E628–E634.
- Vereecken, C. A., Rossi, S., Giacchi, M. V., & Maes, L. (2008). Comparison of a short food-frequency questionnaire and derived indices with a seven-day diet record in Belgian and Italian children. International Journal of Public Health, 53, 297–305.
- Vrtovec, B., Keber, I., Gadzijev, A., Bardorfer, I., & Keber, D. (1999). Carotid intima-media thickness of young coronary patients. Coronary Artery Disease, 10, 407–412.
- World Medical Association (2013). World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. Jama, 310, 2191–2194.
- Xu, X., Wang, B., Ren, C., Hu, J., Greenberg, D. A., Chen, T., … Jin, K. (2017). Recent progress in vascular aging: Mechanisms and its role in age-related diseases. Aging and Disease, 8, 486–505.
