ABSTRACT
This review explores the effects of oestrogen and progesterone fluctuations across the menstrual cycle on fluid and electrolyte balance. The review aims to provide information on this topic for the exercising female but also for researchers working in this field. Beginning with a basic introduction to fluid and electrolyte balance, the review goes on to describe how oestrogen and progesterone have independent and integrated roles to play in the regulation of fluid and electrolyte balance. Despite evidence that oestrogen can influence the osmotic threshold for arginine vasopressin release, and that progesterone can influence aldosterone production, these actions do not appear to influence fluid retention, plasma volume changes at rest and during exercise, or electrolyte losses. However, the large inter-individual variations in hormonal fluctuations throughout the menstrual cycle may mean that specific individuals with high fluctuations could experience disturbances in their fluid and electrolyte balance. During phases of oestrogen dominance (e.g. late-follicular phase) heat dissipation is promoted, while progesterone dominance (e.g. mid-luteal phase) promotes heat conservation with overall higher basal body temperature. However, these responses do not consistently lead to any change in observed sweat rates, heat-stress, or dehydration during exercise. Finally, the literature does not support any difference in fluid retention during post-exercise rehydration periods conducted at different menstrual cycle phases. Although these mean responses largely reveal no effects on fluid and electrolyte balance, further research is required particularly in those individuals who experience high hormonal fluctuations, and greater exploration of oestrogen to progesterone interactions is warranted.
Hormonal variations throughout the menstrual cycle can impact nutritional requirements (Rehrer, McLay-Cooke, & Sims, Citation2017), sport performance (McNulty et al., Citation2020; Janse de Jonge Citation2003), brain function (Shepherd, Citation2001), cardiovascular function (Marsh & Jenkins, Citation2002), and injury rate and ligament laxity (Constantini, Dubnov, & Lebrun, Citation2005) in female exercisers. The cyclical changes in oestrogen and progesterone concentrations across the menstrual cycle also have been associated with fluctuations in body fluid regulation, and may have independent impacts on fluid and electrolyte balance (Giersch, Charkoudian, Steams, & Casa, Citation2020a; Stachenfeld, Citation2008). The focus of the present review will be limited to exploring the evidence for influences of oestrogen and progesterone on fluid and electrolyte balance during the menstrual cycle, primarily considering normally ovulating females. It is worth acknowledging that our review does not extensively cover effects of hormonal contraceptives used by approximately half of female athletes (Martin, Sale, Cooper, & Elliott-Sale, Citation2018). Since, different effects of oral oestrogen and progesterone combinations on fluid and electrolyte balance are evident (Giersch, Morrissey et al., Citation2020; Stachenfeld, Silva, Keefe, Kokoszka, & Nadel, Citation1999) readers are advised to consult these papers.
Regulation of fluid and electrolyte balance
Homeostasis to maintain a constant water and electrolyte balance involves the coordination of many inputs/outputs, including neural pathways and integrative centres in the brain and peripheral effectors (Zimmerman et al., Citation2016; ). Total body water (TBW) can be most simply subdivided into extra cellular fluid (ECF) and intra cellular fluid (ICF) compartments. ECF is composed of three major compartments: plasma, interstitial, and connective tissue water. The largest component is ICF which has been reported to be around 26 litres (59% of TBW) or 34% of total body mass in an average male and around 19 litres (61% of TBW) or 31% of total body mass in an average female (Ritz et al., Citation2008). At rest, a water deficit increases the ionic concentration of the extracellular fluid compartment (increased osmolality, decreased plasma volume) and this draws water from the intracellular compartment (Nose, Mack, Shi, & Nadel, Citation1988). Two receptors also sense this osmotic stimulus in the brain, one regulating drinking behaviour (thirst) and the other controlling renal function (Fitzsimons, Citation1998; Kanbay et al., Citation2019; Leib, Zimmerman, & Knight, Citation2016; Thornton, Citation2010). A fluid deficit will lead not only to a decrease in glomerular filtration rate and a subsequent renin-angiotensin-aldosterone system (RAAS) response to decrease sodium excretion but also will increase the release of arginine vasopressin (AVP) from the posterior pituitary to alter renal tubular water reabsorption (Stockand, Citation2010). These actions counter the reduced effective circulating volume and, when combined with the thirst response, drive increased fluid intake to restore body water balance. If there is an excess of water, the lower ionic concentration of body fluids (reduced osmolality, increased plasma volume) will result in the opposite actions. Thus, the kidneys play a central role in regulating inorganic ion composition and fluid volume in the internal environment.
Figure 1. Normal regulation of fluid and electrolyte balance showing usual processes for gain in body water (INPUT) and loss of body water (OUTPUT) with stimulation of hormonal controls of fluid and electrolyte regulation through arginine vasopressin (AVP) release, and activation of the renin-angiotensin-aldosterone system (RAAS).
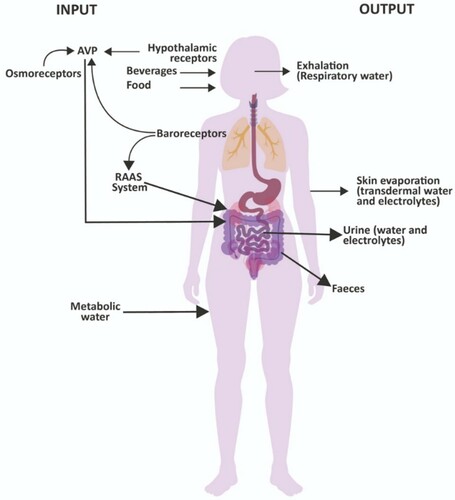
Sodium and water filter freely from the renal glomerular capillaries and undergo considerable reabsorption (usually more than 99%). Most sodium and water reabsorption (about 2/3rds) occurs in the proximal tube, but the primary hormonal regulation of reabsorption occurs in the collecting ducts. Sodium reabsorption is an active process happening in all tubular segments except the descending limb of the loop of Henle, and water reabsorption occurs by diffusion and is dependent upon sodium reabsorption. The kidneys also balance potassium intake with potassium excretion and are primarily responsible for maintaining total body potassium content. The main determinant of permeability, and consequently of water reabsorption, in the collecting tubes is the action of AVP (Hew-Butler, Citation2010). When AVP plasma concentration is high, the water permeability of the collecting tubes is increased. Water reabsorption is maximal, and the final urine volume is minimal (<1% of the filtered water). Without the action of AVP, the water permeability of the collecting tubes is very low and very little water is reabsorbed, allowing a greater amount of water left in the tubule to be excreted in the urine. Cardiovascular baroreceptors mediate posterior pituitary secretion of AVP with a low extracellular volume stimulating AVP secretion, and a high extracellular volume inhibiting it (). AVP is also affected by osmoreceptors in the hypothalamus with a high perfusing osmolality stimulating AVP secretion, and a low osmolality inhibiting it (Perrier et al., Citation2013; Schrier, Berl, & Anderson, Citation1979; Stockand, Citation2010; Thornton, Citation2010). The RAAS also plays a crucial role in body water volume regulation through actions on electrolyte balance. Plasma angiotensin II (ANG II) is elevated during salt depletion and reduced when the individual is sodium replete. Elevation of ANG II induces increased secretion of aldosterone from the adrenal cortex which subsequently stimulates sodium reabsorption by the renal cortical collecting ducts. During exercise, there is primarily a stimulus for the release of AVP caused by an increase in osmolality and a decrease in plasma volume (Hew-Butler, Citation2010; Sollanek, Staab, Kenefick, & Cheuvront, Citation2020) particularly in situations where fluid losses are high, such as with exercise and heat stress.
The role of thirst, sodium balance, and renal function are integrated to help regulate body water volume and maintain circulating blood volume and cardiovascular function (Armstrong & Johnson, Citation2018; Leib et al., Citation2016; McKinley & Johnson, Citation2004). Thirst is a crucial component of this body water regulatory mechanism. Key physiological signals for thirst are plasma hyperosmolality with consequential cellular dehydration and hypovolemia (Thompson, Bland, Burd, & Baylis, Citation1986). The need to drink can be driven by habitual, cultural, and psychogenic drivers, as well as by the regulatory response to a decrease in body water. Hypertonicity of the extracellular fluid, or increases in the circulating concentration of certain dipsogenic hormones (such as angiotensin and aldosterone) and neural signals from low- and high- pressure baroreceptors all regulate the thirst response (Johnson & Thunhorst, Citation1997). Under normal conditions these physiological regulators ensure that plasma volume and osmolality are preserved within normal limits. However, certain situations such as exercise can increase sweat losses and insensible water losses (respiratory water losses). In summary, body fluid and electrolyte balance are regulated by a complex integration of multiple physiological responses (). How these responses are influenced by other hormonal fluctuations such as oestrogen and progesterone will now become the focus in the remaining sections of this review.
Sex hormones and body fluid balance
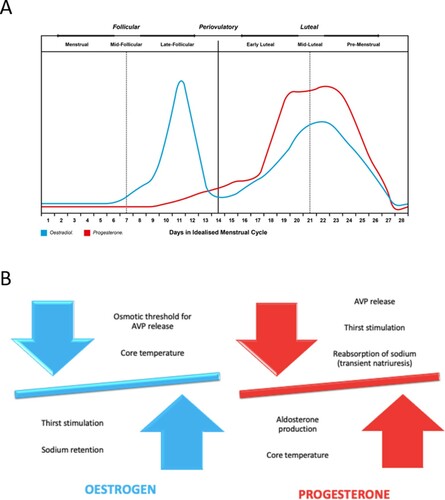
Oestrogen and progesterone have been reported to impact neural and hormonal systems that control thirst, fluid intake, sodium appetite, and renal fluid and sodium regulation (Wenner & Stachenfeld, Citation2017). Hormonal fluctuations over the menstrual cycle (A) lead to phases with elevated oestrogen only (late-follicular), elevated oestrogen and progesterone (mid-luteal), low oestrogen and progesterone (menses), or rising/declining hormone concentrations (early or mid-follicular and early or late luteal). In early studies, oral contraceptives were provided to young women in order to evaluate the influence of hormonal elevation on responses to hypertonic saline infusion (Calzone, Silva, Keefe, & Stachenfeld, Citation2001), and exercise-induced dehydration and subsequent rehydration (Stachenfeld et al., Citation1999). These early studies observed that the osmotic threshold for AVP release and thirst stimulation, during both hypertonic saline infusion and exercise-induced dehydration was lower when using hormonal contraceptives containing oestradiol, compared to either the follicular phase or to hormonal contraceptives that contained only progestins (Calzone et al., Citation2001; Stachenfeld et al., Citation1999). However, there were no effects of oestrogen manipulation on body fluid balance assessed by free water clearance either during hypertonic saline infusion, dehydration, or during rehydration. More recently, it has been suggested that oestrogen could have a significant positive relationship with copeptin (Stachenfeld & Keefe, Citation2002), a stable marker of AVP release (Bolignano et al., Citation2014), which further implies an interaction particularly between oestrogen and mechanisms of body fluid balance. These observations suggest that the shift in osmotic regulation of AVP and thirst represents an alteration in body water regulation to a lower plasma osmolality operating point during periods of high oestradiol exposure (Stachenfeld, Citation2008). While these studies with oral contraceptives in young women indicated a role for oestrogen in the osmotic regulation of AVP, they did not completely isolate the effects of oestrogen because progestins were present at high concentrations under both oral contraceptive conditions (Calzone et al., Citation2001; Stachenfeld, Citation2008; Stachenfeld et al., Citation1999).
Figure 2. Hormonal fluctuations of oestrogen and progesterone during the different phases of the menstrual cycle (A), and influence of oestrogen and progesterone on factors involved in regulation of body fluid and electrolyte balance (B).
To better isolate oestradiol effects from those of progesterone or progestins, Stachenfeld et al. performed a series of studies using a gonadotropin releasing hormone (GnRH) agonist to supress both oestradiol and progesterone. The suppression of endogenous hormone release was followed by controlled administration of oestradiol and progesterone in order to mimic the concentrations attained during the mid to late follicular and mid-luteal phases of a normal menstrual cycle. This ultimately enabled examination of hormone combinations on fluid balance (Stachenfeld, Citation2008). It was observed that oestradiol alone shifted the osmotic threshold for the release of AVP to a lower plasma osmolality. The authors interpreted the oestrogen-associated lowering of the osmotic threshold for AVP release as a lowering of the osmoregulatory set-point, rather than a change in fluid regulation, because the greater plasma levels of AVP did not seem to contribute to greater water retention (Stachenfeld, Citation2008). It is important to note that these studies used hypertonic saline (3% NaCl) infusion rather than exercise-dehydration protocols. Hypertonic saline infusion elevates plasma osmolality in a more dramatic fashion than observed during exercise-dehydration and is quite a different state than dehydration. Despite significant increases in thirst and AVP with hypertonic saline infusion there is also large intravascular fluid volume expansion, under resting conditions at least, as water is drawn from the extravascular space in response to the increased osmotic pressure (Stachenfeld, Citation2008).
Altering the threshold for AVP release could lead to inappropriately high AVP concentration, and may contribute to the onset of hyponatremia, and damaging consequences in the brain (Siegel et al., Citation2007; Speedy, Noakes, & Schneider, Citation2001; Verbalis, Citation2003). Since greater water retention from over-drinking or excessive sodium loss through sweating contribute to exercise associated hyponatremia (EAH), Stachenfeld and Taylor (Citation2009) examined whether sex hormones could influence body water and sodium regulation in women at high risk of EAH during endurance exercise. Their study revealed that more fluid was retained, and more sodium lost, when both oestradiol and progesterone were elevated. The authors suggested that when oestrogen and progesterone were both elevated (e.g. the luteal phase of the menstrual cycle) the monitoring of fluid and electrolyte balance in long duration endurance sports is of particular importance, particularly in women susceptible to symptomatic hyponatremia. It is important to emphasise that women who were most susceptible to EAH had lower body mass which could have exaggerated the impact of excessive water loads and sodium loss during exercise (Stachenfeld & Taylor, Citation2009).
Plasma volume (PV) shifts have been reported during normal menstrual cycles, with PV at its highest during the pre-ovulatory phase (high oestrogen/low progesterone; Sims, Rehrer, Bell, & Cotter, Citation2007). Additionally, Stephenson and Kolka (Citation1988) reported lower resting PV during the mid-luteal phase (high oestrogen/high progesterone) than during the early follicular phase, and reported that PV may fall by up to 8% during the mid-luteal phase when both oestrogen and progesterone are elevated (Rehrer et al., Citation2017; Stephenson & Kolka, Citation1988). However, several investigations have revealed no significant differences in plasma volume across menstrual cycle phases (Chapman et al., Citation1997; Horvath & Drinkwater, Citation1982; Miskec et al., Citation1997). Studies with larger participant numbers and menstrual cycle phase verification through hormone measurements typically observe no change in plasma volume shifts between the phases of the menstrual cycle during exercise (De Souza et al., Citation1990; McCracken, Ainsworth, & Hackney, Citation1994). It is worth noting that these studies have only conducted testing at early- or mid-follicular versus mid-luteal phases (Janse de Jonge, Citation2003). In contrast, plasma volume may be at its highest during the late follicular phase (Sims et al., Citation2007) meaning that further study on these PV shifts may be warranted (B). Therefore, it seems from the evidence available that an elevation in oestrogen, and not progesterone, plays a role in lowering the osmotic threshold for AVP release, but this does not influence fluid retention under the study conditions examined to date. Similarly, hormonal fluctuations do not appear to influence PV at rest consistently, and do not appear to be evident during exercise.
Sex hormones and electrolyte balance
Progesterone has been reported to inhibit aldosterone-dependent sodium reabsorption at distal sites in the nephron and produce a transient natriuresis followed by a compensatory stimulation of the RAAS (Oelkers, Citation1996). Since progesterone is a precursor of aldosterone, it can compete for the mineralocorticoid receptor, leading to reduced aldosterone action or to compensatory increases in aldosterone synthesis followed by increases in renal sodium and water retention (Myles & Funder, Citation1996). Thus, progesterone is postulated to mediate the observed luteal phase increase in aldosterone concentration (Quinkler et al., Citation2002). However, changes in sodium intake across the menstrual cycle could potentially confound results in studies that have reported higher serum or urinary aldosterone concentration during the luteal phase. In these studies, sodium intake was either not controlled (Chapman et al., Citation1997; Hirshoren et al., Citation2002) or not documented (Michelakis, Yoshida, & Dormois, Citation1975). Sodium intake is a primary determinant of aldosterone production via modulation of the RAAS (Adler, Moore, Hollenberg, & Williams, Citation1987). Indeed, luteal phase salt restriction is sometimes advised to ameliorate pre-menstrual symptoms (Grady-Weliky, Citation2003), and a reduction in dietary sodium intake in the pre-menstrual period could potentially affect luteal phase aldosterone synthesis (Szmuilowicz et al., Citation2006). Since differences in sodium balance independently influence RAAS hormones, Szmuilowicz et al. (Citation2006) aimed to compare aldosterone concentration between menstrual phases in those with high and low sodium intake and excretion. These authors demonstrated that urinary and serum aldosterone levels, and the increase in serum aldosterone in response to infused ANG II, were significantly greater in the luteal versus the follicular phase. This effect was evident only among women with high sodium intake and excretion rate and high progesterone levels, but not high oestradiol. Subsequently, using an isolated rat zona glomerulosa cell model, progesterone addition was observed to cause a 2.8-fold increase in aldosterone production, whereas the addition of oestradiol had no effect (Szmuilowicz et al., Citation2006). Therefore, these data suggest that progesterone may directly influence adrenal aldosterone production and that this may be one mechanism underlying increased aldosterone concentration during physiological high-progesterone states (Szmuilowicz et al., Citation2006). Thus, if progesterone independently stimulates aldosterone production, then volume retention could occur through additional sodium reabsorption (Szmuilowicz et al., Citation2006), most likely evident in the mid-luteal phase. Overall, from the observations presented above it could be inferred that, due to progesterone sensitivity, some women might present elevated aldosterone and sodium retention during the mid-luteal phase of the menstrual cycle, especially if they have high sodium intake (B). Nevertheless, previous observations have demonstrated no difference in net sodium balance between trials conducted in the mid-follicular and the mid-luteal phases of the menstrual cycle in active women when studied during a dehydration-rehydration protocol (Rodriguez-Giustiniani & Galloway, Citation2019). However, the large inter-individual variability in hormonal responses within menstrual cycle phases reported across several studies () indicates that considerable differences in progesterone production and adrenal sensitivity to progesterone could contribute to variability in the degree of fluid retention among different individuals. Indeed, many of the null findings reported between phases in experimental studies reflect group mean responses. Thus, it may well be possible that some female athletes will experience fluid and electrolyte balance disturbances.
Table 1. Differences in female sex hormone concentrations reported across menstrual cycle phases in a range of studies assessing menstrual cycle influences on fluid balance, sport performance, injury risk, and thermoregulation. Values are mean ± SD unless otherwise indicated. The overall mean (range) of values highlights the large inter-individual variability in responses across menstrual cycle phases.
Menstrual cycle and perceived fluid retention
Despite the lack of solid experimental evidence, many women perceive changes in fluid retention or “bloating” throughout their menstrual cycle (Sweeney, Citation1934; White, Hitchcock, Vigna, & Prior, Citation2011). Several prospective daily rating studies have reported perceived peak fluid retention at the onset of menstrual flow (Meaden, Hartlage, & Cook-Karr, Citation2005; Taylor, Citation1979), but a hormonal influence underlying these changes remains to be identified. White et al. (Citation2011) reported menstrual and mid-cycle patterns of self-reported “fluid retention” in 765 menstrual cycles of 62 healthy women. This observational prospective study revealed that the temporal pattern of women’s fluid retention experiences across the menstrual cycle was characterised by a perceived peak fluid retention on the first day of menstruation, rather than during the mid-luteal or late-follicular phases of the menstrual cycle, where progesterone and/or oestrogen would be elevated. There was no indication of a relationship between pooled hormone concentrations of oestradiol and progesterone on self-reported fluid retention scores. It has been speculated that perhaps there is a lag of fluid dynamics in response to previous higher hormone concentrations during the mid-luteal phase of the menstrual cycle, leading to perceived fluid retention early in menses. Thus, it seems there is a mismatch between any experimental evidence for effects of oestrogen and progesterone on body fluid and electrolyte balance, with self-reported fluid retention. Further research may need to explore the effects of the menstrual cycle phase transition periods (e.g. declining oestrogen or progesterone hormone concentration) over a few days, and the potential impact on self-reported fluid retention as well as fluid and electrolyte balance.
Sex hormones, thermoregulation, and dehydration
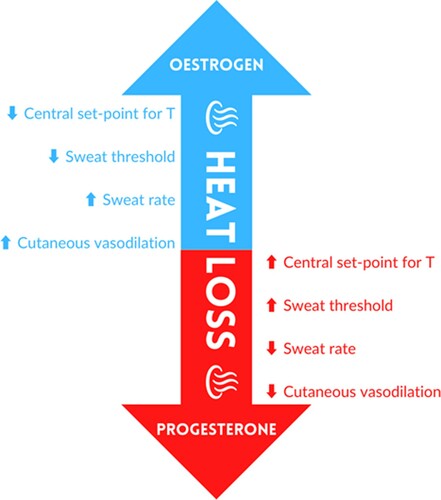
Men and women do not exhibit significant quantitative differences in physiological thermoregulatory responses to exercise or body heating (Charkoudian & Stachenfeld, Citation2014) particularly when factors such as fitness and body size are considered (Gagnon & Kenny, Citation2011; Shapiro, Pandolf, Avellini, Pimental, & Goldman, Citation1980). However, oestrogen and progesterone may have important influences that can alter individual thermoregulatory responses at various points throughout the menstrual cycle (Charkoudian & Stachenfeld, Citation2014). The progesterone peak that occurs during the mid-luteal phase of the menstrual cycle is related to higher resting core temperature (∼0.5 °C increase) and a rightward shift in the core temperature threshold for thermoregulatory peripheral effector responses (Stephenson & Kolka, Citation1993). This shift appears to be due to changes in the onset of active vasodilation to a higher body core temperature (Charkoudian & Johnson, Citation1999). Conversely, during the late-follicular or pre-ovulatory phase of the menstrual cycle, the phase characterised by rising oestradiol concentration, resting core temperature and threshold for cutaneous vasodilation and sweating are shifted to a lower core temperature during exercise (Stephenson & Kolka, Citation1999). This shift potentially augments peripheral vasodilation and sweating response (Charkoudian & Stachenfeld, Citation2015; B & ).
The proposed influence of oestrogen and progesterone on heat loss/heat conservation mechanisms is schematised in . It seems that in terms of basal body temperature, oestradiol dominance (e.g. late-follicular phase) promotes heat dissipation (Charkoudian & Stachenfeld, Citation2014), while progesterone dominance (e.g. mid-luteal phase) appears to promote heat conservation, with overall higher body temperature (Charkoudian & Stachenfeld, Citation2014). The consensus from the existing literature indicates that oestradiol and progesterone have opposite effects on temperature regulation, but that progesterone effects appear to dominate when the two are concomitantly increased in the mid-luteal phase (Brooks-Asplund, Cannon, & Kenney, Citation2000; Charkoudian & Johnson, Citation1999; Charkoudian & Stachenfeld, Citation2014; Kolka & Stephenson, Citation1989; Kolka & Stephenson, Citation1997). The sweating or skin blood flow response’s sensitivity refers to the increment in the response for a given increment in body temperature. Hence, a person with higher sensitivity would be more responsive to a given increase in body temperature (Charkoudian & Stachenfeld, Citation2015). While one study has shown a greater sensitivity of sweating as a function of mean body temperature in the luteal phase (Hessemer & Bruck, Citation1985), there does not appear to be a consistent influence of reproductive hormones on the sensitivity of skin blood flow or sweating responses (when expressed as a function of core body temperature) during exercise or passive body heating (Charkoudian & Johnson, Citation1997; Charkoudian & Johnson, Citation1999; Stephenson & Kolka, Citation1985). Several investigations have indicated that a higher core temperature is maintained during exercise performed in the luteal phase, while sweat rate remains unchanged (Fukuoka et al., Citation2002; Pivarnik, Marichal, Spillman, & Morrow, Citation1992) or reduced (Kuwahara, Inoue, Abe, Sato, & Kondo, Citation2005). Our recent study (Rodriguez-Giustiniani & Galloway, Citation2019), revealed no differences in overnight fluid losses, exercise time to achieve 2% body mass loss, or estimated sweat rate during exercise, between late-follicular and mid-luteal phases of the menstrual cycle. These data support prior observations and suggest that the change in the hormonal milieu across the menstrual cycle does not affect sweat rate during exercise-heat stress. A recent systematic review and meta-analysis on thermoregulation during exercise in the heat, in relation to the menstrual cycle, reported elevated initial internal body temperature pre-exercise and post-exercise in the luteal phase compared to the follicular phase without any observed difference in sweat rate or skin temperature (Giersch, Morrissey et al., Citation2020).
Figure 3. Theoretical influences of oestrogen and progesterone on heat dissipation / conservation mechanisms.
It is known that dehydration (>2% body mass loss) can have a negative effect on aerobic and anaerobic performance (Cheuvront & Kenefick, Citation2014; James, Moss, Henry, Papadopoulou, & Mears, Citation2017), mainly when exercise is performed in the heat. The relationship and interaction between hydration and the menstrual cycle’s hormonal fluctuations are still unclear, but any interaction appears to be small. However, any adverse impacts might compound exercise performance decrements in dehydrated females (Giersch, Charkoudian et al., Citation2020). Although previous investigations have examined the impact of the menstrual cycle phase on exercise performance (Janse de Jonge, Citation2003; McNulty et al., Citation2020), none have assessed performance in different phases of the menstrual cycle as a function of hydration status (Giersch, Charkoudian et al., Citation2020). Giersch, Colburn et al. (Citation2020) recently investigated the impact of the menstrual cycle phase (late-follicular vs mid-luteal) on fluid balance responses to a 24-hour fluid restriction. Their results revealed no menstrual cycle phase differences in body fluid regulation following 24-h fluid restriction for body mass loss, urinary indices, plasma osmolality, or copeptin concentration. These observations suggest that progesterone does not have independent or additive effects on body fluid regulation in the setting of mild passive dehydration. However, although progesterone was increased 4- to 5-fold during the mid-luteal phase of the menstrual cycle, the average progesterone values reported did not reach >6 ng/mL or >19 nmol/L (expected progesterone threshold value for the mid-luteal phase; Speroff & Vande Wiele, Citation1971). The lack of phase differences during fluid restriction may therefore be due to lower-than-expected progesterone concentration in the study participants (Giersch, Morrissey et al., Citation2020), and it cannot be ruled out that progesterone effects may be dose-dependent (Stachenfeld & Keefe, Citation2002; Stachenfeld & Taylor, Citation2005). Taken together these data suggest that there is a change in sweating sensitivity across phases, but this does not appear to result in any greater sweat losses/dehydration or thermoregulatory issues during exercise-heat stress situations. However, it must be acknowledged that there may be considerable individual variability in response dependent upon the magnitude of progesterone elevation during the mid-luteal phase. Several studies (Kuwahara et al., Citation2005; Lei et al., Citation2017; Notley, Dervis, Poirier, & Kenny, Citation2019) have highlighted that training status may directly or indirectly affect absolute oestrogen and progesterone concentrations as well as their fluctuation between phases. Thus, training status may also have an influence on key outcomes such as basal temperature and sweating. Future studies may wish to assess oestrogen to progesterone ratios to gain insights into the interaction of these hormones on key fluid and electrolyte balance outcomes in athletes of differing training status.
Sex hormones and post-exercise rehydration
As previously indicated, few studies have reported differences in fluid and electrolyte balance during the normal menstrual cycle phases at rest, during passive dehydration, or during exercise-heat stress (Rodriguez-Giustiniani & Galloway, Citation2019; Stachenfeld & Keefe, Citation2002; Stachenfeld & Taylor, Citation2005). One further model that may be useful in examining the potential effect of these hormones on fluid and electrolyte balance is the investigation of post-exercise rehydration responses. Three studies have taken this approach. Yasuda, Kawal, Hara, Iide, and Matsamura (Citation2013) evaluated the effects of the menstrual cycle phase on hydration status following exercise in nine eumenorrheic female basketball players. Hydration status in the mid-follicular phase was found to be similar to that of the mid-luteal phase. Similarly, Maughan, McArthur, and Shirreffs (Citation1996) observed that the acute restoration of fluid balance after exercise-induced hypohydration was unaffected between mid-follicular and mid-luteal or late luteal phases in five healthy untrained eumenorrheic young women. These observations suggest that post-exercise rehydration is not directly affected by menstrual cycle hormonal fluctuations; however, neither of these studies verified phases through hormone concentration analyses, nor included the late-follicular phase among the phases examined. Rodriguez-Giustiniani and Galloway (Citation2019) compared the restoration of fluid and electrolyte balance after dehydration during the late-follicular versus the mid-luteal phase (menstrual cycle phase was confirmed through blood hormone concentrations) in a group of ten eumenorrheic active females. Our study revealed no differences, beyond typical test-retest variation, in cumulative urine output, net fluid balance, percentage of fluid retained, electrolyte balance, urine osmolality, or thirst intensity between the phases evaluated. Although it has been reported that greater water retention could be induced by oestrogen dominance (Stachenfeld & Taylor, Citation2009), our data demonstrated that during the late-follicular phase, there was no difference in sweating response, fluid retention or electrolyte balance during rehydration compared with the mid-luteal phase (Rodriguez-Giustiniani & Galloway, Citation2019). These observations were true throughout the range of individual hormonal fluctuations observed in the participants studied. In summary, from the limited number of studies in this area it appears that there is no overall difference in fluid retention/excretion during post-exercise rehydration between phases of the menstrual cycle.
Summary
Current evidence supports a role for female sex hormones to influence thirst and fluid and electrolyte balance. However, studies to date indicate that these do not contribute to whole-body water retention or significantly affect plasma volume at rest or during exercise. Furthermore, despite internal body temperature changes related to progesterone peak during the mid-luteal phase of the menstrual cycle, it seems that there is no significant effect of these fluctuations in core temperature on heat dissipation mechanisms in exercising females. The influence that oestrogen and progesterone variations across the menstrual cycle have on dehydration during exercise is less well studied, so further research is warranted. However, fluctuations in female sex hormones do not appear to affect fluid replacement/retention after exercise. It is worth highlighting that there are considerable inter-individual variations in oestrogen and progesterone fluctuation during the menstrual cycle. This consideration makes it plausible that some women on the upper end of these hormonal ranges may experience disturbances in fluid and electrolyte balance, heat dissipation mechanisms during exercise, and a possibly elevated risk for exercise-induced hyponatremia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abt, J., Sell, T., Laudner, K., McCrory, J., Loucks, T., Berga, S., & Lephart, S. (2007). Neuromuscular and biochemical characteristics do not vary across the menstrual cycle. Knee Surgery, Sports Traumatology, Arthroscopy, 15(7), 901–907. doi:https://doi.org/10.1007/s00167-007-0302-3.
- Adler, G., Moore, T., Hollenberg, N., & Williams, G. (1987). Changes in adrenal responsiveness and potassium balance with shifts in sodium intake. Endocrinology Research, 13(4), 419–445. doi:https://doi.org/10.3109/07435808709035467
- Ansdell, P., Brownstein, C., Škarabot, H. K., Simones, D., Thomas, K., Howatson, G., … Goodall, S. (2019). Menstrual cycle-associated modulations in neuromuscular function and fatigability of the knee extensors in eumenorrheic women. Journal of Applied Physiology, 126(6), 1701–1712. doi:https://doi.org/10.1152/japplphysiol.01041.2018
- Armstrong, L., & Johnson, E. (2018). Water intake, water balance, and the elusive daily water requirement. Nutrients, 10(12), 1928. doi:https://doi.org/10.3390/nu10121928.
- Beidleman, B., Rock, P., Muza, S., Fulco, C., Forte, V., & Cymerman, A. (1999). Exercise VE and physical performance at altitude are not affected by menstrual cycle phase. Journal of Applied of Physiology, 86(5), 1519–1526. doi:https://doi.org/10.1152/jappl.1999.86.5.1519.
- Bolignano, D., Cabassi, A., Fiaccadori, E., Ghigo, E., Pasquali, R., Peracino, A., … Zoccali, C. (2014). Copeptin (CTproAVP), a new tool for under-standing the role of vasopressin in pathophysiology. Clinical Chemistry and Laboratory Medicine, 52(10), 1447–1456. doi:https://doi.org/10.1515/cclm-2014-0379
- Brooks-Asplund, E., Cannon, J., & Kenney, W. (2000). Influence of hormone replacement therapy and aspirin on temperature regulation in post- menopausal women. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 279(3), R839–R848. doi:https://doi.org/10.1152/ajpregu.2000.279.3.R839
- Calzone, W., Silva, C., Keefe, D., & Stachenfeld, N. (2001). Progesterone does not alter osmotic regulation of AVP. American Journal of physiology. Regulatory, Integrative and Comparative Physiology, 281(6), R2011–R2020. doi:https://doi.org/10.1152/ajpregu.2001.281.6.R2011
- Chapman, A., Zamudio, S., Woodmansee, W., Merouani, A., Osorio, F., Johnson, A., … Schrier, R. (1997). Systemic and renal hemodynamic changes in the luteal phase of the menstrual cycle mimic early pregnancy. American Journal of Physiology, 273(Pt 2), F777–F782. doi:https://doi.org/10.1152/ajprenal.1997.273.5.F777
- Charkoudian, N., & Johnson, J. (1997). Modification of active cutaneous vasodilation by oral contraceptive hormones. Journal of Applied Physiology, 83(6), 2012–2018. doi:https://doi.org/10.1152/jappl.1997.83.6.2012
- Charkoudian, N., & Johnson, J. (1999). Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. American Journal of Physiology. Heart and Circulatory Physiology, 276(5), H1634–H1640. doi:https://doi.org/10.1152/ajpheart.1999.276.5.H1634
- Charkoudian, N., & Stachenfeld, N. (2014). Reproductive hormone influences on thermoregulation in women. Comprehensive Physiology, 4(2), 793–804. doi:https://doi.org/10.1002/cphy.c130029
- Charkoudian, N., & Stachenfeld, N. (2015). Sex hormones effect on autonomic mechanisms of thermoregulation in humans. Autonomic Neuroscience, 196, 75–80. doi:https://doi.org/10.1016/j.autneu.2015.11.004
- Cheuvront, S., & Kenefick, R. (2014). Dehydration: Physiology, assessment, and performance effects. Comprehensive Physiology, 4(1), 257–285. doi:https://doi.org/10.1002/cphy.c130017
- Constantini, N., Dubnov, G., & Lebrun, C. (2005). The menstrual cycle and sport performance. Clinics is Sports Medicine, 24(2), e51–e82. doi:https://doi.org/10.1016/j.csm.2005.01.003
- De Souza, M., Maguire, M., Rubin, R., et al. (1990). Effects of menstrual phase and amenorrhea on exercise performance in runners. Medicine and Science in Sports and Exercise, 22(5), 575–580. doi:https://doi.org/10.1249/00005768-199010000-00006
- De Souza, M., Toombs, R., Scheid, J., O’Donell, E., West, S., & Williams, A. (2010). High prevalence of subtle and severe menstrual disturbances in exercising women: Confirmation using daily hormone measures. Human Reproduction, 25(2), 491–593. doi:https://doi.org/10.1093/humrep/dep411.
- Elliott, K., Cable, N., Reilly, T., & Diver, M. (2003). Effect of menstrual cycle phase on the concentration of bioavailable 17-beta oestradiol and testosterone and muscle strength. Clinical Science, 105(6), 663–669. doi:https://doi.org/10.1042/CS20020360
- Fitzsimons, J. (1998). Angiotensin, thirst, and sodium appetite. Physiological Reviews, 78(3), 583–686. doi:https://doi.org/10.1152/physrev.1998.78.3.583.
- Fukuoka, Y., Kaneko, Y., Takita, C., Hirakawa, M., Kagawa, H., & Nakamura, Y. (2002). The effects of exercise intensity on thermoregulatory responses to exercise in women. Physiology and Behavior, 76(4-5), 567–574. doi:https://doi.org/10.1016/S0031-9384(02)00781-3
- Gagnon, D., & Kenny, G. P. (2011). Sex modulates whole-body sudomotor thermosensitivity during exercise. Journal of Physiology, 589(Pt 24), 6205–6217. doi:https://doi.org/10.1113/jphysiol.2011.219220
- Giersch, G., Charkoudian, N., Steams, E., & Casa, D. (2020). Fluid balance and hydration consideration for women: Review and future directions. Sports Medicine, 50(2), 253–261. doi:https://doi.org/10.1007/s40279-019-01206-6
- Giersch, G., Colburn, A., Morrissey, M., Butler, C., Pruchnicki, M., Kavouras, S., … Casa, D. (2020). Effects of sex and menstrual cycle on volume-regulatory responses to 24-h fluid restriction. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 319(5), R560–R565.
- Giersch, G., Morrissey, M., Katch, R., Colburn, A., Sims, S., Stachenfeld, N., & Casa, D. (2020). Menstrual cycle and thermoregulation during exercise in the heat: A systematic review and meta-analysis. Journal of Science and Medicine in Sport, 23(12), 1134–1140. doi:https://doi.org/10.1016/j.jsams.2020.05.014
- Grady-Weliky, T. (2003). Clinical practice. Premenstrual dysphoric disorder. New England Journal of Medicine, 348(5), 433–438. doi:https://doi.org/10.1056/NEJMcp012067
- Hessemer, V., & Brück, K. (1985). Influence of menstrual cycle on thermoregulatory, metabolic, and heart rate responses to exercise at night. Journal of Applied Physiology, 59(6), 1911-1917. doi:https://doi.org/10.1152/jappl.1985.59.6.1911
- Hew-Butler, T. (2010). Arginine vasopressin, fluid balance and exercise: Is exercise-associated hyponatraemia a disorder of arginine vasopressin secretion? Sports Medicine, 40(6), 459–479. doi:https://doi.org/10.2165/11532070-000000000-00000
- Hirshoren, N., Tzoran, I., Makrienko, I., Edoute, Y., Plawner, M., Itskovitz-Eldor, J., & Jacob, G. (2002). Menstrual cycle effects on the neurohumoral and autonomic nervous systems regulating the cardiovascular system. Journal of Clinical Endocrinology and Metabolism, 87(4), 1569–1575. doi:https://doi.org/10.1210/jcem.87.4.8406
- Horvath, S., & Drinkwater, B. (1982). Thermoregulation and the menstrual cycle. Aviation, Space, and Environmental Medicine, 53(8), 790–794.
- James, L., Moss, J., Henry, J., Papadopoulou, C., & Mears, S. (2017). Hypohydration impairs endurance performance: A blinded study. Physiological Reports, 5(12), e13315. doi:https://doi.org/10.14814/phy2.13315
- Janse de Jonge, X. (2003). Effects of the menstrual cycle on exercise performance. Sports Medicine, 33(11), 833–851. doi:https://doi.org/10.2165/00007256-200333110-00004
- Janse de Jonge, X., Thompson, M., Chuter, V., Silk, L., & Thom, J. (2012). Exercise performance over the menstrual cycle in temperate and hot, humid condition. Medicine and Science in Sports and Exercise, 44(11), 2190–2198. doi:https://doi.org/10.1249/MSS.0b013e3182656f13
- Jarvis, S., Vangundy, T., Galbreath, M., Shibata, S., Okazaki, K., Reelick, M., et al. (2011). Sex differences in the modulation of vasomotor sympathetic outflow during static handgrip exercise in healthy young humans. American Journal of Physiology. Regulatory, Integrative, and Comparative Physiology, 301(1), R193–R200. doi:https://doi.org/10.1152/ajpregu.00562.2010
- Johnson, A., & Thunhorst, R. (1997). The neuroendocrinology of thirst and salt appetite: Visceral sensory signals and mechanisms of central integration. Frontiers in Neuroendocrinology, 18(3), 292–353.
- Kanbay, M., Yilmaz, S., Dincer, N., Ortiz, A., Sag, A., Covic, A., … Afsar, B. (2019). Antidiuretic hormone and serum osmolarity physiology and related outcomes: What is old, what is new, and what is unknown? The Journal of Clinical Endocrinology and Metabolism, 104(11), 5406–5420. doi:https://doi.org/10.1210/jc.2019-01049
- Kolka, M., & Stephenson, L. (1989). Control of sweating during the human menstrual cycle. European Journal of Applied Physiology, 58(8), 890–895. doi:https://doi.org/10.1007/BF02332224.
- Kolka, M., & Stephenson, L. (1997). Resetting the thermoregulatory set-point by endogenous estradiol or progesterone in women. Annals of the New York Academy of Sciences, 813, 204–206. doi:https://doi.org/10.1111/j.1749-6632.1997.tb51694.x
- Kubo, K., Miyamoto, M., Tanaka, S., Maki, A., Tsunoda, N., & Kanehisa, H. (2009). Muscle and tendon properties during menstrual cycle. International Journal of Sports Medicine, 30(2), 139–143. doi:https://doi.org/10.1055/s-0028-1104573
- Kuwahara, T., Inoue, Y., Abe, M., Sato, Y., & Kondo, N. (2005). Effects of menstrual cycle and physical training on heat loss responses during dynamic exercise at moderate intensity in a temperate environment. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 288(55), R1347–R1353. doi:https://doi.org/10.1152/ajpregu.00547.2004
- Lei, T., Stannard, S., Perry, B., Schlader, Z., Cotter, J., & Mündel, T. (2017). Influence of menstrual phase and arid vs. Humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. Journal of Physiology, 595(9), 2823–2837. doi:https://doi.org/10.1113/JP273176
- Leib, D., Zimmerman, C., & Knight, Z. (2016). Thirst. Current Biology, 26(24), R1260–R1265. doi:https://doi.org/10.1016/j.cub.2016.11.019
- Marsh, S., & Jenkins, D. (2002). Physiological responses to the menstrual cycle: Implications for the development of heat illness in female athletes. Sports Medicine, 32(10), 601–614. doi:https://doi.org/10.2165/00007256-200232100-00001
- Martin, D., Sale, C., Cooper, S. B., & Elliott-Sale, K. J. (2018). Period prevalence and perceived side effects of hormonal contraceptive use and the menstrual cycle in elite athletes. International Journal of Sports Physiology and Performance, 13(7), 926–932. doi:https://doi.org/10.1123/ijspp.2017-0330
- Maughan, R., McArthur, M., & Shirreffs, S. (1996). Influence of menstrual status on fluid replacement after exercise induced dehydration in healthy young women. British Journal of Sports Medicine, 30(1), 41–47. doi:https://doi.org/10.1136/bjsm.30.1.41
- McCracken, M., Ainsworth, B., & Hackney, A. (1994). Effects of the menstrual cycle phase on the blood lactate responses to exercise. European Journal of Applied Physiology and Occupational Physiology, 69(2), 174–175. doi:https://doi.org/10.1007/BF00609412
- McKinley, M., & Johnson, A. (2004). The physiological regulation of thirst and fluid intake. News Physiological Sciences, 19, 1–6. DOI:https://doi.org/10.1152/nips.01470.2003
- McNulty, K., Elliot-Sale, K., Dolan, E., Swinton, P., Ansdell, P., Goodall, S., … Hicks, K. (2020). The effects of menstrual cycle phase on exercise performance in eumenorrheic women: A systematic review and meta-analysis. Sports Medicine, 50(10), 1813–1827. doi:https://doi.org/10.1007/s40279-020-01319-3
- Meaden, P., Hartlage, S., & Cook-Karr, J. (2005). Timing and severity of symptoms associated with the menstrual cycle in a community-based sample in the midwestern United States. Psychiatry Research, 134(1), 27–36. doi:https://doi.org/10.1016/j.psychres.2005.01.003
- Michelakis, A., Yoshida, H., & Dormois, J. (1975). Plasma renin activity and plasma aldosterone during the normal menstrual cycle. American Journal of Obstetrics and Gynecology, 123(7), 724–726. doi:https://doi.org/10.1016/0002-9378(75)90495-0
- Miskec, C., Potteiger, J., Nau, K., et al. (1997). Do varying environmental and menstrual cycle conditions affect anaerobic power output in female athletes? Journal of Strength and Conditioning Research, 11(4), 219–223.
- Montgomery, M., & Shultz, S. (2010). Isometric knee-extension and knee-flexion torque production during early follicular and postovulatory phases in recreationally active women. Journal of Athletic Training, 45(6), 586–593. doi:https://doi.org/10.4085/1062-6050-45.6.586
- Myles, K., & Funder, J. (1996). Progesterone binding to mineralocorticoid receptors: In vitro and in vivo studies. American Journal of Physiology. Endocrinology and Metabolism, 270 (4 Pt 1), E601–E607. doi:https://doi.org/10.1152/ajpendo.1996.270.4.E601
- Nose, H., Mack, G., Shi, X., & Nadel, E. (1988). Shift in body fluid compartments after dehydration in humans. Journal of Applied Physiology, 65 (1), 318–324. doi:https://doi.org/10.1152/jappl.1988.65.1.318
- Notley, S., Dervis, S., Poirier, M., & Kenny, G. (2019). Menstrual cycle phase does not modulate whole body heat loss during exercise in hot, dry conditions. Journal of Applied Physiology, 126(2), 286–293. doi:https://doi.org/10.1152/japplphysiol.00735.2018
- Oelkers, W. (1996). Effects of estrogens and progestogens on the renin-aldosterone system and blood pressure. Steroids, 61(4), 166–171. doi:https://doi.org/10.1016/0039-128x(96)00007-4
- Perrier, E., Rondeau, P., Poupin, M., Le Bellego, L., Armstrong, L., Lang, F., … Klein, A. (2013). Relation between urinary hydration biomarkers and total fluid intake in healthy adults. European Journal of Clinical Nutrition, 67(9), 939–943. doi:https://doi.org/10.1038/ejcn.2013.93.
- Pivarnik, J., Marichal, C., Spillman, T., & Morrow, J. (1992). Menstrual cycle phase affects temperature regulation during endurance exercise. Journal of Applied Physiology, 72(2), 543–548. doi:https://doi.org/10.1152/jappl.1992.72.2.543
- Quinkler, M., Meyer, B., Bumke-Vogt, C., Grossmann, C., Gruber, U., Oelkers, W., … Bahr, V. (2002). Agonistic and antagonistic properties of progesterone metabolites at the human mineralocorticoid receptor. European Journal of Endocrinology, 146(6), 789–799. doi:https://doi.org/10.1530/eje.0.1460789
- Rehrer, N., McLay-Cooke, R., & Sims, S. (2017). Nutritional strategies and sex hormones interactions on women. In A. C. Hackey (Ed.), Sex hormones, exercise and women (pp. 87–112). Springer: Cham. doi:https://doi.org/10.1007/978-3-319-44558-8_6.
- Ritz, P., Vol, S., Berrut, G., Tack, I., Arnaud, M. J., & Tichet, J. (2008). Influence of gender and body composition on hydration and body water spaces. Clinical Nutrition, 27(5), 740–746. doi: https://doi.org/10.1016/j.clnu.2008.07.010
- Rodriguez-Giustiniani, P., & Galloway, S. (2019). Influence of peak menstrual cycle hormonal changes on restoration of fluid balance after induced dehydration. International Journal of Sports Nutrition and Exercise Metabolism, 29(6), 651–657. doi:https://doi.org/10.1123/ijsnem.2019-0105
- Schrier, R., Berl, T., & Anderson, R. (1979). Osmotic and nonosmotic control of vasopressin release. American Journal of Physiology, 236(4), F321–F332. doi:https://doi.org/10.1152/ajprenal.1979.236.4.F321
- Shapiro, Y., Pandolf, K., Avellini, B., Pimental, N., & Goldman, R. (1980). Physiological responses of men and women to humid and dry heat. Journal of Applied Physiology, 49(1), 1–8. doi:https://doi.org/10.1152/jappl.1980.49.1.1
- Shepherd, J. (2001). Effects of estrogen on cognition, mood and degenerative diseases. Journal of the American Pharmaceutical Association, 41(2), 221–228. doi:https://doi.org/10.1016/s1086-5802(16)31233-5.
- Siegel, A., Verbalis, J., Clement, S., Mendelson, J., Mello, N., Adner, M., … Lewandrowski, K. 2007 Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. The American Journal of Medicine, 120(5), e411–e467. doi:https://doi.org/10.1016/j.amjmed.2006.10.027
- Sims, S., Rehrer, N., Bell, M., & Cotter, J. (2007). Preexercise sodium loading aids fluid balance and endurance for women exercising in the heat. Journal of Applied Physiology, 103(2), 534–541. doi:https://doi.org/10.1152/japplphysiol.01203.2006
- Sollanek, K., Staab, J., Kenefick, R., & Cheuvront, S. (2020). Biological variation of arginine vasopressin. European Journal of Applied Physiology, 120(3), 635–642. doi:https://doi.org/10.1007/s00421-020-04303-x
- Speedy, D., Noakes, T., & Schneider, C. (2001). Exercise-associated hyponatremia: A review. Emergency Medicine, 13(1), 17–27. doi:https://doi.org/10.1046/j.1442-2026.2001.00173.x
- Speroff, L., & Vande Wiele, R. (1971). Regulation of the human menstrual cycle. American Journal of Obstetrics and Gynecology, 109(2), 234–247. doi:https://doi.org/10.1016/0002-9378(71)90872-6
- Stachenfeld, N. (2008). Sex hormone effects on body fluid regulation. Exercise and Sports Science Reviews, 36(3), 152–159. doi:https://doi.org/10.1097/JES.0b013e31817be928
- Stachenfeld, N., & Keefe, D. (2002). Estrogen effects on osmotic regulation of AVP and fluid balance. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 283(4), E711–E721. doi:https://doi.org/10.1152/ajpendo.00192.2002
- Stachenfeld, N., Silva, C., Keefe, D., Kokoszka, C., & Nadel, E. (1999). Effects of oral contraceptives on body fluid regulation. Journal of Applied Physiology, 87(3), 1016–1025. doi:https://doi.org/10.1152/jappl.1999.87.3.1016
- Stachenfeld, N., & Taylor, H. (2005). Progesterone increases plasma volume independent of oestradiol. Journal of Applied Physiology, 98(6), 1011–1018. doi:https://doi.org/10.1152/japplphysiol.00031.2005
- Stachenfeld, S., & Taylor, H. (2009). Sex hormone effects on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. Journal of Applied Physiology, 107(3), 864–862. doi:https://doi.org/10.1152/japplphysiol.91211.2008
- Stephenson, L., & Kolka, M. (1985). Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology, 249(2 Pt 2), R186–R191. doi:https://doi.org/10.1152/ajpregu.1985.249.2.R186
- Stephenson, L., & Kolka, M. (1988). Plasma volume during heat stress and exercise in women. European Journal of Occupational Physiology, 57(4), 373–381. doi:https://doi.org/10.1007/BF00417979
- Stephenson, L., & Kolka, M. (1993). Thermoregulation in women. Exercise and Sport Science Reviews, 21(1), 231–262.
- Stephenson, L., & Kolka, M. (1999). Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. Journal of Applied Physiology, 86(1), 22–28. doi:https://doi.org/10.1152/jappl.1999.86.1.22
- Stockand, J. (2010). Vasopressin regulation of renal sodium excretion. Kidney International, 78(9), 849–856. doi:https://doi.org/10.1038/ki.2010.276
- Sweeney, J. (1934). Menstrual edema: Preliminary report. Journal of the American Medical Association, 103(4), 234–236. doi:https://doi.org/10.1001/jama.1934.02750300008003
- Szmuilowicz, E., Adler, G., Williams, J., Green, D., Yao, T., Hopkins, P., & Seely, E. (2006). Relationship between aldosterone and progesterone in the human menstrual cycle. The Journal of Clinical Endocrinology and Metabolism, 91(10), 3981–3987. doi:https://doi.org/10.1210/jc.2006-1154.
- Taylor, J. (1979). The timing of menstruation-related symptoms assessed by a daily symptom rating scale. Acta Psychiatrica Scandinavica, 60(1), 87–105. doi:https://doi.org/10.1111/j.1600-0447.1979.tb00268.x
- Thompson, C., Bland, J., Burd, J., & Baylis, P. (1986). The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clinical Science, 71, 651–656. doi:https://doi.org/10.1042/cs0710651
- Thornton, S. (2010). Thirst and hydration: Physiology and consequences of dysfunction. Physiology and Behavior, 100(1), 15–21. doi:https://doi.org/10.1016/j.physbeh.2010.02.026.
- Tsampoukos, A., Peckham, E., James, R., & Nevill, M. (2010). Effect of menstrual cycle on sprinting performance. European Journal of Applied Physiology, 109 (4), 659–667. doi:https://doi.org/10.1007/s00421-010-1384-z
- Verbalis, J. (2003). Disorders of body water homeostasis. Best Practice and Research Clinical Endocrinology and Metabolism, 17(4), 471–503. doi:https://doi.org/10.1016/s1521-690x(03)00049-6
- Wenner, M., & Stachenfeld, N. (2017). Sex hormones and environmental factors affecting exercise. In A. C. Hackey (Ed.), Sex hormones, exercise and women (pp. 151–170). Springer: Cham. doi:https://doi.org/10.1007/978-3-319-44558-8_9.
- White, C., Hitchcock, C., Vigna, Y., & Prior, J. (2011). Fluid retention over the menstrual cycle: 1-year data from the prospective ovulation cohort. Obstetrics and Gynecology International, 2011(4), 138551. doi:https://doi.org/10.1155/2011/138451
- Yasuda, N., Kawal, Y., Hara, A., Iide, K., & Matsamura, T. (2013). Evaluation of menstrual cycle phase in hydration status based on urine specific gravity following basketball training. Bulletin of The International Pacific University, 20, 1–5.
- Zimmerman, C., Lin, Y., Leib, D., Guo, L., Huey, E., Daly, G., … Knight, Z. (2016). Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature, 537(7622), 680–684. doi:https://doi.org/10.1038/nature18950
