 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Purpose: Whilst pre-exercise ischaemic preconditioning (IPC) can improve lower-body exercise performance, its impact on upper-limb performance has received little attention. This study examines the influence of IPC on upper-body exercise performance and oxygen uptake (V̇O2) kinetics. Methods: Eleven recreationally-active males (24 ± 2 years) completed an arm-crank graded exercise test to exhaustion to determine the power outputs at the ventilatory thresholds (VT1 and VT2) and V̇O2peak (40.0 ± 7.4 ml·kg−1·min−1). Four main trials were conducted, two following IPC (4 × 5-min, 220 mmHg contralateral upper-limb occlusion), the other two following SHAM (4 × 5-min, 20 mmHg). The first two trials consisted of a 15-minute constant work rate and the last two time-to-exhaustion (TTE) arm-crank tests at the power equivalents of 95% VT1 (LOW) and VT2 (HIGH), respectively. Pulmonary V̇O2 kinetics, heart rate, blood-lactate concentration, and rating of perceived exertion were recorded throughout exercise. Results: TTE during HIGH was longer following IPC than SHAM (459 ± 115 vs 395 ± 102 s, p = .004). Mean response time and change in V̇O2 between 2-min and end exercise (ΔV̇O2) were not different between IPC and SHAM for arm-cranking at both LOW (80.3 ± 19.0 vs 90.3 ± 23.5 s [p = .06], 457 ± 184 vs 443 ± 245 ml [p = .83]) and HIGH (96.6 ± 31.2 vs 92.1 ± 24.4 s [p = .65], 617 ± 321 vs 649 ± 230 ml [p = .74]). Heart rate, blood-lactate concentration, and rating of perceived exertion did not differ between conditions (all p ≥ .05). Conclusion: TTE was longer following IPC during upper-body exercise despite unchanged V̇O2 kinetics.
Highlights
Whilst pre-exercise ischaemic preconditioning can improve lower-body exercise performance and alter V̇O2 kinetics, its impact on upper-limb performance has received little attention.
An acute bout of ischaemic preconditioning prior to arm-crank ergometry exercise significantly improved time to exhaustion compared to a sham control condition.
V̇O2 kinetics in response to ischaemic preconditioning remained unchanged, suggesting alternative mechanisms may explain performance improvements.
| Abbreviations | ||
| BLa | = | blood-lactate concentration |
| ES | = | effect size |
| GET | = | gas exchange threshold |
| GXT | = | graded exercise test |
| HR | = | heart rate |
| IPC | = | ischaemic preconditioning |
| MAP | = | maximum aerobic power |
| MRT | = | mean response time |
| SD | = | standard deviation |
| SHAM | = | sham control |
| SpO2 | = | oxygen saturation |
| TTE | = | time-to-exhaustion |
| V̇O2 | = | pulmonary rate of oxygen uptake |
| VT | = | ventilatory threshold |
| LOW | = | exercise at a power output equivalent to 95%VT1 |
| HIGH | = | exercise at a power output equivalent to VT2 |
Introduction
Ischaemic preconditioning (IPC) is a pre-exercise priming strategy, in which blood flow to the working limbs is restricted, to induce brief, repeated episodes of ischemia followed by reperfusion (De Groot, Thijssen, Sanchez, Ellenkamp, & Hopman, Citation2010). Originally utilised as a method to provide cardiac protection in animal studies (Murry, Jennings, & Reimer, Citation1986) and human myocardium (Yellon, Alkhulaifi, & Pugsley, Citation1993), IPC has subsequently been reported to improve exercise performance in healthy populations (Incognito, Burr, & Millar, Citation2016; Marocolo et al., Citation2019; Salvador et al., Citation2016). Specifically, IPC has been shown to enhance time-trial performance during 5 km cycling (Paradis-Deschênes, Joanisse, & Billaut, Citation2018) and 5 km running (Bailey et al., Citation2012), 100 m swimming (Jean-St-Michel et al., Citation2011), as well as improve cycling time-to-exhaustion (TTE) performance (Cruz, de Aguiar, Turnes, Pereira, & Caputo, Citation2015; Kido et al., Citation2015). On the other hand, only small or no effects on performance have been described following IPC during short-duration activities including high intensity intermittent running (Marocolo et al., Citation2017) and maximal sprinting (Paixão, Da Mota, & Marocolo, Citation2014) that have a significant contribution from anaerobic metabolism.
The physiological mechanisms underlying the potential performance benefits of IPC remain to be fully established. A greater peak rate of oxygen uptake (V̇O2peak) was reported during incremental cycling exercise following IPC (Cruz et al., Citation2015; De Groot et al., Citation2010). Such interventions that may improve oxygen utilisation and/or delivery may also alter V̇O2 kinetics, a key determinant of endurance performance. Faster V̇O2 kinetics lead to a smaller oxygen deficit for any given increase in V̇O2. In addition, this can attenuate development of the V̇O2 slow component (Jones & Burnley, Citation2009). However, findings regarding the influence of IPC on V̇O2 kinetics are inconsistent. For example, during a high-intensity exercise protocol, IPC resulted in an attenuation of the V̇O2 slow component, though 4 km time-trial performance remained unchanged (Kilding, Sequeira, & Wood, Citation2018). On the other hand, no differences in V̇O2 kinetics were reported during moderate- (85% gas exchange threshold; GET) or high-intensity (85% V̇O2peak) cycling (Wiggins, Constantini, Paris, Mickleborough, & Chapman, Citation2019) or during 3-minute all-out exercise (Griffin, Ferguson, Gissane, Bailey, & Patterson, Citation2018). Moreover, despite increased skeletal muscle oxygenation, Kido et al. (Citation2015) reported unchanged V̇O2 kinetics responses during moderate-intensity cycling.
There has been limited investigation into the application of IPC on the upper-limbs, where insight could have practical relevance for individuals primarily relying on their upper-body. The upper-limbs may display a heightened response to IPC, as ∼2–3 times greater deoxygenation in the upper-limbs has been reported compared to the lower-limbs (Brown et al., Citation2018; Cunniffe, Sharma, Cardinale, & Yellon, Citation2017). This may be related to reduced tissue mass in the upper-limbs allowing for a greater occlusion at a given cuff pressure (Cunniffe, Sharma, Cardinale, & Yellon, Citation2017). Furthermore, the upper limbs exhibit a greater proportion of type II muscle fibres (Johnson, Polgar, Weightman, & Appleton, Citation1973) which may impact on V̇O2 kinetics, as individuals with a greater proportion of type II muscle fibres display higher amplitudes of the V̇O2 slow component (Barstow, Jones, Nguyen, & Casaburi, Citation1996). IPC has resulted in increased time-to-task failure and oxygen extraction during handgrip exercise, despite unchanged oxygen delivery (Barbosa et al., Citation2015). Others observed improved forearm oxygen delivery and endothelial functional sympatholysis following IPC (Horiuchi, Endo, & Thijssen, Citation2015). To our knowledge, only one study has investigated the influence of IPC on dynamic upper-body exercise performance (Mota et al., Citation2020). These authors reported that IPC did not change 3-minute, all-out arm-crank ergometry performance in young women. However, the limited effects of IPC may be explained by the all-out nature of this exercise protocol and although the 3-minute, all-out cycling test has been described as valid and reliable (Jones & Burnley, Citation2009), limited corresponding evidence for arm exercise exists. Indeed, participant inexperience with the less conventional arm ergometry all-out protocol could potentially reduce test-retest reliability, hindering the appropriateness of this test for assessing upper-body exercise performance.
Therefore, the aim of this study was to determine the effects of IPC on arm-crank ergometry performance, compared with a sham control (SHAM). A secondary aim was to investigate the effects of IPC on V̇O2 kinetics during arm-crank exercise, compared with SHAM. It was hypothesised that IPC would improve TTE, and that this would be concomitant with an improved V̇O2 mean response time (MRT) and attenuated amplitude of the V̇O2 slow component.
Methods
Participants
Eleven healthy males took part in this study (mean ± SD: 24 ± 2 years, 1.79 ± 0.05 m, 80 ± 7 kg, resting systolic / diastolic blood pressure 133 ± 13 / 74 ± 8 mmHg). Participants were recreationally active (≥ 30-minutes of moderate-intensity exercise ≥ 3 times per week for at least three months, self-reported), not specifically trained in arm-crank ergometry, and non-smoking. Experimental procedures were approved by the institution’s Ethics Committee and conformed to the Declaration of Helsinki in all aspects except for registration in a database. Participants completed a health-screening questionnaire and provided written, informed consent.
Experimental design
Participants attended the laboratory for five separate visits. During visit 1, participants performed a graded exercise test (GXT) to determine V̇O2peak, MAP, and power output corresponding to the ventilatory thresholds (VT1 and VT2), after which they were familiarised with the IPC protocol. Visits 2 and 3 were randomised and counter-balanced, whereby participants performed exercise at a constant power output equivalent to 95% of the first ventilatory threshold (VT1) for 15 minutes (LOW), each preceded by either the experimental (IPC) or sham control (SHAM) protocols. During these visits, following a 10-minute rest, participants were familiarised to and completed the TTE protocol. Subsequently, visits 4 and 5 were also randomised and counter-balanced, whereby participants performed a TTE protocol (HIGH) at a constant power output equivalent to the second ventilatory threshold (VT2) until task failure, each preceded by either the IPC or SHAM protocols. All visits were separated by a minimum of 72 hours to account for the potential latent effects of IPC (Loukogeorgakis et al., Citation2005).
All exercise tests were performed using an electronically braked arm-crank ergometer (Lode Angio, Groningen, The Netherlands). All testing was performed in a seated position; torso movement was not restricted however participants were verbally instructed to limit torso involvement. Arm-crank ergometer height dimensions were individually adjusted, ensuring the handles were aligned just below shoulder height with minor elbow flexion at maximal reach, remaining constant throughout all trials. Participants were instructed to maintain 85 rpm for all exercise trials. For GXT and HIGH, exercise was performed until task failure, determined as crank rate dropping to ≤75 rpm three times or for 5 consecutive seconds, despite strong verbal encouragement which was given when the crank rate was ≤80 rpm. For all trials, crank rate was the only feedback provided to participants; information such as elapsed time, heart rate (HR) and ventilatory data concealed. Participants were instructed to arrive at the laboratory in a rested state (no upper-body exercise in the preceding 24 h), and trials were completed at the same time of day (± 1 h) to mitigate the effects of circadian variation. Participants completed a food diary to record the volumes of food and drink consumed in the 24 h period before visit 2 and replicated their diet prior to subsequent trials. Participants were requested to refrain from alcohol and caffeine 24 h prior to testing. All tests were conducted in the same environmentally-controlled laboratory (ambient temperature 19.0 ± 0.9°C, relative humidity 54.3 ± 4.7%, pressure 1000 ± 8 mmHg).
Graded exercise test and familiarisation
During the first visit, participant height and body mass was measured (Seca, Hamburg, Germany). Following 10-minute seated rest, blood pressure was recorded in duplicate using an automated sphygmomanometer (M2 basic, Omron, Kyoto, Japan). Prior to the GXT, a resting capillary blood sample (20 µL) was obtained from the earlobe, after which participants completed a 3-minute warm-up at 20 W. Thereafter, the GXT commenced at 40 W and increased by an incremental rate of 10 W following each 1-minute stage until task failure. Immediately following the GXT, a further capillary blood sample was collected. Concentration of blood lactate (BLa) was determined using an automatic analyser (Biosen C-Line, EKF Diagnostics, Penarth, UK). Throughout the GXT, pulmonary gas exchange (MetaLyzer 3B, Cortex Medical, Leipzig, Germany) and HR (Polar H10, Polar, Kempele, Finland) were continuously recorded. Immediately upon termination, participants were asked to describe their peripheral (arms and shoulders), central (heart and lungs) and overall (whole body) ratings of perceived exertion (RPE) using the BORG 6–20 scale (Borg, Citation1982). Participants were subsequently familiarised with IPC/SHAM procedures, where occlusion cuffs were inflated to SHAM (20 mmHg) and IPC (220 mmHg) pressures for 2 × 5-minute cycles using an alternating arm cuffing protocol.
V̇O2peak was defined as the highest V̇O2 achieved over a 30-second period. MAP was defined as the power output at task failure. If a full stage was not completed, MAP was set pro rata and calculated as the power of the last stage fully completed plus the fraction of the stage at which the test was terminated. Ventilatory thresholds were determined according to Meyer, Lucía, Earnest, and Kindermann (Citation2005): (VT1) V-slope method: the first disproportionate increase in the rate of carbon dioxide output (V̇CO2) when plotted against V̇O2, together with a relative increase in V̇O2 minute ventilation (V̇E/V̇O2) with no increase in V̇CO2 minute ventilation (V̇E/V̇CO2); VT2: the first disproportionate increase in V̇E when plotted against carbon dioxide output (V̇CO2). Thereafter, V̇O2 was plotted against power output and regression analysis was used to determine the power outputs corresponding to 95%VT1 and VT2 (Metasoft Studio Version 5.8.5, Cortex Medical, Leipzig, Germany) for LOW and HIGH, respectively.
Experimental trials
Upon arrival, participants assumed a semi-supine position and completed either the SHAM or IPC protocol. Occlusion cuffs (SC10D, Hokanson, WA, USA) were positioned as proximal as possible on the upper arm. Alternating between arms, cuffs were inflated to either 220 mmHg (IPC) or 20 mmHg (SHAM) for 5 minutes, followed by 5 minutes of reperfusion, for 4 occlusion/reperfusion cycles per arm, lasting a total of 40 minutes (e.g. Marocolo, da Mota, Pelegrini, & Appell Coriolano, Citation2015). Throughout IPC/SHAM, oxygen saturation (SpO2) was measured continuously at the fingertip of one arm (Pulse Oximeter, Contec, Osaka, Japan) and recorded in the final 15 seconds of the occlusion period for the respective arm. To limit potential placebo/nocebo effects, participants were informed that both occlusion pressures had the potential to improve performance and that despite potential discomfort, neither condition could induce harm.
Following completion of the occlusion protocol, a HR monitor was positioned directly below the sternum, a capillary blood sample was obtained from the earlobe, and the face mask for pulmonary gas sampling fitted. Twelve minutes after the occlusion protocol was completed, the exercise protocol was initiated. This began with 3-min seated rest, followed by 2-min unloaded arm-cranking at 40 rpm. For visits 2 and 3, power output was then instantaneously increased to LOW and performed for a fixed duration of 15 minutes. For visits 4 and 5, power output was increased to HIGH, which was maintained until task failure as previously described. Participants were instructed to maintain 85 rpm and were blinded to any other feedback. Verbal encouragement was standardised and provided every 30 seconds or when the crank rate was ≤ 80 rpm. Pulmonary gas exchange was measured throughout the exercise protocol. Peripheral, central, and overall RPE were recorded immediately at the end of exercise. HR was recorded at rest, during the final 30 seconds of the unloaded phase and at the end of exercise. A capillary blood sample was collected upon termination of exercise. All exercise trials were followed by a 5-min warm-down at 20 W.
Pulmonary gas exchange and V̇O2 kinetics
Breath-by-breath pulmonary gas exchange was continuously measured throughout all exercise trials (MetaLyzer 3B, Cortex Medical, Leipzig, Germany). Prior to each test, the system was calibrated using known gas concentrations (O2: 15.19%; CO2: 4.95%) and a 3 L syringe (Hans Rudolph, Kansas City, MO, USA). V̇O2 data were linearly interpolated to second-by-second data and subsequently smoothed using a moving five of seven-point average. To characterise the overall V̇O2 response during exercise at LOW and HIGH, a single-exponential model, without time delay, with the fitting window commencing at the start of exercise (t = 0), was used as described in the following equation:
where V̇O2 (t) describes the absolute V̇O2 at a given time (t), V̇O2 baseline represents mean V̇O2 over a stable 60-second baseline period, and A represents the amplitude (Bailey, Vanhatalo, DiMenna F, Wilkerson, & Jones, Citation2011). The V̇O2 MRT was quantified with constraints of the fitting window for end-exercise (mean V̇O2 over the final 30-seconds of exercise), and for an iso-time of 275 seconds, representing the minimum completion time of either experimental condition during HIGH trials. The slow component amplitude (ΔV̇O2) was determined as the difference in V̇O2 between 120 seconds and the iso-time or end-exercise, and %V̇O2peak was defined as end V̇O2 relative to V̇O2peak measured during the GXT.
Statistical analysis
All statistical analyses were completed using SPSS V.27 (IBM, Armonk, NY, USA). Normal distribution of data was assessed using the Shapiro–Wilk test. Paired t-tests were conducted to compare TTE, parameters of V̇O2 kinetics, RPE, and SpO2 between IPC and SHAM. Two-factor repeated-measures ANOVA with time and condition (IPC vs SHAM) were utilised to assess differences in HR and BLa. Where significant effects were observed, LSD post-hoc tests were used to locate differences. Effect sizes (ES) for t-test comparisons were calculated using Cohen’s d, with ES magnitudes of ≤0.2, 0.2–0.49, 0.5–0.79 and ≥0.8 interpreted as trivial, small, medium, and large, respectively (Cohen, Citation1969). All data are presented as mean ± SD, with significance accepted at p ≤ .05.
Results
Physiological and performance parameters derived from the graded exercise test to exhaustion were as follows: V̇O2peak was 3.2 ± 0.5 L·min−1 (absolute) and 40.0 ± 7.4 ml·kg−1·min−1 (relative), respectively. MAP was 146 ± 21 W, with power at VT1 and VT2 calculated as 93 ± 19 W and 125 ± 18 W, respectively. Peak HR and End BLa were 178 ± 11 b·min−1 and 8.8 ± 2.4 mmol·L−1.
Mean SpO2 during the IPC procedure was lower compared to SHAM prior to both LOW (74 ± 2% vs 97 ± 1%, p < .001, ES = 1.94) and HIGH (72 ± 2% vs 98 ± 1%, p < .001, ES = 1.94) trials.
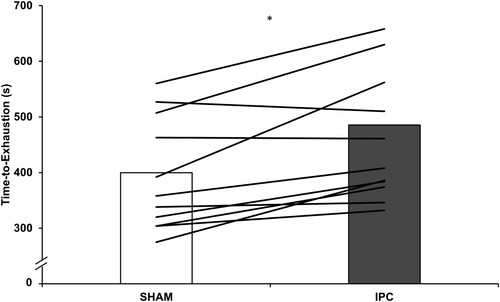
All participants were able to complete the prescribed 15 minutes of exercise at LOW. For HIGH, TTE was longer following IPC compared to SHAM (459 ± 115 s vs 395 ± 102 s, p = .004, ES = 0.58), with 82% of participants (9/11) improving TTE following IPC (). Additionally, no order effects were identified for HIGH TTE trials (p = .69, ES = 0.099).
Figure 1. Time-to-exhaustion (s) during HIGH following ischaemic preconditioning (IPC) and sham control (SHAM). Columns are group mean; lines are individual participant responses. * indicates difference between IPC and SHAM (p = 0.004).
There were no differences in MRT and amplitude between IPC and SHAM during LOW or HIGH (). None of the other V̇O2 kinetic parameters differed between conditions (all p > .05). shows the mean V̇O2 response for LOW and up to the minimum completion time (iso-time: 275 s) of HIGH, respectively. The R2 for the modelled V̇O2 data were 0.66 ± 0.18 (range: 0.30–0.89) for LOW, and 0.83 ± 0.13 (range: 0.55–0.97) for HIGH.
Figure 2. Rate of pulmonary V̇O2 during the final 60 seconds of baseline and throughout the 15-minute constant work rate trials at LOW and to the minimum completion time (275 seconds) of time-to-exhaustion trials at HIGH following ischaemic preconditioning (IPC) and sham control (SHAM).
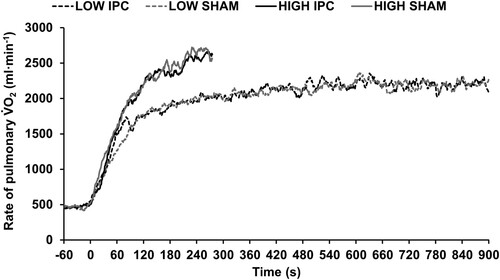
Table 1. V̇O2 kinetic parameters during trials at LOW and HIGH following ischaemic preconditioning (IPC) and sham control (SHAM).
There was a significant main effect of time (p ≤ .001) where HR and BLa increased from rest to end-exercise in all trials. However, there were no differences in HR or BLa between IPC and SHAM (p ≥ .59), nor any interaction effects (p ≥ .25). Additionally, there were no differences in end-exercise RPE responses between IPC and SHAM during exercise at LOW or HIGH (p ≥ .21, ES ≤ 0.44) ().
Table 2. Physiological and perceptual responses prior to and following trials at LOW and HIGH following ischaemic preconditioning (IPC) and sham control (SHAM).
Discussion
The main findings of this study were that IPC improved TTE during arm-crank ergometry at HIGH. However, no change in parameters of V̇O2 kinetics or physiological and perceptual measures were observed at LOW or HIGH.
IPC resulted in a longer TTE during HIGH. To date, no other study has demonstrated an improved TTE during upper-body exercise following IPC. Similar findings of an increased TTE following IPC were reported during maximal (Cruz et al., Citation2015) and severe-intensity (70% difference between GET and V̇O2peak) leg cycling (Kido et al., Citation2015). To our knowledge, the only other study investigating the effect of IPC on arm-crank ergometry found no changes in peak, mean or end power during 3-minute, all-out arm-crank ergometry in young women (Mota et al., Citation2020). However, it is noteworthy that IPC in females may not be as effective as in males; indeed, IPC has been shown to lead to an increase in muscle O2 extraction in males but a decrease in females (Paradis-Deschênes, Joanisse, & Billaut, Citation2017). Conflicting results may also be attributed to the greater effect of IPC during exercises that rely primarily on aerobic metabolism, compared to higher intensity activities with a significant contribution from anaerobic metabolism (Incognito et al., Citation2016; Salvador et al., Citation2016). For example, during lower/whole body exercise, IPC failed to elicit any changes in intermittent endurance performance (Marocolo et al., Citation2017) and detrimental effects of IPC were observed during a maximal 30 second cycling performance (Paixão et al., Citation2014). The IPC-mediated improvement in TTE (16.2%) observed in the present study is greater than what is reported in studies involving lower-body constant work rate exercise performance (∼8%-12%) (Cruz et al., Citation2015; Kido et al., Citation2015). This may be explained by anatomical and morphological differences between the upper and lower limbs, with upper limbs exhibiting a greater proportion of type-II muscle fibres (Johnson et al., Citation1973) and deoxygenation levels during limb occlusion (Brown et al., Citation2018). Indeed, a greater amplitude of the V̇O2 slow component has been reported in individuals with a greater proportion of type-II fibres (Barstow et al., Citation1996). These factors may contribute to the different outcomes to Mota et al. (Citation2020), as our exercise tests required a greater contribution from oxidative metabolism.
Given that parameters of V̇O2 kinetics can be influenced by the overall exercise duration, analysis at an iso-time (275 s) as well as for the full duration during HIGH were completed. The observed performance improvement occurred without a change in V̇O2 kinetics at either intensity or iso-time. We do, nevertheless, report a trend towards a reduced MRT following IPC at LOW, accompanied by a “moderate” effect size (p = .06, ES = 0.47). The influence of IPC on V̇O2 kinetics is ambiguous. For example, Kilding et al. (Citation2018) reported reductions in the V̇O2 slow component during a high-intensity constant work rate protocol (50% difference between GET and V̇O2peak), whereas no changes were observed during submaximal (85% GET and 85% V̇O2max) (Wiggins et al., Citation2019) or all-out (Griffin et al., Citation2018) exercise. Kido et al. (Citation2015) described similar results to the present study, where IPC resulted in improved TTE during severe-intensity constant work rate cycling, despite unchanged V̇O2 kinetics. These contrasting findings regarding V̇O2 kinetics may be attributed to exercise modality and anatomical/morphological differences between upper and lower limbs. However, it is unclear why the presumed association between changes in V̇O2 kinetics and exercise performance was not demonstrated in the present study. These conflicting findings contribute to the lack of clarity surrounding the underpinning mechanisms of IPC.
Improved exercise performance without augmented V̇O2 kinetics may be explained by alternative mechanisms underpinning IPC, such as a reduced energy demand (Beaven, Cook, Kilduff, Drawer, & Gill, Citation2012; Crisafulli et al., Citation2011). In the present study, BLa and %V̇O2peak were similar at the end of HIGH between IPC and SHAM, despite a greater TTE following IPC. This is in line with the observation of a greater maximum aerobic power during incremental cycling (De Groot et al., Citation2010) and similar BLa profiles despite improved TTE following IPC compared to a control group (Cruz et al., Citation2015). Some research has described an improved exercise performance alongside reduced perceptions of fatigue following IPC (Cruz et al., Citation2015; Paradis-Deschênes et al., Citation2018). This is in line with a proposed mechanism potentially underlying IPC which involves a nociceptive augmentation where group III/IV muscle afferents become desensitised to the accumulation of fatigue-associated metabolites, reducing muscle afferent feedback. Indeed, Crisafulli et al. (Citation2011) reported similar findings to this study where IPC increased TTE during incremental cycling despite unchanged V̇O2 kinetics, attributing performance improvements to reduced perception of effort. Moreover, studies investigating the neuromuscular mechanisms of fatigue through surface electromyography and twitch interpolation did not observe any changes in central or peripheral fatigue pathways during submaximal or maximal isometric contractions or maximal isokinetic exercise following IPC (Behrens, Zschorlich, Mittlmeier, Bruhn, & Husmann, Citation2020; Halley, Marshall, & Siegler, Citation2018; Halley, Marshall, & Siegler, Citation2019). Additionally, Halley et al. (Citation2020) demonstrated IPC increased maximal kayaking time-trial performance in which RPE at exhaustion was the same between IPC and control conditions. Again, these positive effects of IPC on subsequent exercise performance may be explained through attenuated perceptions of fatigue, allowing participants to sustain exercise for longer durations.
This study is not without limitations. We quantified parameters of V̇O2 kinetics using second-by-second data which were subsequently smoothed using an objective method (moving five of seven-point average). This objective smoothing approach was chosen as arm-crank ergometry results in lower absolute V̇O2 compared to exercise involving multiple or larger muscle groups, leading to high signal-to-noise ratios. For example, a greater V̇O2peak would be expected during maximal (∼4.0 L·min−1) (Cruz et al., Citation2015) and high-intensity constant work rate cycling protocols (∼4.2 L·min−1) (Kilding et al., Citation2018) compared to arm-cranking completed at LOW (∼2.2 L·min−1) and HIGH (∼2.8 L·min−1) in this study. This makes employing the criterion standard breath-by-breath approach (Bailey et al., Citation2011) difficult, as this approach tends to remove a greater number of outliers the more variable the dataset is, hence resulting in heavy truncation of the most variable datasets. Future research on upper-body exercise could provide insight about the mechanisms of IPC by increasing statistical power for V̇O2 kinetics-related measures (i.e. increase sample size) which may address the possible influence of type II errors. Other limitations include the relative unfamiliarity to the upper-body exercise modality and unconventional nature of arm-crank ergometry, alongside participant motivation during high-intensity exhaustive exercise, may influence day-to-day reproducibility of maximal effort. We attempted to mitigate such confounders by, for example, ensuring verbal encouragement was standardised throughout exercise testing. Furthermore, to reduce the potential learning effect during arm-crank ergometry, two HIGH TTE familiarisation trials were completed. This may have mitigated learning effects during the HIGH trials (visits 4 and 5), as confirmed by the fact that no order effects were identified between TTE tests. We are confident these standardisation procedures have helped to identify the ergogenic effect of IPC on upper-body exercise performance. Another limitation is that the pulse oximetry device used to measure systemic SpO2 had a limited measurement range (70–99%). Although this method can indicate IPC-facilitated arterial occlusion, more sensitive measuring devices are required for accurate assessments of severe reductions in SpO2. For example, implementing near-infrared spectroscopy for measurements of tissue saturation index, an indicator of oxygen delivery and extraction, would help elucidate the physiological mechanisms underpinning IPC. Furthermore, the application of IPC remains challenging, with further investigation warranted to optimise IPC protocols (i.e. number of cycles, time between IPC and exercise) and influence of concurrent or alternative priming strategies (e.g. warm-up), participant characteristics and specific demands of differing types of exercise. Lastly, to address potential placebo/nocebo effects, future IPC research may administer expectancy of belief questionnaires upon completion of experimental procedures.
In conclusion, IPC resulted in improved TTE during arm-crank ergometry at HIGH. However, there were no effects on V̇O2 kinetics during exercise at LOW or HIGH, following IPC compared to a SHAM control condition. Additionally, no changes in physiological and perceptual measures following IPC were observed. These findings highlight the potential efficacy of IPC as a pre-exercise priming strategy for upper-body exercise performance, relevant for populations completing arm-dominant activities.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bailey, S. J., Vanhatalo, A., DiMenna F, J., Wilkerson, D. P., & Jones, A. M. (2011). Fast-start strategy improves VO2 kinetics and high-intensity exercise performance. Medicine & Science in Sports & Exercise, 43(3), 457–467. doi:10.1249/MSS.0b013e3181ef3dce
- Bailey, T. G., Jones, H., Gregson, W., Atkinson, G., Cable, N. T., Thijssen, D. H. J., & Cable, N. T. (2012). Effect of ischemic preconditioning on lactate accumulation and running performance. Medicine & Science in Sports & Exercise, 44(11), 2084–2089. doi:10.1249/MSS.0b013e318262cb17
- Barbosa, T. C., Machado, A. C., Braz, I. D., Fernandes, I. A., Vianna, L. C., Nobrega, A. C., & Silva, B. M. (2015). Remote ischemic preconditioning delays fatigue development during handgrip exercise. Scandinavian Journal of Medicine & Science in Sports, 25(3), 356–364. doi:10.1111/sms.12229
- Barstow, T. J., Jones, A. M., Nguyen, P. H., & Casaburi, R. (1996). Influence of muscle fiber type and pedal frequency on oxygen uptake kinetics of heavy exercise. Journal of Applied Physiology, 81(4), 1642–1650. doi:10.1152/jappl.1996.81.4.1642
- Beaven, C. M., Cook, C. J., Kilduff, L., Drawer, S., & Gill, N. (2012). Intermittent lower-limb occlusion enhances recovery after strenuous exercise. Applied Physiology, Nutrition, and Metabolism, 37(6), 1132–1139. doi:10.1139/h2012-101
- Behrens, M., Zschorlich, V., Mittlmeier, T., Bruhn, S., & Husmann, F. (2020). Ischemic preconditioning Did Not affect central and peripheral factors of performance fatigability after submaximal isometric exercise. Frontiers in Physiology, 11, 371. doi:10.3389/fphys.2020.00371
- Borg, G. A. (1982). Psychophysical bases of perceived exertion. Medicine & Science in Sports & Exercise, 14(5), 377–381.
- Brown, H., Binnie, M. J., Dawson, B., Bullock, N., Scott, B. R., & Peeling, P. (2018). Factors affecting occlusion pressure and ischemic preconditioning. European Journal of Sport Science, 18(3), 387–396. doi:10.1080/17461391.2017.1421712
- Cohen, J. (1969). Statistical power analysis for the behavioral sciences. New York: Academic Press. 101-105.
- Crisafulli, A., Tangianu, F., Tocco, F., Concu, A., Mameli, O., Mulliri, G., & Caria, M. A. (2011). Ischemic preconditioning of the muscle improves maximal exercise performance but not maximal oxygen uptake in humans. Journal of Applied Physiology, 111(2), 530–536. doi:10.1152/japplphysiol.00266.2011
- Cruz, R. S., de Aguiar, R. A., Turnes, T., Pereira, K. L., & Caputo, F. (2015). Effects of ischemic preconditioning on maximal constant-load cycling performance. Journal of Applied Physiology, 119(9), 961–967. doi:10.1152/japplphysiol.00498.2015
- Cunniffe, B., Sharma, V., Cardinale, M., & Yellon, D. (2017). Characterization of muscle oxygenation response to vascular occlusion: Implications for remote ischaemic preconditioning and physical performance. Clinical Physiology and Functional Imaging, 37(6), 785–793. doi:10.1111/cpf.12353
- De Groot, P. C. E., Thijssen, D. H. J., Sanchez, M., Ellenkamp, R., & Hopman, M. T. E. (2010). Ischemic preconditioning improves maximal performance in humans. European Journal of Applied Physiology, 108(1), 141–146. doi:10.1007/s00421-009-1195-2
- Griffin, P. J., Ferguson, R. A., Gissane, C., Bailey, S. J., & Patterson, S. D. (2018). Ischemic preconditioning enhances critical power during a 3 minute all-out cycling test. Journal of Sports Sciences, 36(9), 1038–1043. doi:10.1080/02640414.2017.1349923
- Halley, S. L., Marshall, P., & Siegler, J. C. (2018). The effect of ischaemic preconditioning on central and peripheral fatiguing mechanisms in humans following sustained maximal isometric exercise. Experimental Physiology, 103(7), 976–984. doi:10.1113/EP086981
- Halley, S. L., Marshall, P., & Siegler, J. C. (2019). The effect of IPC on central and peripheral fatiguing mechanisms in humans following maximal single limb isokinetic exercise. Physiological Reports, 7(8), e14063. doi:10.14814/phy2.14063
- Halley, S. L., Peeling, P., Brown, H., Sim, M., Mallabone, J., Dawson, B., & Binnie, M. J. (2020). Repeat application of ischemic preconditioning improves maximal 1,000-m kayak ergometer performance in a simulated competition format. Journal of Strength and Conditioning Research, doi:10.1519/JSC.0000000000003748
- Horiuchi, M., Endo, J., & Thijssen, D. H. J. (2015). Impact of ischemic preconditioning on functional sympatholysis during handgrip exercise in humans. Physiological Reports, 3(2), doi:10.14814/phy2.12304
- Incognito, A. V., Burr, J. F., & Millar, P. J. (2016). The effects of ischemic preconditioning on human exercise performance. Sports Medicine, 46(4), 531–544. doi:10.1007/s40279-015-0433-5
- Jean-St-Michel, E., Manlhiot, C., Li, J., Tropak, M., Michelsen, M. M., Schmidt, M. R., & Redington, A. N. (2011). Remote preconditioning improves maximal performance in highly trained athletes. Medicine & Science in Sports & Exercise, 43(7), 1280–1286. doi:10.1249/MSS.0b013e318206845d
- Johnson, M. A., Polgar, J., Weightman, D., & Appleton, D. (1973). Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. Journal of the Neurological Sciences, 18(1), 111–129. doi:10.1016/0022-510x(73)90023-3
- Jones, A. M., & Burnley, M. (2009). Oxygen uptake kinetics: An underappreciated determinant of exercise performance. International Journal of Sports Physiology and Performance, 4(4), 524–532. doi:10.1123/ijspp.4.4.524
- Kido, K., Suga, T., Tanaka, D., Honjo, T., Homma, T., Fujita, S., & Isaka, T. (2015). Ischemic preconditioning accelerates muscle deoxygenation dynamics and enhances exercise endurance during the work-to-work test. Physiological Reports, 3(5), e12395–e12395. doi:10.14814/phy2.12395
- Kilding, A. E., Sequeira, G. M., & Wood, M. R. (2018). Effects of ischemic preconditioning on economy, VO2 kinetics and cycling performance in endurance athletes. European Journal of Applied Physiology, 118(12), 2541–2549. doi:10.1007/s00421-018-3979-8
- Loukogeorgakis, S. P., Panagiotidou, A. T., Broadhead, M. W., Donald, A., Deanfield, J. E., & MacAllister, R. J. (2005). Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: Role of the autonomic nervous system. Journal of the American College of Cardiology, 46(3), 450–456. doi:10.1016/j.jacc.2005.04.044
- Marocolo, I. C., da Mota, G. R., Londe, A. M., Patterson, S. D., Neto O, B., & Marocolo, M. (2017). Acute ischemic preconditioning does not influence high-intensity intermittent exercise performance. PeerJ, 5, e4118. doi:10.7717/peerj.4118
- Marocolo, M., da Mota, G. R., Pelegrini, V., & Appell Coriolano, H. J. (2015). Are the beneficial effects of ischemic preconditioning on performance partly a placebo effect? International Journal of Sports Medicine, 36(10), 822–825. doi:10.1055/s-0035-1549857
- Marocolo, M., Simim, M., Bernardino, A., Monteiro, I. R., Patterson, S. D., & da Mota, G. R. (2019). Ischemic preconditioning and exercise performance: Shedding light through smallest worthwhile change. European Journal of Applied Physiology, 119(10), 2123–2149. doi:10.1007/s00421-019-04214-6
- Meyer, T., Lucía, A., Earnest, C. P., & Kindermann, W. (2005). A conceptual framework for performance diagnosis and training prescription from submaximal gas exchange parameters–theory and application. International Journal of Sports Medicine, 26(1), S38–S48. doi:10.1055/s-2004-830514
- Mota, G. R., Rightmire, Z. B., Martin, J. S., McDonald, J. R., Kavazis, A. N., Pascoe, D. D., & Gladden, L. B. (2020). Ischemic preconditioning has no effect on maximal arm cycling exercise in women. European Journal of Applied Physiology, 120(2), 369–380. doi:10.1007/s00421-019-04281-9
- Murry, C. E., Jennings, R. B., & Reimer, K. A. (1986). Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation, 74(5), 1124–1136. doi:10.1161/01.cir.74.5.1124
- Paixão, R. C., Da Mota, G. R., & Marocolo, M. (2014). Acute effect of ischemic preconditioning is detrimental to anaerobic performance in cyclists. International Journal of Sports Medicine, 35(11), 912–915. doi:10.1055/s-0034-1372628
- Paradis-Deschênes, P., Joanisse, D. R., & Billaut, F. (2017). Sex-Specific impact of ischemic preconditioning on tissue oxygenation and maximal concentric force. Frontiers in Physiology, 7, 674. doi:10.3389/fphys.2016.00674
- Paradis-Deschênes, P., Joanisse, D. R., & Billaut, F. (2018). Ischemic preconditioning improves time trial performance at moderate altitude. Medicine & Science in Sports & Exercise, 50(3), 533–541. doi:10.1249/MSS.0000000000001473
- Salvador, A. F., De Aguiar, R. A., Lisbôa, F. D., Pereira, K. L., Cruz, R. S., & Caputo, F. (2016). Ischemic preconditioning and exercise performance: A systematic review and meta-analysis. International Journal of Sports Physiology and Performance, 11(1), 4–14. doi:10.1123/ijspp.2015-0204
- Wiggins, C. C., Constantini, K., Paris, H. L., Mickleborough, T. D., & Chapman, R. F. (2019). Ischemic preconditioning, O2 kinetics, and performance in normoxia and hypoxia. Medicine & Science in Sports & Exercise, 51(5), 900–911. doi:10.1249/MSS.0000000000001882
- Yellon, D. M., Alkhulaifi, A. M., & Pugsley, W. B. (1993). Preconditioning the human myocardium. The Lancet, 342(8866), 276–277. doi:10.1016/0140-6736(93)91819-8
