ABSTRACT
Objectives
Elite rugby union players face numerous physiological and psychological stressors which can increase upper respiratory and gastrointestinal illness risk, and in turn can compromise training and competitive performance. This study aimed to investigate the effect of daily prebiotic supplementation on upper respiratory symptoms, gastrointestinal symptoms, and markers of immune function in elite rugby union players.
Methods
Thirty-three elite rugby union players were randomly assigned to consume a prebiotic (2.8 g/day galactooligosaccharide) or placebo (2.8 g/day maltodextrin), daily for 168 days under double-blind conditions. Participants completed daily and weekly questionnaires for self-reported upper respiratory and gastrointestinal symptoms respectively. Blood and saliva samples were collected at 0, 84, and 168 days for assessment of plasma TNF-α and CRP, and saliva IgA respectively.
Results
The prebiotic group experienced a 2-day reduction in upper respiratory symptom duration (P = 0.045). Gastrointestinal symptom severity and incidence were lower in the prebiotic group compared to the placebo group (P < 0.001, P = 0.041) respectively. Salivary immunoglobulin A secretion rate was 42% greater in the prebiotic group compared to the placebo group at day 168 (P = 0.004), no differences in CRP and TNF-α were found (P > 0.05).
Conclusion
A 168-day dietary prebiotic intervention reduced the duration of upper respiratory symptoms and reduced the incidence and severity of gastrointestinal symptoms in elite rugby union players. These findings suggest that seasonal prebiotic interventions may be beneficial for reducing illness in elite rugby union players, improving their availability to train and compete.
Key points
Elite athletes are susceptible to upper respiratory symptoms and gastrointestinal symptoms which may impact upon training availability and competition performance.
For the first time, this study shows that a dietary prebiotic intervention can reduce the duration of upper respiratory symptoms by 2 days in elite rugby union players.
Dietary prebiotic supplementation can improve the incidence and severity of gastrointestinal symptoms experienced by elite rugby union players.
Prebiotic supplementation was able to increase salivary IgA secretion after 168 days.
These findings can inform practice suggesting that seasonal prebiotic use has the potential to modulate immune function and reduce illness in elite rugby union, which may improve a player’s availability to train and compete.
The mechanisms by which prebiotics reduce URS and GIS require further research exploration.
Introduction
Elite rugby union players follow physiologically and psychologically demanding training schedules, with frequent competitive matches, limited recovery time, and regular international travel. Collectively, these stressors may impair immunity and increase the risk of acute upper respiratory symptoms (URS) (e.g. cough, sneezing, sore throat & nasal congestion) and gastrointestinal symptoms (GIS) (e.g. bloating, belching, flatulence, nausea and diarrhoea) (Peters & Bateman, Citation1983; Drew et al., Citation2017; Hellard et al., Citation2015; Svendsen et al., Citation2016; Wentz et al., Citation2018). On average, elite rugby union players experience four episodes of upper respiratory illness and one GI complaint per season (Cunniffe et al., Citation2009) with the greatest incidences reported during pre-season and winter (Cunniffe et al., Citation2009; Tiernan et al., Citation2020; Keaney et al., Citation2021). Therefore, identifying interventions that may help reduce these clinical presentations, or accelerate illness recovery to allow return to play is imperative for optimising players’ health and team performance.
The profile, genetic material, and functional activity of the gut microbial community (the gut microbiome) have a substantial influence on systemic immunity (Roberfroid et al., Citation2010). Manipulation of the gut microbiome is possible through dietary intervention, most commonly through pro- or prebiotic dietary supplements. This may provide a potential strategy to help reduce URS and GIS in team sport athletes. Probiotic supplementation has been shown to reduce URS incidence in active runners (Cox et al., Citation2010; Gleeson et al., Citation2011; Strasser et al., Citation2016). This improvement was attributed to better maintenance of salivary immunoglobulin A (sIgA) (Gleeson et al., Citation2011), an antibody which provides the initial barrier of defence against invading pathogens. Furthermore, a recent 2022 update to a Cochrane meta-analysis concluded that probiotics reduced the number and duration of URS episodes in adults and children (Zhao et al., Citation2022). Similarly in elite rugby union, the use of a multi-strain probiotic showed a trend for ∼2 day reduction in the duration of URS (Haywood et al., Citation2014). Currently, the variety of probiotic strains used across different studies creates uncertainty as to which may be most beneficial for athlete health.
Prebiotics are substrates that are selectively utilised by host microorganisms conferring a health benefit (Gibson et al., Citation2017). Galactooligosaccharides (GOS) are a prebiotic derived from the action of the enzyme β-galactosidase on lactose and provide an alternative to probiotics. Moreover, prebiotics tend to act at the genus level thereby overcoming species variability that exists with probiotics. Bimuno-galactooligosaccharides (B-GOS) have been shown to increase the count and activity of the genus Bifidobacterium (Depeint et al., Citation2008; Vulevic et al., Citation2008) and elicit immunomodulatory effects as shown by reductions in proinflammatory cytokines (C-reactive protein and interleukin-1β) in elderly, overweight, asthmatic, and healthy individuals (Vulevic et al., Citation2013; Vulevic et al., Citation2015; Williams et al., Citation2016). GOS has also previously been shown to reduce the number of URS days, and severity of GIS in a student cohort (Hughes et al., Citation2011) and reduce incidence of travellers’ diarrhoea (Drakoularakou et al., Citation2010; Hasle et al., Citation2017). Whether similar improvements can be replicated in elite rugby union players is currently unknown.
The aim of this study was to assess the effects of a 168-day B-GOS supplementation on the severity, duration and incidence of URS and GIS, sIgA, and plasma concentrations of C-reactive protein and TNF-α in elite rugby union players during a competitive season. It was hypothesised that B-GOS would reduce URS, GIS, TNF-α, CRP and enhance sIgA.
Methods
Study design and participants
The study was a randomised, double-blind, placebo-controlled trial over 168-days during a regular rugby union season in the Gallagher English Premiership. Forty-one healthy, elite rugby union players (age 23 ± 4 years; body mass 103 ± 13 kg; height 186 ± 7 cm) from a single club volunteered to participate in the present study. Participants were non-smokers, had no history of gastrointestinal illness (e.g. irritable bowel syndrome, inflammatory bowel disease, lactose intolerance, and chronic constipation or diarrhoea), and were not regularly consuming foods enriched with probiotics, prebiotics, or vitamins. They were matched into pairs based on body mass and playing position before randomly being allocated an intervention. All data were collected between September 2019 to February 2020 in the English autumn and winter months (temperature range −4 to +25°C). The study duration was originally scheduled for 252 days but was terminated at 168 days due to the Coronavirus-19 pandemic. The study was conducted in accordance with the Declaration of Helsinki, Human Tissue Act 2004 and approved by the Nottingham Trent University Human Invasive Ethics Committee (ethical protocol 612, approved 23rd May 2019). All participants were informed, both verbally and in writing, of the nature of the study before providing written consent to participate.
During the study, a typical week for participants included four to five training days (∼5 h per day), one competitive match, and at least one day of rest. Training included resistance, skills, fitness, tactics, and match play exercises. Players not named in the competitive matchday squad trained an extra day. Participants did not follow individualised diet plans but were provided meals onsite during training days. Similarly, no meal plan was provided when away from the training ground. However, individuals were instructed to avoid any foods and supplements enriched with probiotics (i.e. fermented products), prebiotics (i.e. foods containing prebiotic fibres), and vitamins. In addition, players were asked to report the use of prescribed and/or over the counter cold and flu remedies. Before each data collection visit, players were asked to arrive following an overnight fast and to have avoided using mouthwash.
Supplementation
Players were randomised to consume either 2.8 g/day of the commercially available B-GOS (Bimuno, Clasado Biosciences Ltd, Reading, UK) or 2.8 g/day placebo (maltodextrin) provided as a powder in single dose sachets (Clasado Biosciences Ltd., Reading, UK). Both supplements were identical in taste and colour and were blinded at the site of manufacture (Clasado Biosciences Ltd). The research team, club staff, and participants remained blinded until all statistical analysis was complete. Participants were provided with supplement pre-mixed in water at the training ground and consumed under observation by a member of club staff. On rest days, players were instructed to mix the sachet into water and consume it at breakfast. Participants returned used and unused sachets to assess supplement adherence.
Daily upper respiratory symptoms
To establish the presence of URS, participants completed the Jackson questionnaire daily (Jackson et al., Citation1958). The presence of eight symptoms (headache, chilliness, sneezing, sore throat, malaise, cough, nasal discharge, and nasal obstruction) were rated on a scale of 0–3 (0-none, 1-mild, 2-moderate, and 3-severe). Total symptom scores for each day were summed to give a total Jackson symptom score. An episode of URS was defined using the Jackson criteria as applied by (Martineau et al., Citation2015) with an episode defined by any period lasting ≥ 3 days with a Jackson score ≥ 14 and the presence of nasal discharge, or a symptom score < 14 with a subjective impression of having a cold for at least 3 days. If URS returned within one week it was regarded as the same episode.
Weekly gastrointestinal symptoms
To assess the presence of GIS, participants completed a GIS tool at the end of each week (Gaskell et al., Citation2019). Participants were educated and advised to rate the presence of each symptom over the past week using the 10-point visual analogue scale, with 1–4 indicative of mild GIS, 5–9 indicative of severe GIS, and 10 indicating extremely severe GIS. If no specific GIS was reported, this was rated as zero. The presence of either regurgitation, projectile vomiting, or defaecation was rated as either 0 or 10 as these are extremely severe or not present at all. All symptom scores were summed to give a weekly total and incidence. Symptoms associated with the upper and lower GI tract were also separated to give total symptom scores for both upper and lower regions as previously described (Gaskell et al., Citation2019). To evaluate whether there were any between-group differences when starting the study, players reported GI symptoms for the week prior to day 0 measurements and the start of the intervention.
Collection and analysis of sIgA
At day 0, 84, and 168, all participants provided a saliva sample to determine sIgA. Participants rinsed their mouth using plain water and remained seated for 10-min. An unstimulated, passive saliva sample was produced, whereby participants were instructed to tilt their head forward and release saliva into the collection tube for 2-mins. Samples were immediately frozen at −20°C and then at −80°C within 48 h until analysis. Upon analysis, samples were fully thawed at room temperature and sIgA concentration was determined by enzyme-linked immunosorbent assay (ELISA) (Salimetrics, Philadelphia, PA). The sIgA, intra- and inter-assay variation was 3.2% and 7.3% respectively, and the minimum detectable level of the assay was 2.5 µg/mL which all samples exceeded. sIgA secretion rate was calculated by multiplying the concentration of sIgA (µg/mL) by the flow rate (volume/duration), resulting in a concentration measure per unit of time (µg/min) as per manufacturer’s instructions. For reference, in healthy, illness free athletic populations, mean sIgA secretion rate was previously reported to be 80.3 µg/min (Gleeson et al., Citation2012).
Collection and analysis of blood biomarkers of systemic inflammation
All participants provided a blood sample at day 0 (pre intervention), 84, and 168 to determine TNF-α and CRP concentrations. Samples were drawn from the antecubital vein in two 10 ml vacutainers, one containing heparin and one containing EDTA anticoagulant (BD Vacutainer®). Samples were then centrifuged with the plasma immediately frozen at −80°C until further analysis. TNF-α was assessed using a high-sensitivity ELISA and CRP was assessed using a regular ELISA protocol (R&D systems). For TNF-α, the intra- and inter-assay variation was 7.5% and 5.8% and the minimum detectable level of the assay was 0.022 pg/ml which all samples exceeded. For CRP, the intra- and inter-assay variation was 4.4% and 7.8% and the minimum detectable level of the assay was 0.022 pg/ml which all samples exceeded.
Statistical analysis
Statistical analyses were performed using the statistical package for social sciences (IBM SPSS version 26, Illinois, United States). All data were checked for normal distribution using a Shapiro–Wilk test. Between-group comparisons for body mass, height, age, average weekly workload, and competitive minutes played were conducted using an independent t-test. Between-group comparisons were conducted for the area under the curve (AUC) of daily URS and weekly GIS over 168 days using a Mann–Whitney U test to assess differences in symptom severity. Between group differences in URS incidence were assessed using a Mann–Whitney U test. Between group differences in URS episode duration and GIS-free weeks were assessed using an independent t-test. CRP, TNF-α, and sIgA secretion rate were evaluated using a Mixed-model analysis of variance (ANOVA). All significant interactions and main effects were assessed further with pairwise comparisons using Bonferroni corrections. Data evaluated using parametric tests are presented as mean ± standard deviation. Data presented using non-parametric tests are presented as median (interquartile range). Statistical significance was set at P < 0.05.
Results
Player characteristics
Thirty-three participants (n = 17 Placebo, 16 B-GOS) from the original 41 completed the full 168-day supplementation period. Eight participants withdrew due to non-compliance with taking the supplement. Supplement adherence of the remaining 33 participants did not differ between groups (B-GOS 80.4 ± 13.9% vs. placebo 78.3 ± 14%; P = 0.73). Body mass (B-GOS 103.4 ± 14.0 kg vs. placebo 105.2 ± 13.2 kg), height (B-GOS 186.9 ± 9.4 cm vs placebo 186.6 ± 7.3 cm), and age (B-GOS 22.4 ± 3.3 years vs. placebo 24.5 ± 5.2 years) were not different between groups. There were no between group differences in average weekly workload and competitive minutes played (P > 0.05). No players reported the use of cold and flu remedies throughout the duration of the study.
Upper respiratory symptoms (URS)
A Mann–Whitney U test revealed no differences in the URS incidence rate between B-GOS (1.0 ± 1.4) and Placebo (1.0 ± 1.0) (P = 0.641). The duration of individual URS episodes was shorter in the B-GOS group (7.4 ± 2.8 days) compared to the placebo group (9.8 ± 4.1 days) (P = 0.045) (). There was no difference in AUC of the daily symptom scores between the two groups (P = 0.77).
Table 1. Overview of self-reported URS data
Gastrointestinal symptoms (GIS)
No differences in GIS over the previous 7-days prior to the start of the intervention (day 0) were evident between the two groups (P = 0.53). A Mann–Whitney U test revealed the AUC of total weekly symptom scores was lower in the B-GOS group (50 [10.5-139.5]) compared to the placebo group (149 [69-208]) (P = 0.03) ((a)). AUC for weekly upper GIS scores was lower in the B-GOS group compared to the placebo group (P < 0.001) ((b)), but no differences were found for lower GIS (P = 0.113). The number of symptom free weeks for total GIS (P < 0.041) and upper GIS (P < 0.002) was higher in the B-GOS group compared to the placebo group, whereas no differences were evident for lower GIS (P = 0.151) ().
Figure 1. (a) Weekly GIS scores reported during 24-week study (B-GOS n = 16, Placebo n = 17). AUC analysis revealed between-groups differences in total GIS scores (P = 0.03). b. Weekly upper GIS scores reported during 24-week study (B-GOS n = 16, Placebo n = 17). AUC analysis revealed between-groups differences in upper GIS scores (P < 0.001).
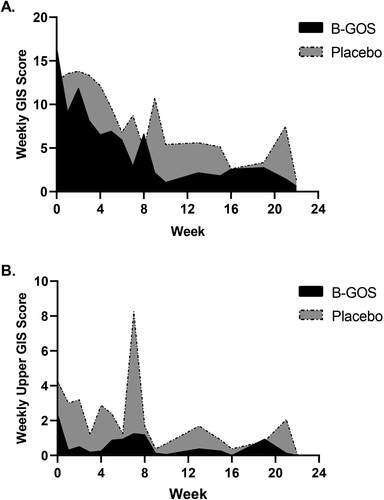
Table 2. Symptom free weeks for GIS during 24-week study.
Systemic inflammation
There was no trial x time interaction for plasma CRP (pooled data, B-GOS 1503.27 ± 1356.32 ng/mL vs. Placebo 1742.67 ± 1486.77 ng/mL) and TNF-α (pooled data, B-GOS 0.82 ± 0.25 pg/mL vs. Placebo 0.90 ± 0.33 pg/mL) (P > 0.05).
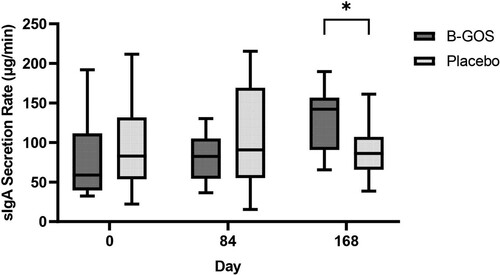
Salivary immunoglobulin A
There was a significant trial x time interaction for sIgA secretion rate (P = 0.001). No differences were observed in sIgA secretion rates at day 0 or 84, but sIgA secretion rate was higher in B-GOS (129.23 ± 38.15 µg/min) than Placebo (90.06 ± 33.45 µg/min) at day 168 (P = 0.004) ().
Discussion
The main findings of this study were that daily supplementation with the dietary prebiotic B-GOS reduced the duration of URS and incidence of GI symptoms in elite rugby union players over a 168-day period. Furthermore, B-GOS increased sIgA secretion rate at 168 days when compared to the placebo group. These findings suggest that B-GOS can potentially modulate the immune and GI system, suppressing URS and GI discomfort experienced by elite rugby union players.
Illness can have detrimental effects on athlete training availability and match preparation (Cunniffe et al., Citation2009; Tiernan et al., Citation2020; Keaney et al., Citation2021). The influence that the gut microbiome can have on the immune system has encouraged research into the potential use of dietary interventions to enhance immune function and mitigate illness risk. To our knowledge, this is the first study to assess the effect of a prebiotic dietary intervention on URS and GIS in an elite-athletic population. The 2-day reduction in URS episode duration is relevant for athletes and coaches, potentially aiding a quicker return to play following a URS episode. Our findings support Hughes et al who observed reductions in GIS incidence and the percentage of days with URS in academically stressed students following a daily dose of either 2.5 g or 5 g of GOS over 8-weeks (Hughes et al., Citation2011). In addition, probiotic interventions in similar athletic cohorts have also reported reductions in URS incidence and duration (Cox et al., Citation2010; Gleeson et al., Citation2011; Strasser et al., Citation2016 ; Haywood et al., Citation2014). Haywood et al also observed a 2-day reduction in URS episode duration and GIS incidence in elite rugby union players following the use of a multi-strain probiotic (Haywood et al., Citation2014). However, not all studies have reported improvements in URS following probiotic supplementation (West et al., Citation2011; Gleeson et al., Citation2012). Differences in study outcomes could be due to variations in URS incidents and chosen probiotic strains. One strength of using prebiotic dietary interventions such as B-GOS in comparison to probiotics, is that prebiotics has been consistently shown to reach the gut undigested and increase the number and activity of beneficial bifidobacteria, alongside the production of beneficial metabolites (SCFA) (Depeint et al., Citation2008; Vulevic et al., Citation2008; Vulevic et al., Citation2013).
The mechanisms by which prebiotics reduce URS and GIS is likely to involve the increase of short chain-fatty acid (SCFA) producing bacteria, such as bifidobacteria. Indeed, elevated faecal SCFA concentrations have been accompanied by enhanced gut epithelial integrity and mucosal immunity (Mariadason et al., Citation1997; Hernot et al., Citation2009). B-GOS has previously been shown to encourage the growth of bifidobacteria in the human gut and subsequently confer numerous health benefits such as reduced systemic inflammation and improved immune response in elderly and overweight populations (Vulevic et al., Citation2008; Vulevic et al., Citation2013). In the current study, B-GOS increased sIgA secretion rates at day 168 when compared to placebo. This finding is consistent with the notion that positive manipulation of the gut microbiome may support mucosal immunity and salivary IgA production. A sixteen-week intervention of the probiotic Lactobacillus casei Shirota maintained salivary IgA during the winter season and reduced URS incidence in active runners (Gleeson et al., Citation2011). However, our findings contrast with a 12-week supplementation of prebiotic B-GOS in obese individuals, which showed an increase in faecal IgA but not saliva (Vulevic et al., Citation2013). Nevertheless, the intervention period in that study was only 12-weeks, thus it is possible that changes in sIgA occur between 12 and 24 weeks.
It should be noted that the total number of URS episodes across both groups in the present study was lower than those previously reported for the winter season. Elite rugby union players have been reported to experience four URS episodes per season (Cunniffe et al., Citation2009), with the greatest incidence during winter. We reported significantly lower rates with higher incidences in the early part of the season. This may explain why B-GOS had little influence on URS incidence and severity. However, it does suggest B-GOS can still improve URS duration even when incidence rates are low. It was also found that participants continued to train despite showing URS. This has been seen elsewhere in a similar cohort and may be because the participants were required to train when at the training site (Cunniffe et al., Citation2009). They may also fear the possibility of being deselected from the upcoming match. It should also be considered that the symptoms could be non-infection related. These are the limitations of collecting self-reported data, and future research should determine infections using molecular testing. Similarly, despite no participants reporting the use of additional URS treatments (e.g. cortisone nasal spray), it was not possible to track whether participants used additional treatments outside of the club. Another limitation was the frequency of sIgA and cytokine measurements. It is possible that B-GOS reduced the duration of URS by enhancing sIgA secretion rate. However, it was beyond the scope of the present study to examine weekly changes in sIgA and their association with URS. This should be considered for future research.
In conclusion, over a 168-day supplementation period, the prebiotic B-GOS reduced the duration of URS, incidence, and severity of GIS and enhanced sIgA secretory rate in elite rugby union players. These findings suggest that prebiotic use may have the potential to modulate immune function and reduce illness, which may improve an athlete’s availability to train and compete. The mechanisms by which B-GOS reduces URS and GIS require further exploration.
Acknowledgements
The research team would like to thank players and club staff for their support and dedication to the study. We would also like to thank Abdel Douiri for statistical advice and Clasado Biosciences Ltd for providing the supplements.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Cox, A. J., Pyne, D. B., Saunders, P. U., & Fricker, P. A. (2010). Oral administration of the probiotic lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. British Journal of Sports Medicine, 44(4), 222–226. https://doi.org/10.1136/bjsm.2007.044628
- Cunniffe, B., Griffiths, H., Proctor, W., Jones, K. P., Baker, J. S., & Davies, B. (2009). Illness monitoring in team sports using a Web-based training diary. Clinical Journal of Sport Medicine, 19(6), 476–481. https://doi.org/10.1097/JSM.0b013e3181c125d3
- Depeint, F., Tzortzis, G., Vulevic, J., I'anson, K., & Gibson, G. R. (2008). Prebiotic evaluation of a novel galactooligosaccharide mixture produced by the enzymatic activity of Bifidobacterium bifidum NCIMB 41171, in healthy humans: A randomized, double-blind, crossover, placebo-controlled intervention study. The American Journal of Clinical Nutrition, 87(3), 785–791. https://doi.org/10.1093/ajcn/87.3.785
- Drakoularakou, A., Tzortzis, G., Rastall, R. A., & Gibson, G. R. (2010). A double-blind, placebo-controlled, randomized human study assessing the capacity of a novel galacto-oligosaccharide mixture in reducing travellers’ diarrhoea. European Journal of Clinical Nutrition, 64(2), 146–152. https://doi.org/10.1038/ejcn.2009.120
- Drew, M. K., Vlahovich, N., Hughes, D., Appaneal, R., Peterson, K., Burke, L., Lundy, B., Toomey, M., Watts, D., Lovell, G., Praet, S., Halson, S., Colbey, C., Manzanero, S., Welvaert, M., West, N., Pyne, D. B., Waddington, G., (2017). A multifactorial evaluation of illness risk factors in athletes preparing for the summer Olympic games. Journal of Science and Medicine in Sport, 20(8), 745–750. https://doi.org/10.1016/j.jsams.2017.02.010
- Gaskell, S. K., Snipe, R. M., & Costa, R. J. (2019). Test–retest reliability of a modified visual analog scale assessment tool for determining incidence and severity of gastrointestinal symptoms in response to exercise stress. International Journal of Sport Nutrition and Exercise Metabolism, 29(4), 411–419. https://doi.org/10.1123/ijsnem.2018-0215
- Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., Stanton, C., Swanson, K. S., Cani, P. D., Verbeke, K., Reid, G. (2017). Expert consensus document: The international scientific association for probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology & Hepatology, 14(8), 491–502. https://doi.org/10.1038/nrgastro.2017.75
- Gleeson, M., Bishop, N., Oliveira, M., McCauley, T., Tauler, P., & Muhamad, A. S. (2012). Respiratory infection risk in athletes: Association with antigen-stimulated IL-10 production and salivary IgA secretion. Scandinavian Journal of Medicine & Science in Sports, 22(3), 410–417. https://doi.org/10.1111/j.1600-0838.2010.01272.x
- Gleeson, M., Bishop, N. C., Oliveira, M., McCauley, T., Tauler, P., & Lawrence, C. (2012). Effects of a lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. International Journal of Sport Nutrition and Exercise Metabolism, 22(4), 235–242. https://doi.org/10.1123/ijsnem.22.4.235
- Gleeson, M., Bishop, N. C., Oliveira, M., & Tauler, P. (2011). Daily probiotic’s (lactobacillus casei shirota) reduction of infection incidence in athletes. International Journal of Sport Nutrition and Exercise Metabolism, 21(1), 55–64. https://doi.org/10.1123/ijsnem.21.1.55
- Hasle, G., Raastad, R., Bjune, G., Jenum, P. A., & Heier, L. (2017). Can a galacto-oligosaccharide reduce the risk of traveller’s diarrhoea? A placebo-controlled, randomized, double-blind study. Journal of Travel Medicine, 24(5). https://doi.org/10.1093/jtm/tax057
- Haywood, B. A., Black, K. E., Baker, D., McGarvey, J., Healey, P., & Brown, R. C. (2014). Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. Journal of Science and Medicine in Sport, 17(4), 356–360. https://doi.org/10.1016/j.jsams.2013.08.004
- Hellard, P., Avalos, M., Guimaraes, F., Toussaint, J., & Pyne, D. B. (2015). Training-related risk of common illnesses in elite swimmers over a 4-yr period. Medicine & Science in Sports & Exercise, 47(4), 698–707. https://doi.org/10.1249/MSS.0000000000000461
- Hernot, D. C., Boileau, T. W., Bauer, L. L., Middelbos, I. S., Murphy, M. R., Swanson, K. S., Fahey, G. C. (2009). In vitro fermentation profiles, gas production rates, and microbiota modulation as affected by certain fructans, galactooligosaccharides, and polydextrose. Journal of Agricultural and Food Chemistry, 57(4), 1354–1361. https://doi.org/10.1021/jf802484j
- Hughes, C., Davoodi-Semiromi, Y., Colee, J. C., Culpepper, T., Dahl, W. J., Mai, V., Christman, M. C., & Langkamp-Henken, B. (2011). Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: A randomized, double-blind, controlled trial in healthy university students. The American Journal of Clinical Nutrition, 93(6), 1305–1311. https://doi.org/10.3945/ajcn.111.014126
- Jackson, G. G., Dowling, H. F., Spiesman, I. G., & Boand, A. V. (1958). Transmission of the common cold to volunteers under controlled conditions: I. The Common Cold as a Clinical Entity. AMA Archives of Internal Medicine, 101(2), 267–278. https://doi.org/10.1001/archinte.1958.00260140099015
- Keaney, L. C., Kilding, A. E., Merien, F., Shaw, D. M., Borotkanics, R. J., & Cupples, B. (2021). Predictors of upper respiratory tract symptom risk: Differences between elite rugby union and league players. Journal of Sports Sciences, 1–8. https://doi.org/10.1080/02640414.2021.1888430
- Mariadason, J. M., Barkla, D. H., & Gibson, P. R. (1997). Effect of short-chain fatty acids on paracellular permeability in caco-2 intestinal epithelium model. American Journal of Physiology-Gastrointestinal and Liver Physiology, 272(4), G705–G712. https://doi.org/10.1152/ajpgi.1997.272.4.G705
- Martineau, A. R., Hanifa, Y., Witt, K. D., Barnes, N. C., Hooper, R. L., Patel, M., Stevens, N., Enayat, Z., Balayah, Z., Syed, A., Knight, A., Jolliffe, D. A., Greiller, C. L., McLaughlin, D., Venton, T. R., Rowe, M., Timms, P. M., Clark, D., Sadique, Z., … Griffiths, C. J. (2015). Double-blind randomised controlled trial of vitamin D3 supplementation for the prevention of acute respiratory infection in older adults and their carers (ViDiFlu). Thorax, 70(10), 953–960. https://doi.org/10.1136/thoraxjnl-2015-206996
- Peters, E. M., & Bateman, E. D. (1983). Ultramarathon running and upper respiratory tract infections-an epidemiological survey. South African Medical Journal, 64(16), 582–584. PMID: 6623247.
- Roberfroid, M., Gibson, G. R., Hoyles, L., McCartney, A. L., Rastall, R., Rowland, I., Wolvers, D., Watzl, B., Szajewska, H., Stahl, B., Guarner, F., Respondek, F., Whelan, K., Coxam, V., Davicco, M.-J., Léotoing, L., Wittrant, Y., Delzenne, N. M., Cani, P. D., … Meheust, A. (2010). Prebiotic effects: Metabolic and health benefits. British Journal of Nutrition, 104(S2), S1–S63. https://doi.org/10.1017/S0007114510003363
- Strasser, B., Geiger, D., Schauer, M., Gostner, J. M., Gatterer, H., Burtscher, M., … Fuchs, D. (2016). Probiotic supplements beneficially affect tryptophan–kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: A randomized, double-blinded, placebo-controlled trial. Nutrients, 8(11). https://doi.org/10.3390/nu8110752
- Svendsen, I. S., Taylor, I. M., Tønnessen, E., Bahr, R., & Gleeson, M. (2016). Training-related and competition-related risk factors for respiratory tract and gastrointestinal infections in elite cross-country skiers. British Journal of Sports Medicine, 50(13), 809–815. https://doi.org/10.1136/bjsports-2015-095398
- Tiernan, C., Lyons, M., Comyns, T., Nevill, A. M., & Warrington, G. (2020). Salivary IgA as a predictor of upper respiratory tract infections and relationship to training load in elite rugby union players. Journal of Strength and Conditioning Research, 34(3), 782–790. https://doi.org/10.1519/JSC.0000000000003019
- Vulevic, J., Drakoularakou, A., Yaqoob, P., Tzortzis, G., & Gibson, G. R. (2008). Modulation of the fecal microflora profile and immune function by a novel trans-galactooligosaccharide mixture (B-GOS) in healthy elderly volunteers. The American Journal of Clinical Nutrition, 88(5), 1438–1446. https://doi.org/10.3945/ajcn.2008.26242
- Vulevic, J., Juric, A., Tzortzis, G., & Gibson, G. R. (2013). A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. The Journal of Nutrition, 143(3), 324–331. https://doi.org/10.3945/jn.112.166132
- Vulevic, J., Juric, A., Walton, G. E., Claus, S. P., Tzortzis, G., Toward, R. E., & Gibson, G. R. (2015). Influence of galacto-oligosaccharide mixture (B-GOS) on gut microbiota, immune parameters and metabonomics in elderly persons. British Journal of Nutrition, 114(4), 586–595. https://doi.org/10.1017/S0007114515001889
- Wentz, L. M., Ward, M. D., Potter, C., Oliver, S. J., Jackson, S., Izard, R. M., Greeves, J. P., & Walsh, N. P. (2018). Increased risk of upper respiratory infection in military recruits who report sleeping less than 6 h per night. Military Medicine, 183(11-12), e699–e704. https://doi.org/10.1093/milmed/usy090
- West, N. P., Pyne, D. B., Cripps, A. W., Hopkins, W. G., Eskesen, D. C., & Jairath, A. (2011). Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: A randomised control trial in athletes. Nutrition Journal, 10(1), 1–11. https://doi.org/10.1186/1475-2891-10-1
- Williams, N. C., Johnson, M. A., Shaw, D. E., Spendlove, I., Vulevic, J., Sharpe, G. R., & Hunter, K. A. (2016). A prebiotic galactooligosaccharide mixture reduces severity of hyperpnoea-induced bronchoconstriction and markers of airway inflammation. British Journal of Nutrition, 116(5), 798–804. https://doi.org/10.1017/S0007114516002762
- Zhao, Q., Dong, B. R., & Hao, Q. (2022). Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews, 8. https://doi.org/10.1002/14651858.CD006895.pub4.