1. Introduction
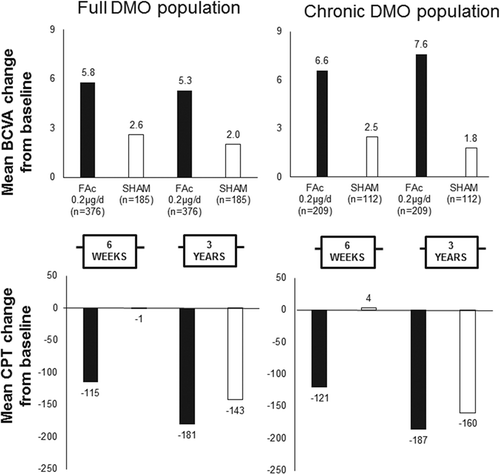
ILUVIEN® has marketing authorizations in 17 European countries for the treatment of chronic diabetic macular oedema (DMO) considered insufficiently responsive to available therapies [Citation1,Citation2]. The granting of the marketing authorizations is based on the pivotal trial results from the Fluocinolone Acetonide in Diabetic Macular OEdema (FAME) trial (ClinicalTrials.gov identifier, NCT00344968) in which patients with DMO, previously having an insufficient response to laser, were treated with ILUVIEN and compared against a sham control-treated population. The trial met its primary endpoint [Citation3] and also revealed that patients, irrespective of chronicity, experienced mean improvements in best-corrected visual acuity that were seen after 6 weeks and were sustained through to 36 months ().
Figure 1. Early (at 6 weeks) and sustained responses in the FAME trial in the full DMO population (left panels) and chronic DMO population (right panels).
Note: Up to week 6, no associated therapies were administered; and, at year 3, for FAc patients, 53 to 55% required no other therapies and 34–37% required macular laser alone. CPT, center point thickness. BCVA: best-corrected visual acuity; FAc: Fluocinolone Acetonide. Data from reference 3.
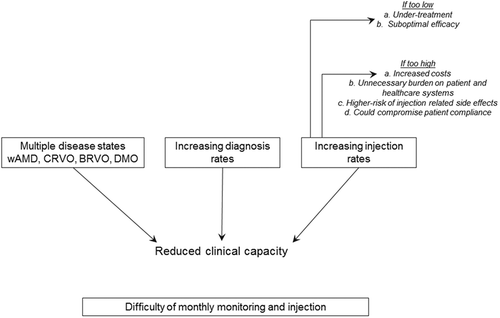
At the sixteenth Congress of the European Retina, Macula and Vitreous Society (EURETINA), Professor Usha Chakravarthy presented the safety results from real-world practice in the ILUVIEN Registry Safety Study (IRISS) trial [Citation4]. This is an open label, registry study assessing the safety of ILUVIEN in the UK, Germany, and Portugal. The first interim analysis results were presented from 328 eyes/292 patients with a mean follow-up period of 281.9 days and showed that 81.6% of patients required no emergent intraocular pressure (IOP)-lowering medication. In terms of changes in IOP, 9.9% had an IOP increase of ≥10 mmHg and 8.2% had an IOP elevation above 30 mmHg. These findings were similar to those reported in the FAME trial at a similar time point and consistent with an on-going audit of electronic medical records using the MedisoftTM auditing tool in the UK. However, there is a paucity of data on the impact of ILUVIEN in the clinic and how being used to improve the management of patients with chronic DMO. Hence, this editorial focuses on the impact of ILUVIEN in day-to-day clinical practice from Queen Alexandra Hospital Portsmouth in the UK, where ILUVIEN has been used in 22 eyes from 20 patients with DMO and discussed how it is helping to improve the service offering by providing an effective therapy from a single injection lasting for up to 36 months. This may have benefits for the practice as well as wider healthcare service provision, helping to reduce burden on the clinic, as patients requiring fewer intravitreal injections and monitoring visits. This may help to improve patient compliance and ultimately would work to better manage the clinic’s capacity and potentially offers cost savings for the clinic and the budget holder.
2. The management of DMO in current clinical practices
The LUMINOUS study [Citation5] is a 5-year observational, noninterventional, multicenter (494 sites), multinational study (43 countries) in 30,514 patients with age-related macula, DMO, branch retinal vein occlusion, central retinal vein occlusion, and choroidal neovascular membrane. Data from the third interim analysis of the LUMINOUS study in DMO patients were extracted in March 2015 and highlight the difficulty of delivering (between 3.7 and 4.0 injections) and monitoring (between 6.4 and 8.4) monthly anti-VEGF injections over the course of a 12-month period [Citation5]. For example, in the case of anti-VEGF injections, there appears to be three principal factors contributing to clinical capacity – the licenced use of the anti-VEGF therapy (e.g. ranibizumab), the rate of disease diagnosis, and the number of injections. In the case of DMO, the total number of injections can be further divided between continuing and newly diagnosed cases, both of which place a burden on clinical capacity and impact the service being offered by healthcare providers.
3. The local management of DMO
In the UK the guidance [Citation6] on the use of ILUVIEN® is determined by the National Institute for health and Care Excellence. It is currently recommended as an option for treating chronic DMO that is insufficiently responsive to available therapies only if the implant is to be used in pseudophakic eyes.
In our department all patients with DMO treated with ILUVIEN had previous cataract surgery. In our settings anti-VEGF injections of ranibizumab accounts for the two-thirds of the injections in the clinic with the numbers of Aflibercept continuously growing.
Patients with anti-VEGF treatment requiring usually monthly appointments after the loading phase and increasing significantly the need for appointments/injections capacity. Potential areas where ILUVIEN may prove to be beneficial in clinical practice are discussed in relation to my own experiences.
3.1. The intravitreal anti-VEGF injection frequency is too low
In real-life practice it is well reported that annual numbers of injections of ranibizumab are fewer than reported in pivotal trials, and fewer injections have been related with poorer visual outcomes after 12 months in DMO [Citation7]. ILUVIEN is indicated for use in patients with chronic DMO who are insufficiently responsive to available therapies [Citation8]. Thus early detection of suboptimal response to intravitreal anti-VEGF injections may be considered for switching to a second-line therapy.
3.2. The intravitreal anti-VEGF injection frequency is too high
Four aspects are cited in . These are explained as follows.
3.2.1. The cost of intravitreal injections
There are limited data available on the actual cost savings from using ILUVIEN. A small independent study in 24 patients compared the cost of treating patients with DMO, refractory to anti-VEGF therapy, with ILUVIEN, or continued anti-VEGF therapy [Citation9]. When extrapolated over a 36-month period, ILUVIEN was estimated to offer a cost saving of at least £8,800 per patient versus continued costs of treating refractory DMO with anti-VEGF therapy. However, this data is from a single National Health Service (NHS) trust and calculation of the potential savings of switching to ILUVIEN has to take in to consideration any additional therapies required.
3.2.2. The burden on the healthcare system
In the FAME trial, 76.1% of patients required only a single implant, which means that additional therapy in the form of >1 ILUVIEN implant was delivered in 23.9% of cases [Citation3]. The possibility of a single injection over 36 months, as opposed to multiple injections, is quite appealing. However, this needs balancing against the known side effects of corticosteroids, even the case of additional therapies. In my own practice, I have treated 20 patients (22 eyes) with ILUVIEN which previously treated with ranibizumab. Once administered, only three patients (four eyes) required further injections of anti-VEGF injections at month 12. Therefore ILUVIEN could help to reduce the burden on the NHS in England as it is based on only a single injection technology. In our experience when additional therapies are being required to treat breakthrough DMO, the number of injections performed appears to be lower.
In line with the Summary of Product Characteristics for ILUVIEN [Citation8] monitoring is required at least quarterly, which could reduce the burden of the clinical follow-ups. In everyday clinical practice, this means that suitable patients treated with ILUVIEN will improve time management and total capacity. Therefore more complex cases can be given the appropriate time needed to properly manage their condition.
3.2.3. The risk of injection-related side effects
The risk of injection-related side effects, as opposed to the known side effects of corticosteroids which include cataract formation and the risk of elevated IOP, is an important consideration. To date, I have not experienced many injection-related side effects simply based on the relatively large volumes of anti-VEGF injections performed compared with the much smaller number of ILUVIEN injections. Again this can potentially be an area where ILUVIEN may prove to be beneficial to the NHS in helping to reduce the burden of managing complications from repeated injections.
Current National Institute for Health and Care Excellence guidance requires all patients undergoing treatment with ILUVIEN must be pseudophakic. Regarding the ocular hypertension, the interim analysis of the IRISS trial [Citation4] seems to support the findings reported in the FAME trial at a similar time-point. Further follow-up is required, to enable a comparison of the longer term outcomes in real-world data and the possible impact in the daily practice.
In our practice, 2 from the 22 eyes developed ocular hypertension and topical treatment is required. Therefore for these patients in addition to the standard monitor visits we had to arrange two more appointments until IOP was controlled. In case of uncontrolled glaucoma requiring surgery (we have no such cases in our department) an additional burden needs to be considered.
3.2.4. Patient compliance
Sivaprasad and Oyetunde [Citation10] surveyed 131 retinal patients in Europe to assess the impact of injection therapy on individuals with DMO or retinal vein occlusion. The aim was to understand the effect of therapies on patient quality of life, which included treatment burden and anxiety/worry as well as practical issues such as the absence from work and the demands of attending an appointment. It was interesting to read that over half of DMO patients had an average of 19.1 appointments with healthcare professionals per se, accounting for around 20 h per patient over a 6-month period. Each injection appointment, including travel time, lasted on average 4.5 h. And the most desired outcome from the patient’s perspective was to achieve the same visual outcomes with fewer injections. In real-life clinical practice, it is also important to ensure physician and patient expectations are aligned as this ultimately dictates if a sufficient response has been achieved. Additionally, around 75% of patients experienced anxiety about their most recent injection and around half reported that they were anxious for the two days prior to the injection.
This data does not quantify patient compliance, but it does indicate the impact of therapy (injection and visit numbers) on the patient. It is generally acknowledged that diabetic patients usually attending multiple hospital appointments. Adding extra appointments for DMO treatment and monitoring, significantly increases their burden. Therefore, treatment with a single injection of sustained effect could help to improve not only their compliance but their quality of life as well.
4. Conclusions
The use of ILUVIEN® is currently recommended as an option for treating chronic DMO that is insufficiently responsive to available therapies in pseudophakic eyes. Treatment with ILUVIEN implant is based on single injection technology providing an effective therapy lasting for up to 36 months. The real world results so far, regarding the efficacy and the safety, from the IRISS study group, corresponding with the outcome from the FAME study.
In a busy clinical setting with on-going increase of demand for intravitreal therapies, the treatment with ILUVIEN implant for chronic DMO in pseudophakic eyes is a suitable option to increase capacity.
Furthermore, diabetic patients usually attending multiple hospital appointments are frequently younger and working people compared with other patients requiring intravitreal therapies. When pseudophakic, treatment with ILUVIEN provides a suitable option in order to reduce their burden and improve their compliance.
This editorial highlights key points regarding the potential benefits from the ILUVIEN use in clinical practice. In our settings, use of ILUVIEN helped to improve our capacity and provide an effective treatment option to our patients. Further studies are necessary in order to compare costs with other therapies in real-world settings.
Declaration of interest
The author is member of the IRISS group and has no conflict of interest relating to this publication. The publication of this article was supported by Alimera Sciences Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgment
Spyridon Mourtzoukos is the guarantor for this article, and take responsibility for the integrity of the work as a whole.
Additional information
Funding
References
- Public Assessment Report Mutual Recognition Procedure ILUVIEN® 190 micrograms Intravitreal Implant in Applicator. Source: http://www.mhra.gov.uk/home/groups/par/documents/websiteresources/con171936.pdf. Accessed 21 September 2016.
- ILUVIEN Summary of Product Characteristics. Source: https://www.medicines.org.uk/emc/medicine/27636. Accessed 21 September 2016.
- Campochiaro PA, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology. 2012;119:2125–2132. DOI:10.1016/j.ophtha.2012.04.030
- Chakravarthy U, Bailey C, Koch F Safety and clinical outcomes in the European registry study of ILUVIEN implant (fluocinolone acetonide; FAc) usage in diabetic macular edema. Presented in session ‘Free Paper Session 4: Vascular Diseases and Diabetic Retinopathy 2ʹ on Thursday 8 September 11am in Auditorium A2 at the The16th Congress of the European Retina, Macula and Vitreous Society.
- Brand C, Mitchell P, Parikh S, et al. Real-world outcomes at 1 year with ranibizumab 0.5 mg in DME patients with low baseline VA: results from the 3rd interim analysis of LUMINOUS study. Presented on Thursday 8th September 2016 at the16th Congress of the European Retina, Macula and Vitreous Society. Source: http://euretina.org/copenhagen2016/programme/free-papers-details.asp?id=4425&day=0
- NICE technology appraisal guidance [TA301]. Fluocinolone acetonide intravitreal implant for treating chronic diabetic macular oedema after an inadequate response to prior therapy. 27 November 2013. Source: https://www.nice.org.uk/guidance/ta301/chapter/1-Guidance.
- Kiss S, Liu Y, Brown J, et al. Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema. Clin Ophthalmol. 2014;26(8):1611–1621.
- Summary of product characteristics for ILUVIEN 190 micrograms intravitreal implant in applicator. Source: https://www.medicines.org.uk/emc/medicine/27636. Accessed 21 September 2016.
- Ch’ng S, Brent A, Banerjee S The economic impact of Intravitreal fluocinolone acetonide (Iluvien®) in refractory diabetic macular oedema in the National Health Service (NHS), United Kingdom. Presented at the European Society of Retina Specialists meeting in Copenhagen, Denmark between 8th and 11th September 2016. Source: http://euretina.org/copenhagen2016/programme/posters-details.asp?id=7555
- Sivaprasad S, Oyetunde S. Impact of injection therapy on retinal patients with diabetic macular edema or retinal vein occlusion. Clin Ophthalmol. 2016;10:939–946.