Abstract
The present study aimed at identifying dysfunctions in brain networks that may underlie disturbed empathic behavior in autism spectrum disorders (ASD). During functional magnetic resonance imaging, subjects were asked to identify the emotional state observed in a facial stimulus (other-task) or to evaluate their own emotional response (self-task). Behaviorally, ASD subjects performed equally to the control group during the other-task, but showed less emotionally congruent responses in the self-task. Activations in brain regions related to theory of mind were observed in both groups. Activations of the medial prefrontal cortex (MPFC) were located in dorsal subregions in ASD subjects and in ventral areas in control subjects. During the self-task, ASD subjects activated an additional network of frontal and inferior temporal areas. Frontal areas previously associated with the human mirror system were activated in both tasks in control subjects, while ASD subjects recruited these areas during the self-task only. Activations in the ventral MPFC may provide the basis for one's “emotional bond” with other persons' emotions. Such atypical patterns of activation may underlie disturbed empathy in individuals with ASD. Subjects with ASD may use an atypical cognitive strategy to gain access to their own emotional state in response to other people's emotions.
INTRODUCTION
Empathy can be defined as the result of psychological inferences about other persons' mental and emotional states allowing for socially appropriate emotional responses. The ability to empathize entails both emotional and cognitive components. On the emotional side, empathy allows for emotional “contagion” (Singer, Citation2006), that is, our ability to share other people's emotions. However, a crucial aspect of empathy is that it includes self-referential emotional cognition in order to evaluate the relationship between other people's emotional states and one's own emotions (Decety & Jackson, Citation2004; Schulte-Rüther, Markowitsch, Fink, & Piefke, Citation2007). Cognitive components of empathy are closely related to the concepts of “theory of mind” (ToM) and “mentalizing” (Frith & Frith, Citation2003). ToM refers to the awareness that mental states of other people may differ from one's own mental state. The ability to adopt others' mental states and evaluate them from one's own mental perspective drives the ability to infer and predict the intentions, beliefs, and feelings of other people and allows for successful social interaction.
Difficulties in social interaction and social cognition are hallmarks of autism spectrum disorders (ASD). It has been suggested that many aspects of the observed problems in social interaction can be explained by an ASD-specific deficit in ToM (Baron-Cohen, Tager-Flusberg, & Cohen, Citation2000) and empathy (Gillberg, Citation1992). It has repeatedly been reported that individuals with ASD have profound difficulties in gaining access to intentions, beliefs, and emotions of other people (see Baron-Cohen et al., Citation2000 for review). Even ASD subjects with high cognitive abilities show impairments in diverse tasks with ToM demands (Happé, Citation1994). Most previous studies of ASD focused solely on the ability to infer other people's thoughts and intentions (for example using false-belief tasks or tasks requiring the inference of emotional states from faces), although the representation of both other and self may be altered in autism (Rogers & Pennington, Citation1991). This idea is supported by several behavioral findings. For example, ASD subjects use fewer descriptions of their own mental or emotional states when talking about personal everyday experiences and daydreams (Hurlburt, Happe, & Frith, Citation1994). Furthermore, ASD subjects do not show the commonly observed memory advantage for self-related materials or self-experienced events (Lombardo, Barnes, Wheelwright, & Baron-Cohen, Citation2007; Millward, Powell, Messer, & Jordan, Citation2000; Toichi et al., Citation2002) and this effect might be intrinsically linked with measures of empathy (Lombardo et al., Citation2007). Finally, many studies report the atypical use of first-person pronouns in autistic children (e.g., Lee, Hobson, & Chiat, Citation1994), suggesting an ASD-related delay of the development of a self-concept.
Little is known about the neural bases of atypical self-reference and empathy in individuals with ASD. In control subjects, tasks requiring self-reference typically activate the medial prefrontal cortex (MPFC), precuneus, and posterior cingulate cortex (PCC) (Amodio & Frith, Citation2006; Cavanna & Trimble, Citation2006; Mitchell, Macrae, & Banaji, Citation2006; Schulte-Rüther et al., Citation2007). Interestingly, mentalizing about other persons and self-referential cognition activate overlapping regions in the MPFC (Amodio & Frith, Citation2006). These findings have led to the suggestion that ToM may involve “simulation” strategies, i.e., the understanding of another person's mind may be mirrored in first-person experiences. In particular, affective mentalizing may strongly draw on self-reference (Mitchell et al., Citation2006). In the context of emotional face-to-face situations, mirror mechanisms (in particular, mirror neurons in the inferior frontal cortex; IFC, BA44/45) have also been proposed as a neural basis of simulation strategies (Carr, Iacoboni, Dubeau, Mazziotta, & Lenzi, Citation2003; Dapretto et al., Citation2006; Schulte-Rüther et al., Citation2007). Recent neuroimaging work revealed dysfunctions of mirror mechanisms in children with ASD (Dapretto et al., Citation2006), which may give rise to the persisting social and empathic deficts in ASD (Williams, Whiten, Suddendorf, & Perrett, Citation2001). However, previous approaches (Dapretto et al., Citation2006) used tasks requiring the imitation and observation of emotional faces which lacked the demand of explicit self-reference and empathizing. Therefore, important behavioral and neurofunctional aspects of self-related emotional processing and its possible disturbance in ASD may have been overlooked.
In the present study, we used a task similar to the one applied in our previous functional magnetic resonance imaging (fMRI) studies of empathy (Schulte-Rüther et al., Citation2007; Schulte-Rüther, Markowitsch, Shah, Fink, & Piefke, Citation2008). Subjects were asked to empathize with facial expressions of emotions and indicate either the emotional state observed in each face (other-task) or their own emotional reaction to the emotional facial expressions (self-task). Happy and sad facial expressions were chosen as stimuli in order to focus on the emergence of contagious emotional responses as one possible outcome of empathic processing. Importantly, our paradigm did not focus on a simple perceptual decision about an emotional face, but rather on the processes of explicit emotional self-reference and emotion identification. The task required interactive switching between the self- and other-perspective and thus allowed for the construction of an interpersonal context in which self- and other-related empathic social cognition could emerge. On the behavioral side, we expected ASD subjects to show fewer contagious emotional responses. On the neural level, we hypothesized to find decreased activation in adults with ASD (compared to control subjects) in the networks supporting ToM (MPFC, temporoparietal regions, temporal poles), self-referential emotional cognition (MPFC, precuneus/PCC), and frontal components (IFC, BA44/45) of the human mirror system (hMS) during both self and other conditions (in comparison to a control-task). Aberrant neural activation of ASD subjects during the self-task (in comparison to a control-task) was expected to reflect atypical strategies of assessing one's own emotions in the absence of contagious emotional responses.
METHODS
Participants
Eighteen male adults (mean age ± SD = 27.40 ± 9.34) with a diagnosis of ASD and 18 male control subjects (mean age ± SD = 25.05 ± 6.69) without a history of neurological or psychiatric disease and matched for age and IQ took part in this study; 14 participants in each group were included in the final fMRI data analysis. Only participants with a general IQ of at least 85 (as assessed with the German version of the WAIS-III) were included. ASD subjects were diagnosed by experienced clinicians for Asperger's syndrome (n = 7) or high-functioning autism (n = 7) according to the criteria of ICD-10 and DSM-IV using a semi-structured diagnostic interview. For all participants, diagnosis was confirmed with the Autism Diagnostic Observation Schedule (ADOS) conducted by trained examiners (EG, IK-B). Furthermore, all participants completed the Autism Spectrum Questionnaire (AQ) and the Empathy Questionnaire (EQ) (Baron-Cohen, Wheelwright, Robinson, & Woodbury-Smith, Citation2005). Demographic and clinical data are summarized in . To exclude confounding psychiatric and neurological disorders, ASD and control subjects completed the Brief Symptom Inventory (Derogatis, Citation1993), and a brief demographic and medical anamnesis was performed. Subjects who scored above threshold for clinically relevant psychiatric problems were excluded from the study. At the time of examination, two subjects of the ASD group were medicated (Subject 1: Venlafaxin® 150 mg, Mirtazapin® 15 mg; Subject 2: Risperdal® 4 mg, Eunerpan® 50 mg). The study was approved by the local ethics committee (according to the Declaration of Helsinki), and all subjects gave written informed consent prior to participation.
TABLE 1 Demographic and clinical characteristics of the ASD and control sample
Experimental paradigm
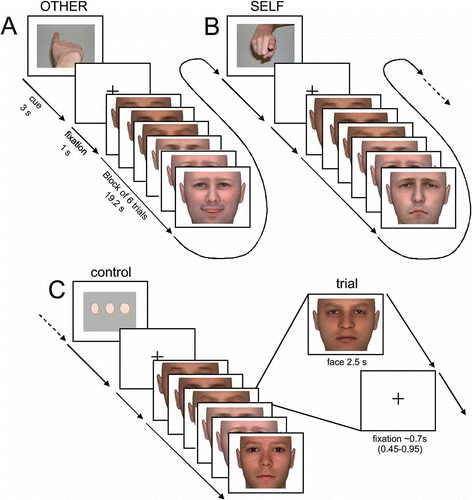
Subjects were asked to empathize with emotional facial expressions presented on a computer screen by “feeling into” the depicted person and either to judge the emotional state of each face (other-task), or to report the emotions elicited in themselves by the emotional faces (self-task). The instructions were as follows. Other-task: “Try to empathize with the depicted person. For each face that appears on the screen you should decide how this person feels.” Self-task: “Try to empathize with the depicted person. For each face that appears on the screen you should decide how you feel yourself when you look at that face.” To reduce potential social desirability bias, subjects were explicitly told that there were no correct or wrong answers in the self-task. Response options were “sad,” “neutral,” or “happy.” For the self- and the other-task, stimulus faces had either a happy or a sad emotional expression with either high or low intensity. A perceptual decision on the width of neutral faces was included as a control condition using “thin,” “normal,” or “wide” as response options. Only neutral faces were used in the control condition to avoid the elicitation of implicit emotional responses during the control-task. We did not include a further low-level baseline (e.g. resting condition) because comparison of experimental tasks against this kind of baseline may yield ambiguous results (Morcom & Fletcher, Citation2007). Importantly, brain activity during resting conditions may be associated with self-referential processing (Gusnard, Akbudak, Shulman, & Raichle, Citation2001) and therefore provides a non-optimal control condition for social cognitive processes such as empathy. The three experimental tasks (self-task, other-task, and control-task) alternated blockwise in a pseudorandomized counterbalanced order. Twelve blocks of each task were presented, resulting in 36 blocks. Each block contained 6 trials, resulting in a total of 192 trials (64 trials per task; see for the exact time course of stimulus presentation). Across self- and other-blocks, intensity (high, low) and quality of emotion (happy, sad) were counterbalanced. A blocked design was chosen to maximize design efficiency for the detection of differences between tasks. Furthermore, as initial pilot testing indicated that switching between tasks on a trial-by trial basis was very difficult even for control subjects, a blocked presentation of tasks was considered as the best choice for our paradigm. A block contained stimuli of either low or high intensity. Low-intensity stimuli were included in the stimulus set to avoid potential ceiling effects. Using only high-intensity stimuli might have rendered the task too easy for controls as well as for ASD subjects. Emotion categories were mixed within blocks, that is, stimulus faces of the same emotion category did not appear more then three times in a row and each emotion appeared at least twice within a block. This procedure was chosen (i) in order to avoid habituation effects related to empathizing with persons displaying the same emotion category; (ii) to prevent subjects from adopting response strategies related to predictable stimulus sequences. Subjects responded with button-presses using three fingers of their right hand while the stimuli were on the screen. Responses were collected for each presented stimulus face and were counted from 150 ms post stimulus-onset until the onset of the next stimulus face. The software Presentation 9 (Neurobehavioral Systems, Albany, CA; http://www.neurobs.com) was used for stimulus presentation and response collection. Prior to scanning, subjects were trained on the experimental tasks to ensure that they were able to respond within the required time window. After the fMRI experiment, subjects were questioned about their strategies used to perform the tasks and other performance-related aspects. Of the 18 ASD and 18 control subjects (CS) who initially participated in the study, 4 participants in each group were not able to describe the difference between the self- and the other-task and indicated that they had always responded “according to how the other person felt” without any reference to their own feelings. These were not included in any analysis, resulting in a final sample of 28 participants (14 ASD, 14 CS) which entered in the fMRI analyses.
Figure 1. Time course of stimulus presentation during the scanning session. Subjects were instructed to empathize with the person presented on the screen and to (A) identify the emotional state observed in the face (other-task) or (B) evaluate their own emotional response to that face (self-task). As a control-task (C) a perceptual decision on the width of neutral faces was used. Each block (19.2 s) was preceded by an instruction cue (3 s) and comprised six stimulus faces (each 2.5 s), separated by a fixation cross (jittered duration: 0.45 – 0.95 s). Instruction cues were pictures of a finger pointing towards the subject (self-task), pointing away from the subject (other-task) or three dots of increasing width (control-task). Each of n = 72 individual faces was presented once displaying a happy expression, once displaying a sad expression and once displaying a neutral expression.
Stimuli
Stimulus faces were constructed using FaceGen 3.1 (Singular Inversions, Vancouver, Canada). Photos of volunteers showing a neutral facial expression were transformed into three-dimensional representations that were subsequently morphed for quality and intensity of emotional expressions. According to established conventions of the Facial Action Coding System (FACS; Ekman & Friesen, Citation1978), each face was morphed to a male adult with a happy and a sad expression (with either high or low intensity), and a neutral expression. Only male stimulus pictures were used because all participants were male and empathizing is facilitated with perceived similarity to the observed person (see, e.g., Preston & de Waal, Citation2002). Furthermore, possible confounds related to differences in empathizing with men or women could be excluded. Validity of emotional expressions was corroborated in a behavioral pilot study. Ten male volunteers rated faces (i) for emotion category (happy, sad, neutral) and intensity (high, low). A total of 72 faces were included in the final stimulus set. These faces had a mean ratio of correct responses for the identification of emotion categories (88.5% ± 17.5 SD) and for the sorting into emotion intensity categories (75.1% ± 19.8 SD).
Eye movement data
Stimuli were presented with a binocular goggle system (Avotec Inc., Stuart, FL) which allowed for simultaneous eye-movement recording (iView X, SensoMotoric Instruments GmbH, Berlin, Germany) at a sampling rate of 50 Hz and a resolution of 600 × 800 pixels. Due to technical problems, eye movement data were not available for seven participants. Eye movement data from the remaining 21 participants (9 of 14 ASD, 12 of 14 CS) were further processed with eye-movement data analysis software (ILAB 3.6.0, Gitelman, Citation2002). Eye blinks were filtered out and fixations during the presentation of facial stimuli were determined (minimum duration of 50 ms and no consecutive dispersion of more than 20 pixels). Mean durations of fixation for each experimental condition within predefined regions of interest (eyes, mouth, and whole face region) were determined for each participant.
MR technical parameters
MR imaging was accomplished on a 1.5-T Avanto MR scanner (Siemens, Erlangen, Germany) using a standard head coil. For functional imaging, gradient-echo, echoplanar T2*-weighted images (EPI) were acquired (TE = 60 ms, TR = 3000ms, α3 = 90°, FOV = 200mm, voxel size = 3.1 × 3.1 × 4 mm3, matrix size = 64 × 64, 30 transversal slices, slice acquisition: ascending) in one session (∼14 min). Anatomical images were acquired using a T1-weighted 3D magnetization-prepared, rapid acquisition gradient echo (MP-RAGE) pulse sequence (TE = 3.93 ms, TR = 2200 ms, α = 15°, FOV = 256 mm, voxel size = 1 × 1 × 1 mm3, matrix size = 256 × 256, 160 sagittal slices, slice thickness = 1 mm).
Image processing and data analysis
Twenty-eight subjects (14 CS, 14 ASD) were included in the final sample for the analysis of fMRI data. Functional volumes were analyzed with SPM5 (Wellcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB 7 (The Mathworks, Inc., Natick, MA). The first four volumes of each functional time-series were discarded to allow the MR signal to reach a steady state. The remaining 285 images were realigned using rigid body transformation, normalized into the Montreal Neurological Institute (MNI) coordinate space and resampled at 2 × 2 × 2 mm3. Normalization parameters were determined by applying the “unified segmentation” routine (Ashburner & Friston, Citation2005) to each individual subject's mean EPI image. This routine gives normalization parameters that are at least as precise as the standard normalization routine in SPM5, but may even provide more precision due to the parallel and recursive segmentation and normalization procedure. Anatomical scans were normalized into MNI space using the same method. Prior to statistical analysis, functional volumes were smoothed with an 8 × 8 × 8 mm3 Gaussian kernel (full width half maximum) to compensate for residual variations in individual anatomy and to meet the requirements of the Gaussian random fields theory.
Boxcar functions of 19.2 s duration (corresponding to the onset of each experimental block, starting with the first presentation of a face) were convolved with a model of the hemodynamic response (canonical HRF implemented in SPM) and its first-order temporal derivative (to compensate for timing differences in slice acquisition). Movement parameters were included as additional regressors of no interest. A high-pass cut-off filter of 128 s was used to account for low-frequency drifts in the imaging data. To handle within-subject autocorrelations an approximate AR(1) model was estimated at omnibus F-significant voxels (p < .001), used globally over the whole brain. Parameter estimates of the resulting general linear model were calculated for each voxel and each regressor.
For population inference, the contrast estimates for the simple effect of each experimental condition were taken to the second level (using the first regressor of the first-level HRF model as an estimate of response height) and a random effects analysis was performed (mixed ANOVA, factors: condition × group × subject). Departures from sphericity assumptions were accommodated using the non-sphericity correction in SPM5 (modeling of variance components). For this procedure, unequal variance was assumed for all factors; non-independence was assumed for the factor condition (repeated measures). Specific effects at each voxel were tested by applying appropriate linear contrasts to the parameter estimates. Experimental conditions containing high- and low-intensity stimuli were modeled separately. However, since initial assessment of results related to stimulus intensity did not reveal differential effects, high- and low-intensity trials were collapsed for subsequent data analysis. Further analysis related to group differences in empathizing focused on the separate comparison of both empathizing tasks with the control-task.
To constrict the analysis of group differences to brain regions that play a role in empathizing, we used the respective within-group contrasts as functional regions of interest for the assessment of interactions with the factor group (i.e., group differences of the self- or other-task relative to the control-task). These analyses were performed using the SPMs of a within-group contrast as an inclusive mask (threshold used for masking: p < .01) and as a functional region of interest (ROI) for the interaction contrast. The statistical threshold for both within- and between-group comparisons (interactions) was set to p < .05, corrected for multiple comparisons at the cluster level (for cluster-level inference, SPMs were thresholded at p < .001, voxel level). For a-priori anatomical ROIs, small volume corrections were applied across each respective region (p < .05, voxel level, family-wise error (FWE) correction). Anatomical ROIs were constructed using the software WFU Pickatlas (Maldjian, Laurienti, Burdette & Kraft, Citation2003). Using AAL-labels (Tzourio-Mazoyer et al., Citation2002), the following ROIs were defined according to our a-priori hypotheses (see “Introduction”): IFC (BA44/45) (inferior frontal gyrus pars opercularis and triangularis), MPFC (superior frontal cortex pars medialis, middle frontal gyrus pars orbitalis, anterior cingulate cortex, gyrus rectus), STS (superior temporal gyrus, middle temporal gyrus), temporal pole (temporal pole: superior temporal gyrus, temporal pole: middle temporal gyrus), and precuneus/PCC (precuneus, posterior cingulate cortex). A ROI of the TPJ was constructed using the coordinates of rTPJ given in a recent meta-analysis on empathy, ToM, and attention (Decety and Lamm, Citation2007) and the corresponding mirrored coordinate of lTPJ, each surrounded by a 10 mm sphere. These ROIs were used for small volume corrections in SPM. Peak activated voxels resulting from these analyses were further inspected in a whole brain analysis to ensure that these voxels represented peak activations within the respective ROI and not merely an overlapping activation cluster from neighboring regions.
To assess correlations between brain activation and individual empathic abilities (as measured by the EQ), whole brain regression models were constructed using individual EQ values and first-level contrast estimates of either the other-control or the self-control comparison. Areas showing positive correlations between brain activation and EQ values across all subjects were identified within brain regions that were reliably activated in the respective contrast of the ANOVA analysis (see above), either for ASD or for CS subjects. These analyses were performed using the combined SPMs (logical OR) of both within group contrasts of the ANOVA analysis as an inclusive mask (threshold used for masking p < .01) and as a ROI for the regression analyses. The statistical threshold for the regression analyses was set to p < .05, corrected for multiple comparisons at the cluster level (for cluster-level inference, SPMs were thresholded at p < .005, voxel level).
Localization of activations
SPMT maps resulting from the group analysis were superimposed onto a group mean MR image calculated from the normalized anatomical T1-images of each subject (see above). MNI coordinates of the local maxima within areas of significant relative changes in neural activity were determined and anatomically localized by comparing activation maps superimposed on the anatomical group mean brain with a standard atlas of brain anatomy (Duvernoy, Citation1999). In addition, an SPM toolbox (Eickhoff et al., Citation2005) was applied which allows for the integration of probabilistic cytoarchitectonic maps of the brain and functional neuroimaging data.
Analysis of behavioral data
Behavioral data were analyzed with the software package SPSS 15 (SPSS Inc., Chicago, IL). For each experimental condition and each subject, percentage of correct (i.e., correct attribution of the emotional state of a stimulus face in the other-task) and congruent responses (i.e., responses during the self-task mirroring the emotional state of a stimulus face), as well as mean reaction times (RTs) were calculated. Since Kolmogorov-Smirnoff tests indicated normal distribution of all variables of interest, parametric analyses (mixed ANOVAs and t-tests) were employed to test for statistically significant differences between groups and experimental conditions. For all behavioral analyses, significance was determined using two-tailed testing.
RESULTS
Behavioral data
RTs were analyzed with a 2 × 2 × 2 mixed ANOVA (task × intensity × group) and a 3 × 2 mixed ANOVA (task × group). RTs were faster for the other- than for the self-task, F(1, 26) = 6.94, MSE = 21.95, p < .05, and faster for the high than the low emotion intensity stimuli, F(1, 26) = 22.65, MSE = 31.06, p < .001. Interactions and the main effect of group were nonsignificant. RTs of the three tasks differed, F(2, 52) = 3.339, MSE = 59.10, p < .05, but self- and other-task did not differ significantly from the control-task, respectively (post-hoc pairwise comparisons, p > .262). Interactions and the main effect of group were also nonsignificant.
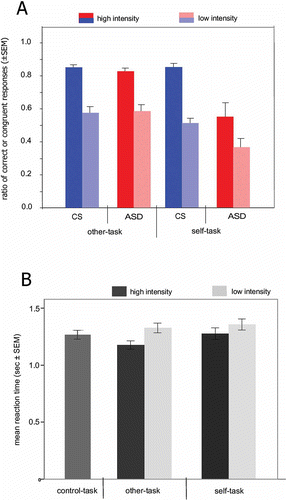
For the analyses of correct/congruent responses, 2 × 2 × 2 mixed ANOVAS were calculated (task × intensity × group).The number of correct responses for the other-task was higher than the number of congruent responses for the self-task, F(1, 26) = 12.83, MSE = 0.085, p < .001, and higher for the high emotional intensity than the low emotional intensity stimuli, F(1, 26) = 104.73, MSE = 0.037, p < .001. There was also a main effect of group, F(1, 26) = 5.936, MSE = 0.125, p < .05, and a significant task × group interaction, F(1, 26) = 7.78, MSE = 0.085, p < .010. These effects were due to a group difference of congruent responses in the self-task (t-test for independent samples, t = 2.906, df = 15.47, p < .05) but no differences in the other-task (t = 0.159, df = 22.931, p = .875) (see ). Other interactions were not significant. In the other- and in the self-task, errors or incongruent responses were mostly due to the selection of “neutral” instead of the target emotion. Selection of the opposite emotional state (“sad” for happy faces or “happy” for sad faces) occurred on average in less than 2.3% of the trials. A 2 × 2 × 2 ANOVA (intensity × emotion × group) of the number of such choices revealed no significant effects or interactions. Across experimental groups, mean congruent responses during the self-task were correlated with EQ values (Spearman's rho = .414, p < .028), confirming the interrelationship between empathic abilities and performance in the experimental task. To relate performance in the self-task (as measured by the percentage of congruent responses) to performance in the other task (as measured by the percentage of correct responses) a correlation analysis was performed. Behavioral data for the self- and the other-task were significantly correlated for control subjects (r = .610, p < .01), but not for ASD subjects (r = .163, p < .289).
Figure 2. A. Emotional responses in control subjects (CS, n = 14) and subjects with autism spectrum disorders (ASD, n = 14) in the self- and the other-task. Average correct responses during the other-task (i.e., correct attribution of the emotional state of a stimulus face) and congruent responses during the self-task (i.e., responses mirroring the emotional state of a stimulus face) in control subjects and ASD subjects depending on task (self, other) and the intensity of the emotional expression (high, low). Error bars index the standard error of means (SEM). Note that the label “correct” is used only for the other-task as the emotional state of the observed person is defined by an objective criterion (facial expression, validated in a behavioral pilot-study). B. RTs during the control-, self- and other-tasks. Average RTs of correct responses during the control-task, other-task, and self-task. For the self- and other-tasks, separate RTs are given for stimuli with high and low emotional intensity. Since ANOVAs neither indicated a significant main effect of group nor significant interactions, RTs were collapsed across groups. Error bars index the standard error of means (SEM).
Eye movement data
Unlike previous studies reporting atypical visual scanning patterns in ASD for emotional faces (Dalton, Nacewicz, Alexander, & Davidson, Citation2007), we did not find differences between groups in the time spent on fixating the face, the eye, or the mouth region during any experimental condition (t-tests for independent samples; t(19) < 0.707, p > .489). Neuroimaging data are thus not confounded by aberrant fixation patterns that may occur in ASD subjects (see also Dapretto et al, Citation2006). Eye movement data are summarized in .
TABLE 2 Eye movement data
FMRI data
In the following paragraphs, we report fMRI results for each group separately, as well as results of the direct statistical comparisons between groups (interaction analyses). Note that differences in the activation patterns of the respective group results are not indicative of a difference between groups, unless significant in the direct comparison, and that post-hoc exclusion of medicated subjects from the fMRI data analysis did not change the pattern of results reported here.
Other-task vs. control-task
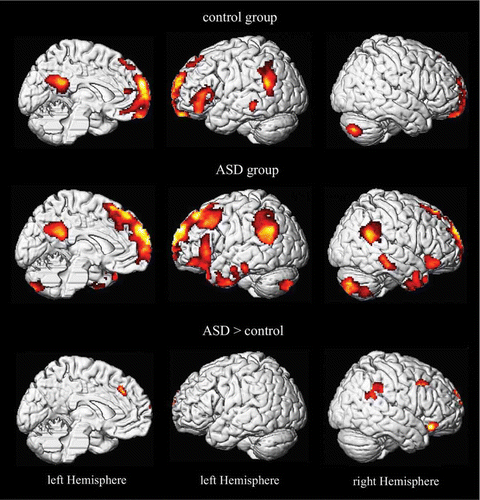
In control subjects, significant increases in neural activity were observed in bilateral medial cortical structures (ventral portions of the MPFC (vMPFC), precuneus/PCC), the left lingual gyrus, bilateral middle temporal gyrus/superior temporal sulcus (STS), left temporoparietal junction (TPJ), and right IFC (BA44/45). ASD subjects showed increased neural activation in the dorsal part of the left MPFC (dMPFC), bilateral precuneus, right middle temporal gyrus/STS and left TPJ. There was no significant activation in ASD subjects in the right IFC even at an uncorrected threshold (p < .001, voxel level). Significant differences in brain activation between groups could be revealed in the direct comparison (interaction analysis). Control subjects showed differential activation in the vMPFC and precuneus/PCC while there was differential activation in the dMPFC in ASD subjects (see ).
TABLE 3 Peaks of activation in the experimental tasks
Self-task versus control-task
In the control group, increased neural activity was evident in areas similar to those observed for the other-task. However, additional activations were located in the dMPFC, left IFC, left TPJ, and right cerebellum. In contrast, ASD subjects showed increases in neural activity which extended into widespread frontal areas (left superior frontal gyrus, bilateral middle frontal gyrus, bilateral IFC), bilateral TPJ, inferior temporal gyrus (ITG), and temporal pole. Significant differences in brain activation between groups could be revealed in the direct comparison (interaction analysis). ASD subjects showed differential activation in the right IFC (pars orbitalis, BA47), right dMPFC, right middle frontal gyrus, and the right TPJ (see ). No significant differential activations were observed for the control group at the pre-defined statistical threshold. At a more liberal, exploratory threshold (p < .05, uncorrected) the biggest cluster of activation emerged in the vMPFC.
Conjunction of other-task vs. control-task and self-task vs. control-task
The conjunction of the two experimental tasks (compared to the control-task) revealed activations bilaterally in the vMPFC and precuneus/PCC, right STS, and left TPJ in control subjects. In ASD subjects, conjoint activation could be observed in bilateral precuneus/PCC and left dMPFC. Using a ROI analysis, a trend towards significance could be observed for a cluster in right STS (p < .0689, FWE-corrected).
Correlations of brain activation and empathy
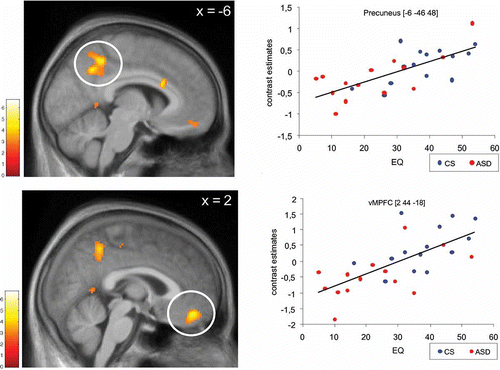
Whole brain regression analyses yielded significant correlations between empathic abilities and brain activation during the other task in the vMPFC (MNI coordinates of peak activated voxel: [2, 44, −18]) and precuneus bilaterally (MNI coordinates of peak activated voxel: [−6, −46, 48], see ). Whole brain analyses were performed across the whole group. However, to elucidate potential group differences in correlations, individual contrast estimates were extracted at peak activated voxels and tested for a positive correlation with EQ values separately for ASD and CS subjects. These analyses revealed significant correlations for each group in the vMPFC (ASD: R = 0.575, p < .05; CS: R = 0.538, p < .05) and precuneus (ASD: R = 0.725, p < .01; CS: R = 0.598, p < .05). The whole brain regression analysis of EQ and the self-task did not reveal foci of activation at the selected threshold. However, using the same precuneus and vMPFC coordinates reported above, we found significant correlations between activation in the self-task and EQ values in both vMPFC (R = 0.449, p < .01) and precuneus (R = 0.387, p < .05) across all participants. Separate correlation analyses for each group yielded marginally significant results for the precuneus in both groups (CS: R = 0.428, p = .0633; ASD: R = 0.452, p = .0522) and for the vMPFC only in control subjects (CS: R = 0.398, p = .0801; ASD: R = 0.289, p = .1578).
Figure 3. Covariation of brain activation and empathic abilities. Brain activity during the other task (vs. high-level baseline) correlated with individual EQ values (Baron-Cohen and Wheelwright, Citation2004) across all participants. SPM is thresholded at p < .005 (voxel-level). Circled clusters are significant at p < .05, corrected for multiple comparisons at the cluster level. Scatterplots illustrate the correlation in the peak activated voxels of both significant clusters in vMPFC and precuneus, respectively. Solid lines represent the linear best fit.
DISCUSSION
The paradigm of the present study is unique in that it enabled us to assess emotional self- and other-related processing in ASD subjects during an interactive empathic situation. Other studies have examined emotional self- or other-related social cognition in ASD using a paradigm that required the recognition of one's own face (Uddin et al., Citation2008) or abstract evaluation of trait adjectives (Kennedy & Courchesne, Citation2008), or have examined resting-state conditions that are considered to be associated with self-referential processing (Kennedy, Redcay, & Courchesne, Citation2006). To our knowledge, this is the first fMRI study that examines subjects with ASD in an explicit empathizing task. Previous imaging studies on empathy in ASD either focused on brain activation related to imitation and observation of facial expressions as indirect measures of empathy (Dapretto et al., Citation2006) or used affective pictures to induce emotion and correlate respective brain activation with empathy questionnaires (Silani et al., Citation2008).
The present study aimed at identifying brain dysfunctions underlying atypical self- and other-related emotional processing in adults with ASD in the context of facial expressions of emotions. With respect to behavioral performance, there were no significant differences in RTs between ASD subjects and control subjects for any experimental condition. It is thus unlikely that differences in neural activations are related to domain-general performance deficits (such as differences in perceptual processing speed). Moreover, the percentage of correct responses in the other-task did not differ between groups, suggesting that ASD subjects were able to infer other persons' emotions from facial displays. In contrast to previous studies (Dalton et al., Citation2007), we did not observe differences in eye-movements and visual scanning patterns between groups. One can thus exclude that our neuroimaging results are confounded by aberrant fixation patterns in ASD subjects. Consistent with previous findings of reduced emotional contagion (Scambler, Hepburn, Rutherford, Wehner, & Rogers, Citation2007) and empathy (Baron-Cohen & Wheelwright, Citation2004), individuals with ASD reported fewer contagious emotional responses during the self task. Note that incongruent responses were mostly “neutral” responses. Choosing an opposite emotion was rare, suggesting an absence of emotional contagion rather than the emergence of inappropriate incongruent emotional responses. The finding that in control subjects the amount of emotional contagion was correlated with the correct identification of emotional expressions suggests that these two processes are closely interrelated components of empathic processing.
Brain networks involved in ToM and empathy
The MPFC, STS, and TPJ, which are typically involved in ToM tasks (Frith & Frith, Citation2003), were activated in both controls and ASD subjects. Our data thus corroborate earlier studies demonstrating that ToM areas are recruited in face-to-face situations where emotional states are inferred from facial displays (Baron-Cohen et al., Citation1999; Schulte-Rüther et al., Citation2007). In contrast to previous reports (Castelli, Frith, Happé, & Frith, Citation2002; Happé et al., Citation1996), we did not observe significant hypoactivation of the ToM network in subjects with ASD. Our data suggest that individuals with ASD appear to activate ToM-related brain regions when they receive the instruction to intentionally empathize. Correspondingly, Wang, Lee, Sigman, and Dapretto (Citation2007) reported that the explicit instruction to focus on the social contents of stimuli may diminish hypoactivation of ToM areas in ASD subjects. In our experiment, ASD subjects showed increased activation in the right TPJ during the self-task, indicating that ASD subjects may use different cognitive resources to evaluate and gain access to their own emotional state in response to other persons' emotions. Various neuroimaging studies have demonstrated that the right TPJ is typically associated with the self–other distinction (Decety & Grezes, Citation2006; Schulte-Rüther et al., Citation2007; Vogeley & Fink, Citation2003) and plays a prominent role in differentiating between self-produced actions and actions caused by others (Blakemore & Frith, Citation2003). For example, during movement observation this brain region is associated with the awareness of not being the source of the action (Farrer & Frith, Citation2002), and it is modulated by the degree of mismatch between self-experienced and observed actions (Farrer et al., Citation2003). Thus, the observed differential activation of the TPJ in ASD subjects in our study may depend on a dysfunctional coupling of diminished “mirroring” of observed emotions and a state of enhanced cognitive distinction between one's own emotions and those expressed by the facial stimuli. Such enhanced distinction between oneself and other persons may contribute to a diminished capability of showing contagious emotional reactions. Other authors have argued that overlapping regions of the right TPJ are implicated in social cognitive processing (such as empathy and ToM) but also in lower-level attentional processing and that such attentional processing might be a prerequisite for higher-level social cognition (Decety and Lamm, Citation2007). For example, it has been suggested that the right TPJ is part of a ventral attention network which is involved in directing attention from internal states to external stimuli, and vice versa (Corbetta, Patel, & Shulman, Citation2008). During the self-task, the continuous shift between the assessment of one's own emotions and the observation of emotional faces may thus account for the activation of the TPJ in both groups. It might be speculated that for ASD subjects, such switching processes need more attentional resources than in control subjects (as indicated by stronger activation of the TPJ and the ventral inferior frontal cortex) because of their lack of emotional contagion (i.e., their internal emotional state was more often incongruent with the observed emotional faces).
Using a similar paradigm, Schulte-Rüther et al. (Citation2008) showed that males (in comparison to females) also show enhanced recruitment of the TPJ during the assessment of their own emotions in an empathic situation. Furthermore, emotional reactions to the observed faces were less pronounced in males than in females. Taken together, our results demonstrate that gender differences in these brain functions and behavior show similarities with ASD-related neurofunctional and behavioral deviations. The data thus provide preliminary support for theories that relate ASD to an extreme variant of a typical “male brain” (Baron-Cohen, Knickmeyer, & Belmonte, Citation2005).
Medial prefrontal cortex
The MPFC has been implicated in diverse emotional and non-emotional social tasks. Recently, it has been proposed that the MPFC can be segregated into neurofunctional submodules along a caudal–rostral axis (Amodio & Frith, Citation2006). According to the model, the most ventral parts of the MPFC (approximately defined by z < 2) are involved in autonomic and visceral aspects of emotional responses (Koski & Paus, Citation2000), which are typically associated with the monitoring of the value of future outcomes. In contrast, dorsal (posterior rostral) MPFC areas are considered to be primarily engaged in action monitoring and the evaluation of observed actions. Moreover, Amodio and Frith (Citation2006) argue that processing within the MPFC proceeds from the most dorsal and most ventral parts towards an anterior rostral transition zone. In this transition zone, more abstract metacognitive representations supporting self-reference and mentalizing are supposed to be implemented. Overall, this model is in accordance with the idea that social cognitive judgments rely primarily on the dMPFC while the vMPFC is more related to self-referential emotional cognition (D'Argembeau et al., Citation2007; Mitchell et al., Citation2006; Schulte-Rüther et al., Citation2007). Consistently, a large number of neuroimaging studies have implicated the vMPFC in self-referential thinking (e.g., Macrae, Moran, Heatherton, Banfield, & Kelley, Citation2004; Schmitz, Kawahara-Baccus, & Johnson, Citation2004), especially in the context of emotions (Moran, Macrae, Heatherton, Wyland, & Kelley, Citation2006). Since the vMPFC is strongly interconnected with emotion processing areas including the amygdala, ventral striatum, and orbitofrontal cortex (Ongur, Ferry, & Price, Citation2003), it is conceivable that self-related cognition, emotion processing, and external socially significant cues are integrated in this region. Such integration may allow for one's “emotional bonding” with other persons in empathic situations. This view is corroborated by our finding of a positive correlation between empathic abilities and activation in the vMPFC during empathizing (see ).
Note, however, that recent meta-analyses of brain imaging studies investigating theory of mind (Spreng, Mar, & Kim, Citation2009) and experience of emotion (Kober et al., Citation2008) demonstrated similar activations of ventral and dorsal portions of the MPFC during tasks that require emotional social cognition. These findings speak against the view of a clear-cut ventral/dorsal neurofunctional segregation within the MPFC. However, one needs to consider that many studies included in the meta-analysis (Kober et al., Citation2008) used facial expressions or pictures showing complex social scenes (e.g. the International Affective Picture System, IAPS) as stimulus materials which may evoke both empathic reactions and ToM reasoning. Furthermore, ToM paradigms typically contain not only cognitive, but also affective components. Lesion studies are better suited than meta-analyses to differentiate between effects of emotional processing and ToM within subregions of the MPFC. It has recently been demonstrated that lesions of the vMPFC selectively affect performance in ToM tasks that require the empathic understanding of other people's feelings (e.g., detecting “faux-pas” situations), but do not impair cognitive aspects of ToM (e.g., understanding of second-order false belief; Shamay-Tsoory, Tomer, Berger, Goldsher, & Sharon-Peretz, Citation2005; Stone, Baron-Cohen, & Knight, Citation1998). Moreover, patients with vMPFC lesions rate themselves as having less empathic ability than other people (Shamay-Tsoory, Sharon-Peretz, & Perry, Citation2009).
Our fMRI results show further evidence for the concept of a neurofunctional segregation in the MPFC in that we demonstrate a dissociation of activation along the caudal–rostral axis in the comparison of control subjects and subjects with ASD during empathizing (see ). These data underline the notion of a particular role of the vMPFC in affective ToM and empathy. Across groups, there is activation in the anterior rostral MPFC, the part of MPFC that has been associated with diverse abstract mentalizing tasks. However, the peak of activation was located more dorsally in subjects with ASD and more ventrally in control subjects. In the direct comparison between ASD subjects and control subjects, the activation patterns show a clear dissociation, with differential dMPFC recruitment in ASD subjects and differential vMPFC recruitment in control subjects. This brain activation pattern is paralleled by a reduction of contagious emotional responses in subjects with ASD. In controls, empathizing with other persons is thus likely to be triggered by emotional self-referential cognition instantiated in vMPFC regions, whereas in ASD subjects cognitive components of ToM (e.g., detection of intentions) and action monitoring (relying on dMPFC regions) may predominate. As the vMPFC plays an important role for monitoring the value of future outcomes (Amodio and Frith, Citation2006), it is conceivable that ASD subjects lack the direct link between metacognitive representations and the emotional value of social interactions. Clinical observations indicating that individuals with ASD can develop ToM abilities at an abstract level, but lack intuitive ToM abilities in dyadic social interactions (Bowler, Citation1992; Happé, Citation1994), are consistent with this suggestion. Note that the differential activation of dMPFC in ASD subjects for the other-task was at least in part also driven by a deactivation in control subjects for this task (in comparison to the control task) (see ). Enhanced emotional self-referential cognition as evidenced by vMPFC activation may perhaps have suppressed processing in the dMPFC. However, further studies investigating functional connectivity patterns of dMPFC and vMPFC are needed to substantiate such a claim.
Figure 4. Brain activity in the MPFC during the self- and other-task (relative to the control-task). In the other-task, activation was located in the dMPFC in ASD-subjects, while the control subjects (CS) activated portions of the vMPFC. Direct between-group contrast (CS > ASD; ASD > CS) corroborated this pattern of activations. Likewise, in the self-task, the peak of activation for ASD subjects was restricted to the dMPFC while activations extended into the vMPFC in control subjects. In the figure, activations are superimposed on the anatomical group mean image. For illustrative purposes, SPMs are thresholded at p < .001, uncorrected. Note however that all depicted peaks of activation were significant when correction for multiple comparisons was applied (see ), except for the comparison control > ASD in the self-task which is shown here at an exploratory level (p < .05 uncorrected).
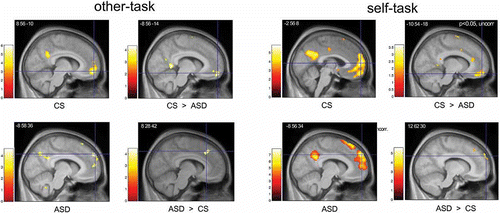
Figure 5. Contrast estimates of significant between-group differences. Mean contrast estimates for the autism spectrum group (ASD) and the control group (CS). Contrast estimates were calculated for statstically significant between-group comparisons for the other-task (A) and the self-task (B) relative to the control condition (interaction analyses, see ). Coordinates of peak activated voxels are given in MNI-space. Contrast estimates were determined for each group separately and reflect the relative contribution of each condition to the amplitude of the adjusted blood-oxygen-level-dependent (BOLD) signal relative to the fitted mean in the respective brain region. Note that contrast estimates are in arbitrary units. They do not reflect an estimate of effect size and are depicted here for visualization purposes. For statistical inference, an SPM analysis was conducted and all depicted areas were significant in an interaction contrast ([otherCS – controlCS]– [otherASD – controlASD] or [otherASD – controlASD] – [otherCS – controlCS ] for panel A or [selfASD – controlASD] – [selfCS – controlCS] for panel B), corrected for multiple comparisons (see and “Methods” section for further details). Error bars indicate the standard error of means; self = self-task; other = other-task, control = control-task.
![Figure 5. Contrast estimates of significant between-group differences. Mean contrast estimates for the autism spectrum group (ASD) and the control group (CS). Contrast estimates were calculated for statstically significant between-group comparisons for the other-task (A) and the self-task (B) relative to the control condition (interaction analyses, see Table 3). Coordinates of peak activated voxels are given in MNI-space. Contrast estimates were determined for each group separately and reflect the relative contribution of each condition to the amplitude of the adjusted blood-oxygen-level-dependent (BOLD) signal relative to the fitted mean in the respective brain region. Note that contrast estimates are in arbitrary units. They do not reflect an estimate of effect size and are depicted here for visualization purposes. For statistical inference, an SPM analysis was conducted and all depicted areas were significant in an interaction contrast ([otherCS – controlCS]– [otherASD – controlASD] or [otherASD – controlASD] – [otherCS – controlCS ] for panel A or [selfASD – controlASD] – [selfCS – controlCS] for panel B), corrected for multiple comparisons (see Table 3 and “Methods” section for further details). Error bars indicate the standard error of means; self = self-task; other = other-task, control = control-task.](/cms/asset/1fcca140-f788-4c5c-976d-6638771da333/psns_a_471325_o_f0005g.gif)
Self-related emotional cognition in ASD
Besides the MPFC, ASD and control subjects also recruited the precuneus and the adjacent PCC during both the self- and other-tasks, and precuneus activation was also positively correlated with empathic abilities, as assessed with the EQ. These regions have been implicated in a broader range of self-referential cognitive and emotional processes such as first-person perspective taking (Vogeley et al., Citation2001), representation of the mental self (Lou et al., Citation2004), and autobiographical memory (Piefke et al., Citation2008; Piefke, Weiss, Zilles, Markowitsch, & Fink, Citation2003). Interestingly, activation in these areas could be observed during both self- and other-conditions. This pattern of results is in accordance with other studies indicating that overlapping brain areas are implicated in judging other people's and one's own mental states, especially in cases where the other person is perceived as similar to oneself (Mitchell, Banaji, & Macrae, Citation2005; Mitchell et al., Citation2006). Mitchell et al. (Citation2006) conclude that the judgment of other persons may be built on the simulation of judging oneself. Together with these data, our results suggest that empathizing with other people may draw on simulation mechanisms by activating the neural networks underlying self-referential cognitive and emotional processing. In the present study, this idea is supported by the results of the conjunction analysis (self- and other-tasks vs. control-task): During both tasks, conjoint neural activation could be observed in PCC/precuneus and MPFC (dMPFC in ASD and vMPFC in healthy controls). This simulation mechanism may be disturbed in individuals with ASD. In support of this conclusion, our behavioral data demonstrate for control subjects that the tendency for emotionally congruent responses (self-task) was positively correlated with the ability to correctly infer an emotion in the other person (other-task). This was not the case in ASD subjects. Thus, in typical adults the capacity to identify the emotions of other people may benefit from the capacity of emotional contagion. These behavioral data are also consistent with the view that self-reflection may facilitate sensitive judgments about the mental states of other persons (Dimaggio, Lysaker, Carcione, Nicolo, & Semerari, Citation2008).
We observed significantly less activation of the vMPFC and precuneus/PCC in ASD subjects relative to the control group. Similar aberrant activation patterns in subjects with ASD have been reported for resting state conditions (Kennedy et al., Citation2006), which may be linked to automatic processes of self-referential cognition (Gusnard et al., Citation2001). Further evidence for altered activation patterns in ASD in these medial cortical areas has been observed during a task that required subjects to make judgments about the relationship of trait adjectives to oneself or a well-known other person (Kennedy & Courchesne, Citation2008). In combination with these data, our results point to a deficiency in the neural networks subserving self-referential processing in ASD as one reason for reduced empathic abilities. This may in particular be based on dysfunctions of MPFC regions and the precuneus/PCC. Our findings thus show a neurofunctional mechanism for the frequently observed impairments of self-referential cognition in ASD (Hurlburt et al., Citation1994; Lombardo et al., Citation2007; Toichi et al., Citation2002) and the related deficits in empathic behavior.
Due to a lack of self-referential emotional processing (possibly resulting in diminished emotional contagion), individuals with ASD may recruit different strategies to infer emotional states of other persons. ASD subjects showed activation in a widespread frontal network (including mid-dorsolateral and ventrolateral areas) and inferior temporal regions (including the temporal poles) during the self-task (see ). Mid-dorsolateral and ventrolateral areas of the prefrontal cortex have been implicated in diverse executive demands (e.g., monitoring cognitive processes and problem solving (Duncan & Owen, Citation2000). These activations may reflect the need for additional cognitive resources for resolving the cognitive–emotional requirements of the self-task.
The ITG has been implicated in feature-based analysis of visual stimuli (Gauthier, Anderson, Tarr, Skudlarski, & Gore, Citation1997). Schultz et al. (Citation2000) reported that ASD subjects show increased activation in the ITG during face perception, whereas control subjects differentially activate the fusiform gyrus, a brain region that has been implicated in the processing of configuration-related aspects of complex visual stimuli such as faces (Gauthier, Tarr, Anderson, Skudlarski, & Gore, Citation1999). Accordingly, other studies demonstrated that individuals with ASD gain access to complex visual displays using a feature-based strategy that overlooks the overall configuration of the stimulus (Manjaly et al., Citation2007). As a convergent zone for multiple sensory modalities, the temporal poles are involved in concept formation (Damasio, Tranel, Grabowski, Adolphs, & Damasio, Citation2004) and episodic autobiographical recollections (Piefke et al., Citation2003). It is most likely that they make available semantic and episodic materials for ongoing information processing in the brain (Frith & Frith, Citation2003). Since ASD subjects appear to have difficulties in establishing an intuitive emotional link to other persons, one may speculate that they activate these additional regions in an effortful attempt to make sense of the other's emotional facial expression with reference to themselves, for example, by recruiting their personal memories of past emotional interactions with other people. Note, however, that ITG and temporal poles did not show significantly higher activations for ASD subjects in the direct statistical comparison to controls. Thus, definite conclusions regarding the involvement of ITG and temporal poles in compensatory strategies will require further investigation.
Mirror mechanisms
The human mirror system (hMS) may play an important role in social cognition, especially in the context of emotional face-to-face interactions (Carr et al., Citation2003; Dapretto et al., Citation2006; Schulte-Rüther et al., Citation2007; but also see Hickok, Citation2009, for a critique). In support of this notion, we demonstrate that areas previously associated with the hMS (e.g., BA44/45 in the IFC) are activated when empathy is elicited by facial expressions of emotions. It is currently a matter of debate whether an early deficiency of the hMS in individuals with ASD may lead to their typical social and emotional deficits (Williams et al., Citation2001). Dapretto et al. (Citation2006) demonstrated that children with ASD show less activation in frontal components of the hMS during the observation and imitation of emotional facial expressions. However, children with ASD are not necessarily impaired in the understanding and imitation of non-emotional actions (Hamilton, Brindley, & Frith, Citation2007). Furthermore, it remains unclear at which level of imitative process problems may arise in individuals with ASD (Southgate & Hamilton, Citation2008). It has thus been argued that the claim of a direct link between imitation deficits and a core dysfunction of the mirror system in autism is speculative, to date. In the present study, we did not observe a significant difference between the two groups in the IFC (BA44/45) at the selected statistical threshold, in either the self- or the other-task. There was right-hemispheric activation in the IFC (BA44/45) during the other-task in the control group, but no activation above threshold in ASD subjects. In the self-task, however, left-hemispheric activation in frontal parts of the hMS was evident in ASD subjects as well. In adults with ASD, components of the hMS may thus become engaged in emotional face-to-face interactions especially when subjects are explicitly instructed to attend to their own emotional reaction to other people's emotions. The data suggest that ASD does not necessarily affect basic functions of the hMS. Rather, hMS recruitment during social interaction appears to be modulated by a combination of task, context, and instruction in individuals with ASD. Further studies are needed to clarify under which circumstances aberrant activations in the hMS may occur in ASD subjects and under which circumstances hMS function in this patient group is comparable to that of control subjects.
Limitations
Several potential limitations of the paradigm should be kept in mind. Our choice of computerized faces warranted high naturalism and optimum standardization of stimuli. More natural stimuli (e.g., videoclips of emotional faces in a naturalistic context) may perhaps trigger stronger empathic reactions; however, they are not well controlled experimentally. Potential differences between computerized facial stimuli and real faces have not been investigated in ASD yet, and should be explored in future studies. For example, ASD subjects might find it harder to empathize with a computerized person, and perceive such persons as more dissimilar to themselves. Such effects could contribute to our observed dorsal/ventral dissociation in MPFC (Mitchell et al., Citation2005, Citation2006).
An important aspect of the paradigm is that the behavioral responses during the self-task can be interpreted as an index of emotional contagion. Though this self-report response might be biased (e.g. by social desirability), our interpretation of reduced emotional contagion in ASD is in line with several previous studies (Scambler et al., Citation2007; Baron-Cohen & Wheelwright, Citation2004). Furthermore, a recent study systematically investigated social desirability bias in subjects with Asperger syndrome and control subjects, and found no group differences (Dziobek et al., Citation2008). However, more objective measures of emotional contagion (e.g. skin conductance, video recordings of facial reactions) should be employed in future studies to rule out such potential biases. Another issue related to the self-task refers to conditions of alexithymia. Subjects suffering from alexithymia have profound difficulties in verbalizing and identifying their own emotional states. It has been suggested that there may be an overlap between ASD and alexithymia with respect to social difficulties, affective interaction, and emotional awareness. (Fitzgerald & Bellgrove, Citation2006). However, fMRI studies did not reveal a direct relationship between difficulties in emotional awareness and self-reflection and mentalizing (Silani et al., Citation2008). Future studies should therefore investigate the role of alexithymia in ASD in more detail.
CONCLUSION
The present data provide novel insights into the brain networks involved in explicit emotional self-reference and emotion identification, two processes closely related to empathy. Furthermore, we demonstrate atypical neural activation associated with these processes in individuals with ASD. Importantly, our findings support the idea of a ventral–dorsal neurofunctional segregation in the MPFC. Atypical MPFC function during emotional face-to-face interactions in individuals with ASD may at least in part contribute to the impairment in self-referential emotional processing associated with the disease.
Acknowledgments
We are grateful to all volunteers who took part in this study. We wish to thank our colleagues in the Brain Imaging Physics (INM-4) and Cognitive Neurology groups (INM-3) at the Research Center Jülich (Institute of Neuroscience and Medicine) and the Department of Child Neuropsychology of the University Hospital Aachen for their support and helpful advice. This work was supported by the Hans-Lungwitz-Foundation (grant to MP), the Deutsche Forschungsgemeinschaft (EC 277 to MP and HJM, IRTG 1328), the European Commission (FP6-043460 to MP and HJM), and the German Max Planck Society (research award to HR).
REFERENCES
- Amodio , D. M. and Frith , C. D. 2006 . Meeting of minds: The medial frontal cortex and social cognition . Nature Reviews Neuroscience , 7 : 268 – 277 .
- Ashburner , J. and Friston , K. J. 2005 . Unified segmentation . NeuroImage , 26 : 839 – 851 .
- Baron-Cohen , S. , Knickmeyer , R. C. and Belmonte , M. K. 2005 . Sex differences in the brain: Implications for explaining autism . Science , 310 : 819 – 823 .
- Baron-Cohen , S. , Ring , H. A. , Wheelwright , S. , Bullmore , E. T. , Brammer , M. J. Simmons , A. 1999 . Social intelligence in the normal and autistic brain: An fMRI study . European Journal of Neuroscience , 11 : 1891 – 1898 .
- Baron-Cohen , S. , Tager-Flusberg , H. and Cohen , D. 2000 . Understanding other minds: Perspectives from autism and developmental cognitive neuroscience , Oxford, , UK : Oxford University Press .
- Baron-Cohen , S. and Wheelwright , S. 2004 . The empathy quotient: An investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences . Journal of Autism and Developmental Disorders , 34 : 163 – 175 .
- Baron-Cohen , S. , Wheelwright , S. , Robinson , J. and Woodbury-Smith , M. 2005 . The Adult Asperger Assessment (AAA): A diagnostic method . Journal of Autism and Developmental Disorders , 35 : 807 – 819 .
- Blakemore , S. J. and Frith , C. 2003 . Self-awareness and action . Current Opinion in Neurobiology , 13 : 219 – 224 .
- Bowler , D. M. 1992 . “Theory of Mind” in Asperger's Syndrome . Journal of Child Psychology and Psychiatry, and Allied Disciplines , 33 : 877 – 893 .
- Carr , L. , Iacoboni , M. , Dubeau , M. C. , Mazziotta , J. C. and Lenzi , G. L. 2003 . Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas . Proceedings of the National Academy of Sciences of the United States of America , 100 : 5497 – 5502 .
- Castelli , F. , Frith , C. , Happé , F. and Frith , U. 2002 . Autism, Asperger syndrome and brain mechanisms for the attribution of mental states to animated shapes . Brain , 125 : 1839 – 1849 .
- Cavanna , A. E. and Trimble , M. R. 2006 . The precuneus: A review of its functional anatomy and behavioural correlates . Brain , 129 : 564 – 583 .
- Corbetta , M. , Patel , G. and Shulman , G. L. 2008 . The reorienting system of the human brain: From environment to theory of mind . Neuron , 58 : 306 – 324 .
- Dalton , K. M. , Nacewicz , B. M. , Alexander , A. L. and Davidson , R. J. 2007 . Gaze-fixation, brain activation, and amygdala volume in unaffected siblings of individuals with autism . Biological Psychiatry , 61 : 512 – 520 .
- Damasio , H. , Tranel , D. , Grabowski , T. , Adolphs , R. and Damasio , A. 2004 . Neural systems behind word and concept retrieval . Cognition , 92 : 179 – 229 .
- Dapretto , M. , Davies , M. S. , Pfeifer , J. H. , Scott , A. A. , Sigman , M. Bookheimer , S. Y. 2006 . Understanding emotions in others: Mirror neuron dysfunction in children with autism spectrum disorders . Nature Neuroscience , 9 : 28 – 30 .
- D'Argembeau , A. , Ruby , P. , Collette , F. , Degueldre , C. , Balteau , E. Luxen , A. 2007 . Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking . Journal of Cognitive Neuroscience , 19 : 935 – 944 .
- Decety , J. and Grezes , J. 2006 . The power of simulation: Imagining one's own and other's behavior . Brain Research , 1079 : 4 – 14 .
- Decety , J. and Jackson , P. L. 2004 . The functional architecture of human empathy . Behavioral and Cognitive Neuroscience Reviews , 3 : 71 – 100 .
- Decety , J. and Lamm , C. 2007 . The role of the right temporoparietal junction in social interaction: How low-level computational processes contribute to meta-cognition . The Neuroscientist , 13 ( 6 ) : 580 – 593 .
- Derogatis , L. R. 1993 . Brief Symptom Inventory (BSI) administration, scoring, and procedures manual , 3rd , Minneapolis, MN : National Computer Services .
- Dimaggio , G. , Lysaker , P. H. , Carcione , A. , Nicolo , G. and Semerari , A. 2008 . Know yourself and you shall know the other … to a certain extent: Multiple paths of influence of self-reflection on mindreading . Consciousness and Cognition , 17 : 778 – 789 .
- Duncan , J. and Owen , A. M. 2000 . Common regions of the human frontal lobe recruited by diverse cognitive demands . Trends in Neurosciences , 23 : 475 – 483 .
- Duvernoy , H. M. 1999 . The human brain: Surface, three-dimensional sectional anatomy with MRI, and blood supply , Vol. 2 , New York : Springer .
- Dziobek , I. , Rogers , K. , Fleck , S. , Bahnemann , M. , Heekeren , H. Wolf , O. T. 2008 . Dissociation of cognitive and emotional empathy in adults with Asperger syndrome using the Multifaceted Empathy Test (MET) . Journal of Autism and Developmental Disordors , 38 : 464 – 473 .
- Eickhoff , S. B. , Stephan , K. E. , Mohlberg , H. , Grefkes , C. , Fink , G. R. Amunts , K. 2005 . A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data . NeuroImage , 25 ( 4 ) : 1325 – 1335 .
- Ekman , P. and Friesen , W. V. 1978 . Facial Action Coding System: Manual , Palo Alto, CA : Consulting Psychologists Press .
- Farrer , C. , Franck , N. , Georgieff , N. , Frith , C. D. , Decety , J. and Jeannerod , M. 2003 . Modulating the experience of agency: A positron emission tomography study . NeuroImage , 18 : 324 – 333 .
- Farrer , C. and Frith , C. D. 2002 . Experiencing oneself vs another person as being the cause of an action: The neural correlates of the experience of agency . NeuroImage , 15 : 596 – 603 .
- Fitzgerald , M. and Bellgrove , M. A. 2006 . The overlap between alexithymia and Asperger's syndrome . Journal of Autism and Developmental Disorders , 36 ( 4 ) : 573 – 576 .
- Frith , U. and Frith , C. D. 2003 . Development and neurophysiology of mentalizing . Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences , 358 : 459 – 473 .
- Gauthier , I. , Anderson , A. W. , Tarr , M. J. , Skudlarski , P. and Gore , J. C. 1997 . Levels of categorization in visual recognition studied using functional magnetic resonance imaging . Current Biology , 7 : 645 – 651 .
- Gauthier , I. , Tarr , M. J. , Anderson , A. W. , Skudlarski , P. and Gore , J. C. 1999 . Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects . Nature Neuroscience , 2 : 568 – 573 .
- Gillberg , C. L. 1992 . The Emanuel Miller Memorial Lecture 1991. Autism and autistic-like conditions: subclasses among disorders of empathy . Journal of Child Psychology and Psychiatry, and Allied Disciplines , 33 : 813 – 842 .
- Gitelman , D. R. 2002 . ILAB: A program for postexperimental eye movement analysis . Behavior Research Methods, Instruments, & Computers , 34 : 605 – 612 .
- Gusnard , D. A. , Akbudak , E. , Shulman , G. L. and Raichle , M. E. 2001 . Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function . Proceedings of the National Academy of Sciences of the United States of America , 98 : 4259 – 4264 .
- Hamilton , A. F. , Brindley , R. M. and Frith , U. 2007 . Imitation and action understanding in autistic spectrum disorders: How valid is the hypothesis of a deficit in the mirror neuron system? . Neuropsychologia , 45 : 1859 – 1868 .
- Happé , F. G. E. 1994 . An advanced test of theory of mind: Understanding of story characters' thoughts and feelings by able autistic, mentally-handicapped, and normal children and adults . Journal of Autism and Developmental Disorders , 24 : 129 – 154 .
- Happé , F. , Ehlers , S. , Fletcher , P. , Frith , U. , Johansson , M. Gillberg , C. 1996 . ‘Theory of mind’ in the brain: Evidence from a PET scan study of Asperger syndrome . NeuroReport , 8 : 197 – 201 .
- Hickok , G. 2009 . Eight problems for the mirror neuron theory of action understanding in monkeys and humans . Journal of Cognitive Neuroscience , 21 ( 7 ) : 1229 – 1243 .
- Hurlburt , R. T. , Happé , F. and Frith , U. 1994 . Sampling the form of inner experience in three adults with Asperger syndrome . Psychological Medicine , 24 : 385 – 395 .
- Kennedy , D. P. and Courchesne , E. 2008 . Functional abnormalities of the default network during self- and other-reflection in autism . Social Cognitive and Affective Neuroscience , 3 : 177 – 190 .
- Kennedy , D. P. , Redcay , E. and Courchesne , E. 2006 . Failing to deactivate: Resting functional abnormalities in autism . Proceedings of the National Academy of Sciences of the United States of America , 103 : 8275 – 8280 .
- Kober , H. , Barrett , L. F. , Joseph , J. , Bliss-Moreau , E. , Lindquist , K. Wager , T. D. 2008 . Functional grouping and cortical–subcortical interactions in emotion: A meta-analysis of neuroimaging studies . NeuroImage , 42 ( 2 ) : 998 – 1031 .
- Koski , L. and Paus , T. 2000 . Functional connectivity of the anterior cingulate cortex within the human frontal lobe: A brain-mapping meta-analysis . Experimental Brain Research , 133 : 55 – 65 .
- Lee , A. , Hobson , R. P. and Chiat , S. 1994 . I, you, me, and autism: An experimental study . Journal of Autism and Developmental Disorders , 24 : 155 – 176 .
- Lombardo , M. V. , Barnes , J. L. , Wheelwright , S. J. and Baron-Cohen , S. 2007 . Self-referential cognition and empathy in autism . PLoS ONE , 2 : e883
- Lou , H. C. , Luber , B. , Crupain , M. , Keenan , J. P. , Nowak , M. Kjaer , T. W. 2004 . Parietal cortex and representation of the mental self . Proceedings of the National Academy of Sciences of the United States of America , 101 : 6827 – 6832 .
- Macrae , C. N. , Moran , J. M. , Heatherton , T. F. , Banfield , J. F. and Kelley , W. M. 2004 . Medial prefrontal activity predicts memory for self . Cerebral Cortex , 14 : 647 – 654 .
- Maldjian , J. A. , Laurienti , P. J. , Burdette , J. B. and Kraft , R. A. 2003 . An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets . NeuroImage , 19 : 1233 – 1239 .
- Manjaly , Z. M. , Bruning , N. , Neufang , S. , Stephan , K. E. , Brieber , S. Marshall , J. C. 2007 . Neurophysiological correlates of relatively enhanced local visual search in autistic adolescents . NeuroImage , 35 : 283 – 291 .
- Millward , C. , Powell , S. , Messer , D. and Jordan , R. 2000 . Recall for self and other in autism: Children's memory for events experienced by themselves and their peers . Journal of Autism and Developmental Disorders , 30 : 15 – 28 .
- Mitchell , J. P. , Banaji , M. R. and Macrae , C. N. 2005 . The link between social cognition and self-referential thought in the medial prefrontal cortex . Journal of Cognitive Neuroscience , 17 : 1306 – 1315 .
- Mitchell , J. P. , Macrae , C. N. and Banaji , M. R. 2006 . Dissociable medial prefrontal contributions to judgments of similar and dissimilar others . Neuron , 50 : 655 – 663 .
- Moran , J. M. , Macrae , C. N. , Heatherton , T. F. , Wyland , C. L. and Kelley , W. M. 2006 . Neuroanatomical evidence for distinct cognitive and affective components of self . Journal of Cognitive Neuroscience , 18 : 1586 – 1594 .
- Morcom , A. M. and Fletcher , P. C. 2007 . Does the brain have a baseline? Why we should be resisting a rest . NeuroImage , 37 : 1073 – 1082 .
- Ongur , D. , Ferry , A. T. and Price , J. L. 2003 . Architectonic subdivision of the human orbital and medial prefrontal cortex . Journal of Comparative Neurology , 460 : 425 – 449 .
- Piefke , M. , Prestinger , M. , Arin , T. , Kohl , B. , Kastrau , F. Schnitker , R. 2008 . The neurofunctional mechanisms of traumatic and non-traumatic memory in patients with acute PTSD following accident trauma . Neurocase , 13 : 342 – 357 .
- Piefke , M. , Weiss , P. H. , Zilles , K. , Markowitsch , H. J. and Fink , G. R. 2003 . Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory . Brain , 126 : 650 – 668 .
- Preston , S. D. and de Waal , F. B. M. 2002 . Empathy: Its ultimate and proximate bases . Behavioral and Brain Sciences , 25 : 1 – 20 .
- Rogers , S. J. and Pennington , B. F. 1991 . A theoretical approach to the deficits in infantile autism . Development and Psychopathology , 3 : 137 – 162 .
- Scambler , D. J. , Hepburn , S. , Rutherford , M. D. , Wehner , E. A. and Rogers , S. J. 2007 . Emotional responsivity in children with autism, children with other developmental disabilities, and children with typical development . Journal of Autism and Developmental Disorders , 37 : 553 – 563 .
- Schmitz , T. W. , Kawahara-Baccus , T. N. and Johnson , S. C. 2004 . Metacognitive evaluation, self-relevance, and the right prefrontal cortex . NeuroImage , 22 : 941 – 947 .
- Schulte-Rüther , M. , Markowitsch , H. J. , Fink , G. R. and Piefke , M. 2007 . Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: A functional magnetic resonance imaging approach to empathy . Journal of Cognitive Neuroscience , 19 : 1354 – 1372 .
- Schulte-Rüther , M. , Markowitsch , H. J. , Shah , N. J. , Fink , G. R. and Piefke , M. 2008 . Gender differences in brain networks supporting empathy . NeuroImage , 42 : 393 – 403 .
- Schultz , R. T. , Gauthier , I. , Klin , A. , Fulbright , R. K. , Anderson , A. W. Volkmar , F. 2000 . Abnormal ventral temporal cortical activity during face discrimination among individuals with autism and Asperger syndrome . Archives of General Psychiatry , 57 : 331 – 340 .
- Shamay-Tsoory , S. G. , Tomer , R. , Berger , B. D. , Goldsher , D. and Sharon-Peretz , J. 2005 . Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage . Cognitive and Behavioral Neurology , 18 : 55 – 67 .
- Shamay-Tsoory , S. G. , Sharon-Peretz , J. and Perry , D. 2009 . Two systems for empathy: A double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions . Brain , 132 : 617 – 627 .
- Silani , G. , Bird , G. , Brindley , R. , Singer , T. , Frith , C. and Frith , U. 2008 . Levels of emotional awareness and autism: An fMRI study . Social Neuroscience , 3 : 97 – 112 .
- Singer , T. 2006 . The neuronal basis and ontogeny of empathy and mind reading: Review of literature and implications for future research . Neuroscience and Biobehhavioral Reviews , 30 : 855 – 863 .
- Southgate , V. and Hamilton , A. F. 2008 . Unbroken mirrors: Challenging a theory of autism . Trends in Cognitive Sciences , 12 : 225 – 229 .
- Spreng , R. N. , Mar , R. A. and Kim , A. S. N. 2009 . The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis . Journal of Cognitive Neuroscience , 21 : 489
- Stone , V. E. , Baron-Cohen , S. and Knight , R. T. 1998 . Frontal lobe contributions to theory of mind . Journal of Cognitive Neuroscience , 10 : 640 – 656 .
- Toichi , M. , Kamio , Y. , Okada , T. , Sakihama , M. , Youngstrom , E. A. Findling , R. L. 2002 . A lack of self-consciousness in autism . American Journal of Psychiatry , 159 : 1422 – 1424 .
- Tzourio-Mazoyer , N. , Landeau , B. , Papathanassiou , D. , Crivello , F. , Etard , O. Delcroix , N. 2002 . Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain . NeuroImage , 15 ( 1 ) : 273 – 89 .
- Uddin , L. Q. , Davies , M. S. , Scott , A. A. , Zaidel , E. , Bookheimer , S. Y. Iacoboni , M. 2008 . Neural basis of self and other representation in autism: An FMRI study of self-face recognition . PLoS ONE , 3 : e3526
- Vogeley , K. , Bussfeld , P. , Newen , A. , Herrmann , S. , Happé , F. Falkai , P. 2001 . Mind reading: Neural mechanisms of theory of mind and self-perspective . NeuroImage , 14 : 170 – 181 .
- Vogeley , K. and Fink , G. R. 2003 . Neural correlates of the first-person-perspective . Trends in Cognitive Sciences , 7 : 38 – 42 .
- Wang , A. T. , Lee , S. S. , Sigman , M. and Dapretto , M. 2007 . Reading affect in the face and voice: Neural correlates of interpreting communicative intent in children and adolescents with autism spectrum disorders . Archives of General Psychiatry , 64 : 698 – 708 .
- Williams , J. H. , Whiten , A. , Suddendorf , T. and Perrett , D. I. 2001 . Imitation, mirror neurons and autism . Neuroscience and Biobehavioral Reviews , 25 : 287 – 295 .