ABSTRACT
Objectives
It is hypothesized that the risk of hypersensitivity reactions (HSRs) may be lower with ferric carboxymaltose than iron dextran because of its non-dextran carbohydrate moiety. This study compares the risk of HSRs between iron dextran and ferric carboxymaltose.
Methods
This was a retrospective pharmacoepidemiological study with a case–population design covering 2008–2017. Global exposure data were estimated using IQVIA™ sales data. Spontaneously reported HSR data were retrieved from the World Health Organization database (VigiBase™) using different search criteria including: the Standardized MedDRA® Query (SMQ) ‘Anaphylactic reaction’; type I–IV HSR terms; narrow terms for anaphylactic/anaphylactoid reactions; and cases with a fatal outcome.
Results
Total exposure in 100 mg doses was 117.3 million for iron dextran and 84.2 million for ferric carboxymaltose. The relative risk (with 95% confidence interval) for ferric carboxymaltose versus iron dextran was 4.18 (3.88–4.50) for SMQ Anaphylactic reaction; 12.9 (9.90–16.7) for type I–IV HSRs; 1.72 (1.45–2.04) for anaphylactic/anaphylactoid reactions; and 1.92 (1.24–2.99) for death.
Conclusion
The risk of spontaneously reported HSRs was consistently higher with ferric carboxy-maltose than with iron dextran over the period 2008–2017. Thus, this study does not support that dextran-free intravenous irons are associated with fewer HSRs than iron dextran.
1. Introduction
According to the Global Burden of Disease Study 2017, anemia is the most common medical impairment in the world, affecting 1.95 billion people [Citation1]. Anemia is most prevalent in the poorest regions of the world, though it is also problematic in developed countries, with the most vulnerable population groups being young children, pregnant women, and women of childbearing age [Citation2,Citation3]. The most common cause of anemia is iron deficiency, responsible for more than half of cases [Citation3]. There are many causes of iron deficiency, including malnutrition, the increased iron demand of pregnancy, heavy menstrual bleeding, chronic diseases such as chronic kidney disease or inflammatory bowel disease, and the increasing prevalence of bariatric surgery [Citation4]. Iron deficiency anemia is harmful, being associated with impaired cognitive development in children, increased morbidity, adverse outcomes in pregnancy, and reduced work capacity [Citation5]. Oral iron formulations are beneficial for some patients with iron deficiency anemia, but their use is limited by gastrointestinal side effects which can result in nonadherence to treatment [Citation4,Citation6]. Intravenous (IV) iron formulations, consisting of iron–carbohydrate complexes, are generally recommended in cases where oral iron is inefficient, poorly tolerated, or poorly absorbed [Citation7,Citation8].
Early IV iron formulations were associated with rare but serious hypersensitivity reactions (HSRs), including anaphylactic reactions and death [Citation9]. Other formulations of IV iron with improved adverse event profiles have since been developed, including low-molecular-weight iron dextran (LMWID), ferumoxytol, ferric carboxymaltose (FCM), and ferric derisomaltose/iron isomaltoside 1000 [Citation9]. Based on rare ‘dextran-induced anaphylactoid/anaphylactic reactions’ seen with some dextran fractions used as plasma volume expanders, it has been hypothesized that IV iron formulations containing dextran may have a higher risk of HSRs than dextran-free formulations (e.g., FCM) [Citation10–Citation13]. However, this hypothesis overlooks the myriad of mechanisms which can lead to hypersensitivity besides anti-dextran antibody reactions [Citation14], and that similar antibody-mediated anaphylactic reactions have been documented for starch-based IV products [Citation15], which share a branched-polysaccharide backbone with the dextran-free FCM [Citation16]. Serious, potentially fatal HSRs have been reported following administration of FCM [Citation17–Citation22].
Studies comparing iron dextran (ID) and FCM are small and not designed or powered to estimate the incidence of HSRs [Citation23–Citation25]. In the absence of robust head-to-head data, the aim of the present study is to compare the risk of HSRs between ID and FCM using spontaneously reported adverse event data from a global safety database (VigiBase™). A comprehensive approach is taken, incorporating eight different categories of HSR events, including the Standardized MedDRA® Query (SMQ) for Anaphylactic reaction used by FDA, and fatal outcomes.
2. Methods
This was a retrospective pharmacoepidemiological study with a case–population design [Citation26], covering the ten-year time period from January 2008 to December 2017. Global exposure data and frequency of spontaneously reported HSRs were collected for two types of IV iron formulation, ID and FCM. For ID, high- and low-molecular-weight formulations, and high- and low-dose formulations, were all included and grouped together.
2.1. Data sources
2.1.1. Estimating exposure to intravenous iron
Global exposure to ID and FCM was estimated using sales data for these products. Sales volumes were retrieved from MIDAS®, an IQVIA™ analytics platform, which is validated annually by calculation of a ‘global precision index’ (94.3% in 2017) [Citation27]. Data were included for all available countries (82 countries across six continents). In most countries, sales were captured for hospital and retail settings. Coverage varied by country. Sales data were normalized to 100 mg dose equivalents, termed 1 defined daily dose (DDD) of iron, as has been done in similar studies of this nature [Citation13,Citation28–Citation32].
2.1.2. Estimating the incidence of spontaneously reported hypersensitivity reactions
Global data on spontaneously reported HSRs were retrieved from VigiBase™, the safety surveillance database of the World Health Organization (WHO), run by the Uppsala Monitoring Centre [Citation33]. Reports are continuously submitted by more than 150 member countries of the WHO Programme for International Drug Monitoring.
For this study, spontaneously reported HSR events associated with an administration of ID or FCM were identified in the database using sets of Medical Dictionary for Regulatory Activities (MedDRA®) preferred terms for hypersensitivity. Duplicate events were excluded so that each event was counted just once. Event reports were included in the analyses where the drug–MedDRA® term combination was reported with basis, ‘suspect’. The following sets of terms were used:
i) The SMQ ‘Anaphylactic reaction’, as used by FDA in their regulatory review of FCM [Citation17]. This SMQ comprises the following four groups of MedDRA® terms: Group A: narrow terms pertaining to HSRs, Group B: broad terms pertaining to respiratory reactions potentially related to hypersensitivity, Group C: broad terms pertaining to skin reactions potentially related to hypersensitivity, and Group D: broad terms pertaining to cardiovascular reactions potentially related to hypersensitivity. SMQs are validated, pre-determined sets of terms that were developed based on extensive review, testing, analysis, and expert discussion [Citation34]. The specific terms in each group are listed in the supplemental online material (Table S1).
ii) Type I–IV hypersensitivity terms (hypersensitivity, type I hypersensitivity, type II hypersensitivity, type III immune complex mediated reaction, and type IV hypersensitivity reaction), as these terms are not fully captured in the other search approaches, and to explore potential differences in coding practices across companies.
iii) Anaphylactic/anaphylactoid reaction terms (anaphylactic reaction, anaphylactic shock, anaphylactoid reaction, and anaphylactoid shock), as used in two prior studies of spontaneously reported adverse reactions [Citation31,Citation32].
iv) Cases with a fatal outcome.
2.2. Statistical analysis
Descriptive statistics were used to evaluate exposure and reported rates of HSRs. Data were aggregated across all countries. The number of unique spontaneously reported HSRs was determined for eight different categories of HSR, based on the groups defined above: 1. SMQ Anaphylactic reaction (Group A–D combined, primary analysis); 2. Group A; 3. Group B; 4. Group C; 5. Group D; 6. Type I–IV hypersensitivity terms; 7. Anaphylactic/anaphylactoid reaction terms; 8. Death. Additionally, the type I–IV hypersensitivity terms were evaluated excluding reactions coded as ‘hypersensitivity’ without specification of type, and also excluding ‘type I hypersensitivity’ to create a set of type II–IV hypersensitivity terms. A sensitivity analysis included only the HSR events that had a time to onset within 24 hours of IV iron administration. To investigate geographic variability, the share of exposure and HSR events were also determined by region.
The number of reports per IV iron formulation was divided by the exposure for that formulation (expressed as 100,000 DDDs) to yield a rate of number of reported adverse drug reactions per 100,000 doses. Rates were calculated by year and also for the total period 2008–2017. Relative risks and 95% confidence intervals were estimated by Poisson regression, for FCM versus ID.
The effect of baseline covariates on the likelihood of experiencing at least one HSR event (across any of the eight categories) was determined by stratifying HSR reports by patient age (0–27 days, 28 days–23 months, 2–11 years, 12–17 years, 18–44 years, 45–64 years, 65–74 years, ≥75 years, unknown) and sex (male, female, unknown). The time to onset of the HSR, the IV iron dose administered, and the reason for administration (i.e., indication) were also determined, where possible.
All statistical calculations were performed with SAS® 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Exposure to iron dextran and ferric carboxymaltose
The annual global exposure to ID decreased from 14.4 million DDDs in 2008 to 9.7 million DDDs in 2017 (). Over the same period, the annual global exposure to FCM increased from 0.6 million DDDs to 24.2 million DDDs (). The cumulative exposure over this ten-year period was 117.3 million DDDs for ID and 84.2 million DDDs for FCM.
Figure 1. Global sales of intravenous iron products (iron dextran and ferric carboxymaltose) from 2008 to 2017 in million DDDs (IQVIA™ MIDAS® data).
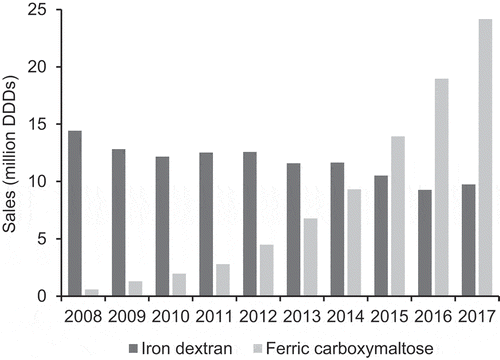
By region, ID was predominantly used in Asia (39.5% of total exposure), Europe (32.3%), and the Americas (21.4%), whereas FCM was predominantly used in Europe (64.0%).
3.2. Reported number and characteristics of hypersensitivity reactions
In total, from 2008 to 2017, 928 events of HSR (SMQ Anaphylactic reaction Group A–D) were reported for ID, and 2788 for FCM. Of these events, 250 with ID and 514 with FCM were severe Group A events. For all eight categories of HSR, more events occurred with FCM than with ID (). Thirty-four and 47 fatalities were reported in relation to ID and FCM administration, respectively.
Table 1. Global reported number and rate of hypersensitivity reactions and death with iron dextran (ID) and ferric carboxymaltose (FCM).
Considering yearly data, the number of Group A–D events with ID fluctuated in the range of 59 to 125. For FCM, the number of Group A–D events increased from 69 in 2008 to 670 in 2017. Events by year for the other categories of HSR are presented in .
By region, the majority of HSR events reported for ID were in Europe (34.9% of all events), the Americas (33.5%), and Asia (25.2%); HSR events reported for FCM were predominantly in Europe (72.1%).
Patients who experienced at least one HSR event were most likely to be in the age bracket of 18–44 years (ID: 37.9%; FCM: 54.9%), and to be female (ID: 67.9%; FCM: 85.5%). A greater proportion of patients who experienced an HSR event were aged ≥45 years for ID (44.5%) compared with FCM (26.9%). Time to onset of the HSR was generally within 24 hours of treatment (ID: 49.5%; FCM: 68.7%), though in many cases time to onset was not reported (ID: 42.9%; FCM: 23.4%). The most commonly administered doses for ID were 25 to <100 mg (11.5%) and 100 to <200 mg (9.8%), and for FCM were 1000 mg (29.4%) and 500 mg (26.9%); again, in many cases, dose was not reported (ID: 61.9%; FCM: 31.6%). HSRs were predominantly associated with an administration to treat iron deficiency, iron deficiency anemia, or some other anemia (ID: 54.0%; FCM: 52.5%); the reason for administration was not stated in 41.6% of ID and 42.7% of FCM cases.
3.3. Reporting rates for hypersensitivity reactions
Over the period between 2008 and 2017, the number of reported HSRs (SMQ Anaphylactic reaction Group A–D) per 100,000 DDDs (the ‘reporting rate’) was fairly constant for ID, ranging from 0.59 to 1.00 each year (). The reporting rate for FCM was consistently higher than for ID, and showed a pattern of decrease from 2008 (12.1) to 2011 (4.14), then relative constancy from 2012 to 2017 (range 2.77 to 3.85) (). In total over the ten-year period, the Group A–D reporting rate was 0.79 for ID and 3.31 for FCM. The corresponding relative risk of HSRs for FCM versus ID was 4.18 (). In the sensitivity analysis of events that had a time to onset within 24 hours of administration, the Group A–D reporting rate was 0.41 for ID and 2.27 for FCM, with a relative risk of 5.57.
Figure 2. Global reporting rate of hypersensitivity reactions (SMQ Anaphylactic reaction Group A–D) per 100,000 DDDs of iron dextran and ferric carboxy-maltose (VigiBase™ and IQVIA™ MIDAS® data).
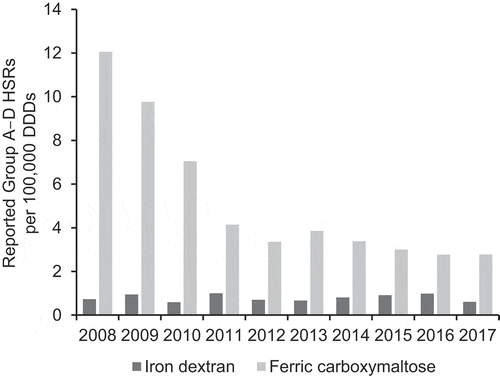
Figure 3. Global reported number, rate, and relative risk of hypersensitivity reactions and death with iron dextran and ferric carboxymaltose from 2008 to 2017. Abbreviations: DDD, defined daily dose; Group A, narrow terms pertaining to hypersensitivity reactions; Group B, broad terms pertaining to respiratory reactions potentially related to hypersensitivity; MedDRA®, Medical Dictionary for Regulatory Activities; n, number of reported adverse drug reactions (VigiBase™ data); rate, number of reported adverse drug reactions per 100,000 DDDs; SMQ, Standardized MedDRA® Query.
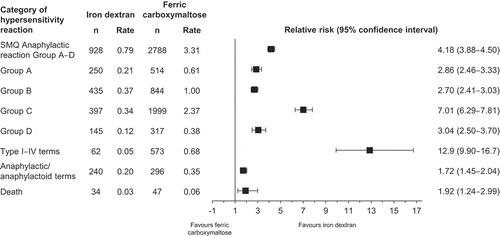
For all eight categories of HSR, including death, the rate of events over 10 years was consistently greater with FCM than with ID (). The relative risk of HSRs for FCM versus ID was greatest for type I–IV hypersensitivity terms (12.9), and smallest for anaphylactic/anaphylactoid reaction terms (1.72) (). In the additional analysis of type I–IV hypersensitivity terms excluding reactions coded as hypersensitivity without specification of type, the relative risk of HSRs for FCM versus ID was 151. Hypersensitivity type II–IV terms were reported for FCM but not for ID, meaning that a relative risk could not be calculated.
Reporting rates by year for all eight categories of HSR are presented in .
For context, the reporting rate of all adverse drug reactions (i.e., HSR and non-HSR events) from 2008 to 2017 was 1.3 per 100,000 DDDs for ID, and 6.0 per 100,000 DDDs for FCM.
4. Discussion
The results of this pharmacoepidemiological study show that the spontaneous reporting frequency of HSRs in the WHO’s safety database was higher with FCM than with ID over the ten-year period from 2008 to 2017. Thus, this study does not support the theory that dextran-free IV irons such as FCM are safer than ID. For robustness, we used eight different categories of HSR events, all of which favoured ID, by up to 13 times. This was despite a conservative approach in which high-molecular-weight ID (HMWID) and LMWID were grouped together, thereby biasing against LMWID because HMWID is generally associated with a much higher rate of life-threatening adverse reactions than LMWID [Citation28]. Where such information was reported, HSRs were generally associated with typical doses of the respective formulations, and with administrations for the indicated conditions of iron deficiency/anemia.
While these data consistently indicate that FCM had a higher risk of HSRs than ID over the period 2008–2017, the results must be considered in the context of the study’s limitations. The dataset relied on the number of suspected adverse reaction reports spontaneously submitted by healthcare professionals and patients, which depends on a wide range of parameters that cannot be controlled. For example, spontaneous reporting will be impacted by the geographical region (due to differences in healthcare organization and local reporting systems), the amount of focus on a particular adverse reaction from the authorities or medical community, the environment in which the product is administered (hospital or elsewhere), the number of people receiving the product, the target population and its demographics (female sex, younger age, and presence of comorbidities are risk factors for HSRs [Citation35]), the nature and frequency of the adverse reactions, and the physician’s choice of MedDRA® term when reporting adverse reactions. Underreporting of HSR events was apparent with FCM in the present study, since the observed rate of SMQ Anaphylactic reaction Group A–D HSRs (3.31 per 100,000 DDDs) is orders of magnitude below that stated in the FCM US label (1.5%) [Citation16,Citation17]. Considering geographic region, the majority of FCM exposure (and HSR reporting) was in Europe, whereas ID was used more globally. Compared to the share of exposure, the share of HSR events was relatively lower in Asia (for both formulations) and higher in the Americas (for ID), which indicates differential reporting of HSRs between regions. With regard to differences in target population, patients who experienced an HSR with ID versus FCM were more likely to be older (suggesting a population with more severe illness who may be at greater risk of HSRs), and less likely to be female (suggesting a lower proportion of obstetrics/gynecology/pregnancy/postpartum cases).
The amount of time a product has been on the market is relevant to this study because reporting rates of spontaneously reported adverse reactions tend to be highest in the introductory phase of a new drug, and to subsequently decrease over time (the ‘Weber effect’) [Citation36]. Consequently, it can be hypothesized that studies of spontaneously reported adverse reactions show bias in favour of formulations which have been on the market for longer [Citation37]. FCM was launched in 2007 (EU approval, 2007; US approval 2013), and the present study ran from 2008 to 2017. We observed that the greatest rate of spontaneously reported HSRs associated with FCM occurred in 2008, and the rate decreased with time. In contrast, ID, available in low-molecular-weight form since the 1990s, had a constant, low rate of spontaneously reported HSRs. Thus, the timing of the present study was biased in favour of ID. However, it should be noted that even at the end of the study period, many years after the launch of FCM, the reporting rates of HSRs were higher with FCM than with ID.
The time on the market also differed between formulations in relation to the European Medicines Agency (EMA) special warning for HSRs with IV iron products, issued in 2013 [Citation38]. However, there was no obvious effect of this warning on spontaneously reported HSRs in the present study.
Another limitation is that exposure data may have been underestimated, as direct sales to clinics and private offices were not captured, and in some countries not all distribution channels were captured. Underestimation of exposure will lead to an overestimation of hypersensitivity reporting frequency. In addition, whereas IQVIA™ MIDAS® data are available at a country level, VigiBase™ data are available by WHO region only, and thus the degree of geographic overlap and the validity of dividing one value by the other is unknown.
Taking all of these limitations into account, it may not be valid to conclude that one product is safer than another based on the number of spontaneous reports alone. The Uppsala Monitoring Centre states that comparisons between medicinal products based on VigiBase™ data may be misleading [Citation39], and the EMA has stated (in relation to a pandemic flu vaccine) that it is not scientifically valid to suggest that one treatment is less safe than another based purely on a comparison of the number of spontaneous reports of adverse reactions [Citation40]. Instead, all available data, in particular data from clinical trials and robust epidemiological studies, should be taken into consideration when evaluating safety profiles and potential differences between products.
These same criticisms apply to other real-world database studies that have looked at the incidence of HSRs with IV iron products. Whereas one study of spontaneously reported anaphylactic reactions among IV iron products concluded that relative risk estimates cannot be calculated due to under-reporting, possible differential reporting, absence of product brand names, and incomplete usage data [Citation41], other studies have attempted to make comparisons between different IV iron formulations based on spontaneously reported adverse reaction data [Citation13,Citation28–Citation31]. Considering ID, a retrospective cohort study of 688,183 IV iron recipients in the US from 2003–2013 (prior to the availability of FCM) showed that the risk of ‘anaphylaxis’ was higher with ID than with non-dextran IV iron products [Citation42]. However, data adapted from the manuscript’s supplemental material showed that the incidence of death on the day of IV iron administration favoured ID over the non-dextran products, suggesting that many of the so-called ‘anaphylaxis’ reactions were actually milder infusion symptoms, not anaphylaxis [Citation9,Citation42–Citation44]. Such false-positive coding is the result of confusing and inconsistent terminology surrounding the reporting of HSR events [Citation45].
Further on this point, the rate of HSRs coded with a specific type of hypersensitivity (i.e., type I–IV) in the present study was strikingly different for FCM versus ID, with a relative risk of 151, and type II–IV reactions were reported for FCM only. This discrepancy may be attributed to a systematic difference in how adverse events are coded between companies. This issue merits further research as it may represent a substantial bias in analyses of post-marketing data. For example, neither the MedDRA® SMQ Anaphylactic reaction nor the SMQ Angioedema contain the preferred terms for type II, III, or IV hypersensitivity. Thus, analyses using these SMQs will result in a systematic underestimation of HSRs with FCM.
The hierarchy of evidence in medicine has been established for many years, placing meta-analyses of randomized controlled trials (RCTs) at the top, followed by RCTs, and with non-randomized studies and case studies/reports as the weakest form of evidence [Citation46]. Analyses of spontaneous reports can be used to identify a possible signal that will need to be further examined and validated [Citation47], but such analysis does not necessarily reflect the occurrence of events in clinical practice. Such data cannot determine causation, and should not be used to compare the frequency of adverse reactions between products, or to determine the benefit–risk balance. Head-to-head RCTs are essential in order to assess the relative risk of adverse drug reactions between formulations in an unbiased fashion. There is limited evidence from RCTs comparing the incidence of HSRs with ID and FCM. One head-to-head study (N = 55) found that HMWID and FCM had a similar incidence of adverse events, and no serious adverse events [Citation23]. Although no RCTs have compared LMWID with FCM, a retrospective case review study (N = 92) found no serious adverse events associated with the administration of LMWID or FCM in pregnancy [Citation25]. Similarly, a systematic review of RCTs of IV iron did not identify any serious treatment-related HSRs with LMWID (N = 83) or FCM (N = 543) [Citation48]. Finally, considering other forms of IV iron, three large RCTs have investigated the incidence of HSRs as a primary outcome, finding no meaningful differences between FCM and ferumoxytol [Citation49] or ferric derisomaltose/iron isomaltoside 1000 and iron sucrose [Citation50,Citation51]. Thus, there are inadequate data from RCTs to assess the hypothesis that ID has a higher risk of HSRs than FCM, and spontaneously reported data provide no support for this hypothesis.
5. Conclusions
Over a ten-year period (2008 to 2017), the spontaneous reporting frequency of HSRs in the WHO’s safety database was consistently higher with FCM than with ID. Thus, this study does not support the theory that dextran-free IV irons, such as FCM, are associated with fewer HSRs than ID.
Author Contributions
DD, PSM and CCS contributed to the design of the study and were involved in the acquisition, analysis, and interpretation of data. All authors participated in the drafting or the critical review of the article; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Data Availability
The data that support the findings of this study are available from VigiBase™ and IQVIA™ MIDAS® but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of VigiBase™ and IQVIA™ MIDAS®.
Disclaimer
Data obtained from VigiBase™, the World Health Organization (WHO) global database of individual case safety reports. As the information comes from a variety of sources, the probability that the suspected adverse reaction is drug-related is not the same in all cases, and the information does not represent the opinion of the Uppsala Monitoring Centre or the WHO.
Declaration of Interest
All authors are employees of Pharmacosmos A/S. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download PDF (56.5 KB)Acknowledgments
Writing support was provided by Chris Watling, PhD, assisted by his colleagues at Cambridge Medical Communication Ltd (Cambridge, UK), and funded by Pharmacosmos A/S.
Supplementary Material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858.
- Stevens GA, Finucane MM, De-Regil LM, et al.; Nutrition Impact Model Study Group (Anaemia). Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995–2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25.
- Kassebaum NJ. GBD 2013 Anemia Collaborators. The global burden of anemia. Hematol Oncol Clin North Am. 2016;30:247–308.
- Camaschella C. Iron deficiency. Blood. 2019;133:30–39.
- World Health Organization. Iron deficiency anaemia: assessment, prevention, and control. A guide for programme managers. Geneva: World Health Organization; 2001.
- Tolkien Z, Stecher L, Mander AP, et al. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10:e0117383.
- Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia. Am J Hematol. 2016;91:31–38.
- Peyrin-Biroulet L, Williet N, Cacoub P. Guidelines on the diagnosis and treatment of iron deficiency across indications: a systematic review. Am J Clin Nutr. 2015;102:1585–1594.
- Auerbach M, Deloughery T. Single-dose intravenous iron for iron deficiency: a new paradigm. Hematology Am Soc Hematol Educ Program. 2016;2016:57–66.
- Hedin H, Richter W. Pathomechanisms of dextran-induced anaphylactoid/anaphylactic reactions in man. Int Arch Allergy Appl Immunol. 1982;68:122–126.
- Ljungström KG. Pretreatment with dextran 1 makes dextran 40 therapy safer. J Vasc Surg. 2006;43:1070–1072.
- Neiser S, Wilhelm M, Schwarz K, et al. Assessment of dextran antigenicity of intravenous iron products by an immunodiffusion assay. Port J Nephrol Hypert. 2011;25:219–224.
- Bailie GR, Clark JA, Lane CE, et al. Hypersensitivity reactions and deaths associated with intravenous iron preparations. Nephrol Dial Transplant. 2005;20:1443–1449.
- Szebeni J, Fishbane S, Hedenus M, et al. Hypersensitivity to intravenous iron: classification, terminology, mechanisms and management. Br J Pharmacol. 2015;172:5025–5036.
- Kreimeier U, Christ F, Kraft D, et al. Anaphylaxis due to hydroxyethyl-starch-reactive antibodies. Lancet. 1995;346:49–50.
- Injectafer® (ferric carboxymaltose injection). Prescribing information; 2013 [cited 2019 Nov 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/203565s000lbl.pdf
- Center for Drug Evaluation and Research. Medical review of Injectafer (VIT-45, ferric carboxymaltose injection; FCM) for the treatment of iron deficiency anemia (203565Orig1s000); 2013 [cited 2019 Nov 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/203565Orig1s000MedR.pdf.
- Prescrire editors. Fer polymaltose intraveineux: angioedèmes, etc. [Intravenous iron polymaltose: angioedema, etc.]. Rev Prescrire. 2013;31(361):829.
- Chenaf C, Pagani M, Parry E, et al. Post-marketing safety profile of ferric carboxymaltose (Ferinject®). Fundam Clin Pharmacol. 2013;27(Suppl 1):101.
- Swissmedic. Vigilance News; 2013:11.
- Drake S. Precaution for Ferinject® vials. DrugAlert; 2018:404 [cited 2019 Nov 12]. Available from: https://www.hps.com.au/knowledge-centre/drugalert/precaution-for-ferinject-vials/.
- Australian Government Department of Health. Therapeutic Goods Administration. Database of adverse event notifications – medicines. Ferinject: Medicine summary; 2018 [cited 2019 Nov 12]. Available from: http://hpscorp.boylentest.com.au/wp-content/uploads/2018/05/20180524_daen-report.pdf.
- Wolf M, Koch TA, Bregman DB. Effects of iron deficiency anemia and its treatment on fibroblast growth factor 23 and phosphate homeostasis in women. J Bone Miner Res. 2013;28:1793–1803.
- Hussain I, Bhoyroo J, Butcher A, et al. Direct comparison of the safety and efficacy of ferric carboxymaltose versus iron dextran in patients with iron deficiency anemia. Anemia. 2013;169107.
- Myers B, Myers O, Moore J. Comparative efficacy and safety of intravenous ferric carboxymaltose (Ferinject) and iron(III) hydroxide dextran (Cosmofer) in pregnancy. Obstet Med. 2012;5:105–107.
- Théophile H, Laporte JR, Moore N, et al. The case-population study design: an analysis of its application in pharmacovigilance. Drug Saf. 2011;34:861–868.
- IQVIA™. ACTS 2018. IQVIA quality assurance. 2018 [cited 2019 Nov 12]. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/library/publications/acts-2018-32nd-edition.pdf.
- Chertow GM, Mason PD, Vaage-Nilsen O, et al. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–382.
- Bailie GR, Hörl WH, Verhoef JJ. Differences in spontaneously reported hypersensitivity and serious adverse events for intravenous iron preparations: comparison of Europe and North America. Arzneimittelforschung. 2011;61:267–275.
- Bailie GR. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am J Health Syst Pharm. 2012;69:310–320.
- Ehlken B, Nathell L, Gohlke A, et al. Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxy-maltose and iron (III) isomaltoside 1000 in Europe based on data from EudraVigilance and VigiBase™ between 2014 and 2017. Drug Saf. 2019;42:463–471.
- Nathell L, Gohlke A, Wohlfeil S. Reported severe hypersensitivity reactions after intravenous iron administration in the European Economic Area (EEA) before and after implementation of risk minimization measures. Drug Saf. 2020;43:35–43.
- Uppsala Monitoring Centre. VigiBase: the unique global resource at the heart of the drive for safer use of medicines [cited 2019 Nov 12]. Available from: https://www.who-umc.org/vigibase/vigibase/.
- Medical Dictionary for Regulatory Activities (MedDRA®). Standardised MedDRA Queries [cited 2019 Nov 12]. Available from: https://www.meddra.org/standardised-meddra-queries.
- Mulder MB, van den Hoek HL, Birnie E, et al. Comparison of hypersensitivity reactions of intravenous iron: iron isomaltoside-1000 (Monofer®) versus ferric carboxy-maltose (Ferinject®). A single center, cohort study. Br J Clin Pharmacol. 2019;85:385–392.
- Weber JCP. Epidemiology of adverse reactions to nonsteroidal anti-inflammatory drugs. In: Rainsford KD, Velo GD, editors. Side-effects of anti-inflammatory drugs, advances in inflammation research. New York: Raven Press; 1984. p. 1–7.
- Schaffalitzky de Muckadell P, Strom CC. Comment on ‘Evaluation of the reported rates of severe hypersensitivity reactions associated with ferric carboxy-maltose and iron (III) isomaltoside 1000 in Europe based on data from EudraVigilance and VigiBase™ between 2014 and 2017ʹ. Drug Saf. 2019;42:689–691.
- European Medicines Agency. Assessment report for: Iron containing intravenous (IV) medicinal products (EMA/549569/2013); 2013 [cited 2019 Nov 12]. Available from: https://www.ema.europa.eu/en/documents/referral/intravenous-iron-containing-medicinal-products-article-31-referral-assessment-report_en.pdf.
- Uppsala Monitoring Centre. Caveat document. Statement of reservations, limitations and conditions relating to data released from VigiBase, the WHO global database of individual case safety reports (ICSRs). 2018 [cited 2019 Nov 12]. Available from: https://www.who-umc.org/media/164610/umc_caveat.pdf.
- European Medicines Agency. Rapid response to BMJ. Re: Pandemrix vaccine: why was the public not told of early warning signs? (EMA/659264/2018). 2018 [cited 2019 Nov 12]. Available from: https://www.ema.europa.eu/en/documents/other/european-medicines-agency-rapid-response-british-medical-journal-pandemrix_.pdf.
- Wysowski DK, Swartz L, Borders-Hemphill BV, et al. Use of parenteral iron products and serious anaphylactic-type reactions. Am J Hematol. 2010;85:650–654.
- Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA. 2015;314:2062–2068.
- Therapeutics Initiative. Intravenous (IV) iron for severe iron deficiency. Vancouver, British Columbia, Canada: Therapeutics Letter; 2015. p. 97.
- DeLoughery TG, Auerbach M. Is low-molecular weight iron dextran really the most risky iron? Unconvincing data from an unconvincing study. Am J Hematol. 2016;91:451–452.
- Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99:1671–1676.
- Cochrane Consumer Network. Levels of evidence [cited 2019 Nov 12]. Available from: https://consumers.cochrane.org/levels-evidence.
- European Medicines Agency. Guideline on good pharmacovigilance practices (GVP). Module IX Addendum I – Methodological aspects of signal detection from spontaneous reports of suspected adverse reactions (EMA/209012/2015); 2017 [cited 2019 Nov 12]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-gvp-module-ix-addendum-i-methodological-aspects-signal_en.pdf.
- Aksan A, Işık H, Radeke HH, et al. Systematic review with network meta-analysis: comparative efficacy and tolerability of different intravenous iron formulations for the treatment of iron deficiency anaemia in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1303–1318.
- Adkinson NF, Strauss WE, Macdougall IC, et al. Comparative safety of intravenous ferumoxytol versus ferric carboxymaltose in iron deficiency anemia: a randomized trial. Am J Hematol. 2018;93:683–690.
- Auerbach M, Henry D, Derman RJ, et al. A prospective, multi-center, randomized comparison of iron isomaltoside 1000 versus iron sucrose in patients with iron deficiency anemia; the FERWON-IDA trial. Am J Hematol. 2019;94:1007–1014.
- Bhandari S, Kalra PA, Berkowitz M, et al. Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: the FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant. 2020. DOI:10.1093/ndt/gfaa011.
