1. Introduction
Spain is one of the countries most strongly affected by the coronavirus (COVID-19) pandemic with more than 800,000 cases reported in the country as of October 5 [Citation1]. In Madrid, the first reported case was diagnosed at La Paz University Hospital on February 25. At the peak of the pandemic, on the 3 April 0941 patients were hospitalized with COVID-19. On that date, patients with COVID-19 occupied approximately 80% of the available beds in the Hospital. The Hematology Unit was one of the few units that did not have infected patients, and we were able to continue conducting our regular work while minimizing our patients’ risk of infection. This is especially relevant in the hematopoietic cell transplantation (HCT) setting, given the high mortality rates reported for hematologic patients with COVID-19, as well as the initial lack of practical experience or availability of guidelines [Citation2].
In a study carried out by Piñata et al., the mortality rate for patients infected with SARS-CoV-2 who underwent hematopoietic cell transplantation was 18% [Citation2]. In accordance with the initial recommendations, many centers which carry out HCT chose to delay non-urgent procedures during the outbreak and thus avoid potential infections [Citation3]. The Acute Leukemia Working Party of the European Society for Bone Marrow Transplantation (EBMT) state that during the pandemic, allogeneic transplantation (allo-HCT) may be postponed in patients with standard and low risk leukemia [Citation4]. Spanish guidelines also discourage autologous hematopoietic cell transplantation (auto-HCT) in low-grade lymphomas and allo-HCT in refractory high-grade lymphomas and Hodgkin lymphoma [Citation5]. In multiple myeloma, the European Myeloma Network recommends delaying autologous transplantation and extending induction therapy, especially in standard risk patients and those who respond adequately to induction [Citation6]. Nonetheless, a failure to provide the most effective therapy in a timely manner may often result in irreversible relapse or progression of hematologic malignancies [Citation7]. Some HCT centers have therefore decided to continue carrying out transplants, especially for patients without COVID-19 who have malignant disease [Citation8,Citation9]. Recently released guidelines do not recommend delaying transplantation for acute leukemias, high-risk myelodysplastic syndromes or auto-HCT, if performed for curative intent [Citation10].
Between February 25, when the first patient with COVID-19 was admitted to our center, and June 15, when there were no new cases, we performed 16 HCTs (9 allogeneic and 7 autologous). None of the patients who received a transplant were infected with SARS-CoV-2. By implementing rigorous preventive and supportive measures, we were able to protect our patients from COVID-19, and continue to perform potentially curative procedures.
2. Preventive and supportive measures
General preventive measures were established in our Department on March 6, one week before the national lockdown began. We designed a rigorous protocol aimed at minimizing the risk of SARS-CoV-2 infection among our patients. The precautions which apply to the general population (frequent hand hygiene, use of face mask and social distancing) were universally recommended in hematologic immunocompromised patients, even before the onset of the COVID-19 outbreak. We expanded these measures to include all caregivers. A full list of the measures taken which are still in place is summarized below.
2.1. Outpatients
From the beginning of the pandemic, we implemented telemedicine for patients in follow-up and a home drug-delivery system for frail patients. A COVID-19 free circuit was implemented around hematology areas. Our day-care Unit and the Hematology outpatient areas are physically apart from the general hospital, and no suspected or known COVID-19 patient was accepted into these areas. When patients had to visit our center, a hematology nurse went through a checklist of respiratory symptoms, measured the patient’s temperature and provided hand sterilization before they entered the building. Use of a FFP2 face mask was strongly recommended and companions were not allowed in. Every patient suspected to have COVID-19 was transferred to the Emergency Room, with the exception of those receiving HCT, who would wait in a separate area where a hematologist evaluated whether they were suffering from HCT- rather than COVID-19 related complications. Outpatients underwent PCR testing for SARS-CoV-2 before each chemotherapy cycle and they were also given a recommendation sheet.
2.2. Inpatients
Only patients with negative PCR test results were admitted, and visitors were not allowed to accompany them. Handwashing and disinfection of shoes with Virkon® was mandatory before entering the Hematology Unit. The door to the Unit remained closed and could only be opened with Hematology identification. The number of medical examinations carried out outside the Hematology Unit were kept to a minimum. Patients did not undergo PCR testing after they were admitted, unless they experienced respiratory symptoms or unexplained fever. They were encouraged to wear masks when a health care provider entered the room to minimize the possibility of transmission from asymptomatic carriers. No nosocomial infections were reported.
2.3. Donors
National and international guidelines were followed for stem cell donors [Citation11,Citation12]. Donors underwent PCR testing of nasopharyngeal swabs twice: prior to mobilization and before apheresis. A positive result meant that the donor was excluded from donation for at least 28 days after they received a negative nasopharyngeal PCR test result. The risk for both the donor and the recipient was further reduced by postponing donations by 28 days if the donor had been in close contact with a person who was confirmed to have COVID-19.
2.4. Recipients
Recipients were also tested 24–48 h before commencing conditioning. If the patient tested positive, the procedure was postponed for between 30 and 90 days, depending on the severity of the infection and the risk of relapse. HCT was delayed for at least 14 days (ideally 21 days) if the recipient had been in close contact with someone who was confirmed to have COVID-19 [Citation3]. Due to the high prevalence of SARS-CoV-2 in our city, visits to the Transplantation Unit were not allowed. Patients receiving HCT from a matched unrelated donor could not commence conditioning until the product had arrived at our facilities. This was especially important as international travel regulations resulted in some products being delayed.
2.5. Apheresis and graft cryopreservation
All hematopoietic stem cells were collected by peripheral blood apheresis and no ex-vivo manipulation of the graft was performed. After the Spanish National Organization of Transplants (ONT) published their official statement on March 11, we started cryopreserving the product for at least 14 days. However, later evidence did not support such cryo-quarantine [Citation11].
2.6. Medical staff
Human resources have been a limiting factor during the pandemic. Staff with comorbidities did not take care of patients other than through telemedicine. Over 55% of our staff was transferred to COVID-19 areas. Professionals working in COVID-19 areas did not visit hematologic patients and clinical meetings continued, but with the fewest number of staff members possible. Everyone was required to wear fresh hospital clothing, which had to be changed daily. Surgical face masks were mandatory. When carrying out physical examinations, we kept the hygiene measures already in place, such as the use of disposable coat and gloves. Asymptomatic staff were not monitored. Doctors and nurses who experienced mild symptoms were encouraged to isolate at home, returning to work after COVID-19 was excluded with a negative PCR test. Keeping nurses and senior medical staff working in the Transplantation Unit was fundamental for conducting successful HCT procedures.
3. Results and discussion
We performed 16 HCT procedures on patients with hematologic malignancies between February 25 (when the first COVID-19 patient was diagnosed in our hospital) and June 15 (when there were no new cases). Their details are summarized in . Seven transplants were autologous and 9 were allogeneic. Five were donated by a matched sibling donor (MSD), 3 from an haploidentical donor (Haplo) and 1 from a matched unrelated donor (MUD). We continued to perform HCT during the pandemic for several reasons: firstly HCT is the only curative option for many patients with hematologic malignancies; secondly, we could not predict the length of the first wave; and thirdly several expert reports predicted that there would be a new peak in the fall and that the pandemic could last until 2022 [Citation13]. We therefore decided that in addition to high-risk acute leukemia, we would also treat standard-risk AML patients for whom transplantation was indicated. We also performed two haplo-HCTs in refractory Hodgkin lymphoma, an allo-HCT from an MSD in relapsed mantle cell lymphoma, and three autologous HCTs in diffuse large B lymphoma, Hodgkin lymphoma, and follicular lymphoma. This was despite the recommendations made by the Grupo Español de Linfomas y Trasplante Autólogo de Médula Ósea (GELTAMO) to delay transplantation in patients with low-grade lymphomas and Hodgkin lymphoma. Conversely, Australian and Brazilian guidelines do not recommend delaying HCT in these patients [Citation14,Citation15]. The most common indication for auto-HCT was multiple myeloma. Most guidelines recommend delaying transplantation and prolonging the induction regimen by up to 8 cycles [Citation6]. In these patients, our main concern was the cumulative delay of transplants, so we decided to keep treating patients with this indication. Transplantation was delayed for only one frail patient, who was awaiting a second transplant as part of a tandem regimen. This patient received maintenance treatment with lenalidomide.
Table 1. Patients undergoing HCT at La Paz University Hospital during COVID-19 pandemic
Many patients were already scheduled to receive HCT at the start of the pandemic. We decided to continue treating them, while implementing rigorous infection control measures. It is worth noting that even before the COVID-19 outbreak began, patients receiving HCT have generally been more careful than the general population with infection control measures such as hand hygiene, face masks and social distancing. It is important to remember that the prognosis for HCT recipients with COVID-19 may be worse than for the general population due to HCT-associated immunosuppression. This is especially true in the first year after the procedure [Citation16]. However, these patients have lower mortality than those with uncontrolled or progressive disease [Citation2]. It is thus important to decide whether to perform the transplant (at the risk of potentially exposing the patient to a SARS-CoV-2 infection during admission and immediately after the procedure), or to delay it (increasing the risk of relapse), even though the patient may still become infected at that time. Overcrowding in the Intensive Care Unit (ICU) is another factor to keep in mind when scheduling HCT. One of the ICUs in our hospital is reserved for patients who do not have COVID-19, and is therefore available for HCT recipients. In our center, only 1 out of the 16 transplanted patients required admission to ICU.
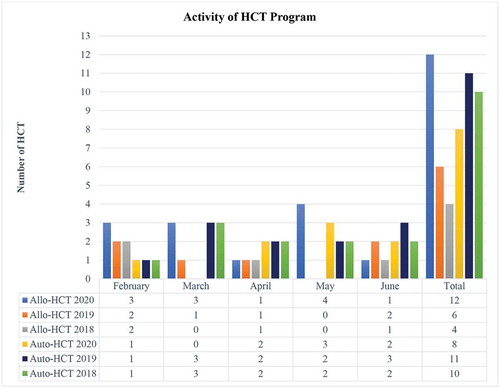
As shown in , the number of HCT procedures carried out in 2020 was slightly higher than during the same period in previous years (2020 n = 20, 2019 n = 17 and 2018 n = 16).
Figure 1. Activity of HCT program at La Paz University Hospital during the COVID-19 outbreak and in the same period the two previous years
None of our patients had COVID-19 either when they were admitted, or in the following 3 months. We performed 22 PCR tests at admission on 9 patients undergoing allo-HCT and 16 PCR tests on 7 patients undergoing auto-HCT. All of the test results were negative. Although some experts recommend carrying out chest X-Rays or CT scans on asymptomatic patients before conditioning, we did not do this as the rate of SARS-CoV-2 infection at our hospital was 80% [Citation10]. We reasoned that the risk of becoming infected during the imaging procedure was higher than the risks incurred by a false-negative test in a population which was already confined. It has been suggested that patients who have undergone allo-HCT have higher rates of false-negative results with RT-PCR based assays [Citation17]. However, the clinical and analytical data were not suggestive of SARS-CoV-2 infection in any patient and the pretest probability of infection in this population was already very low.
Between February and June, only one of our patients, who had previously undergone allo-HCT, was diagnosed as being infected with SARS-CoV-2. The patient was a 42-year-old male with a 6 year history of AML. He presented with typical mild symptoms of SARS-CoV-2 infection, but he had a negative result with a nasopharyngeal PCR test. His diagnosis was based on clinical and analytical data, as well as positive IgM and IgG serology. He was managed on an outpatient basis and was treated with hydroxychloroquine. Oxygen and heparin were not required due to an absence of radiological abnormalities and normal D-dimer levels.
The EBMT recommends excluding donors with COVID-19 for 3 months, while the World Marrow Donor Association (WMDA) and the American Society for Transplantation and Cellular Therapy (ASTCT) suggest excluding them for at least 28 days [Citation3,Citation10,Citation11]. Although appropriate, these recommendations may not be realistic in some clinical situations. In our center, apheresis was performed 14 days after obtaining a negative PCR test result in a completely asymptomatic haploidentical donor who had previously had a positive result for SARS-CoV-2 with a routine PCR test. The decision was taken to carry out the procedure as the recipient was a pediatric patient with ALL with poor cytogenic prognosis and a high risk of relapse. Before these recommendations were published, we performed an HCT from an MSD who was diagnosed with COVID-19 three days after apheresis. The recipient did not get COVID-19. Past cases and current evidence suggest that the SARS-CoV-2 is not transmitted through blood products (including hematopoietic stem cells) [Citation10]. Given the current outlook, it does not seem viable to exclude donors who had had COVID-19. The approach recommended by the WMDA (exclusion for 28 days) seems more reasonable, with a risk assessment being carried out for both the donor and recipient.
After the ONT released its recommendations on March 11, we started cryopreserving the products from related and unrelated donors for a period of 14 days. This allows us to monitor for the onset of symptoms in the donor and to keep the stem cell product in the center’s facilities when initiating conditioning [Citation11]. In our hospital, all of the products were obtained from peripheral blood apheresis, and their quality was tested before the start of conditioning. For hematologic malignancies, there is no proof that cryopreservation negatively affects product survival. However, given the current evidence about safety of blood products and the flexibilization of travel regulations, it is no longer recommended that the product be frozen, as long as the donor maintains a social distance from other people and receives two negative test results (before starting mobilization and prior to apheresis). In some cases, when social distancing cannot be ensured, or when there is a high prevalence of COVID-19 in the donor’s area, planned cryopreservation may still be a possibility. The WDMA does not currently recommend cryo-quarantine, and we no longer cryopreserve the product for 14 days [Citation12].
4. Expert opinion
In conclusion, we have shown that as long as rigorous infection prevention measures are implemented, an adult HCT program can continue without infecting patients with SARS-CoV-2. Our experience in a hospital that was badly affected by the COVID-19 pandemic may be useful to other centers given its concerning evolution.
In our opinion, curative procedures such as HCT, must not be delayed. Recommendations should be dynamic and they should change in accordance with the available evidence.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Centro Nacional de Epidemiología (CNE), Ministerio de Sanidad de España. National center of epidemiology, ministry of health, Spain [Internet] [cited 2020 Oct 5]. Available from: https://cnecovid.isciii.es/covid19/#declaración-agregada
- Piñana JL, Martino R, García-García I, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol. 2020 [cited 2020 Oct 9]: [16p.]. 9:21.
- Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020 [ update 2020 Jun 8; cited 2020 Oct 5]: [6p.]. DOI:10.1038/s41409-020-0919-0.
- Brissot E, Labopin M, Baron F, et al. Management of patients with acute leukemia during the COVID-19 outbreak: practical guidelines from the acute leukemia working party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2020 [cited 2020 Oct 9]: [4p.]. DOI:10.1038/s41409-020-0970-x.
- Grupo Español de Linfomas y Trasplante Autólogo de Médula Ósea (GELTAMO). Recomendaciones del Comité Científico del grupo GELTAMO para el manejo de los pacientes con linfoma durante la crisis del Covid-19. [Recommendations from the scientific committee of the GELTAMO group for managing patients with lymphoma during COVID-19 outbreak] [Internet] Spanish. [cited 2020 Oct 5]. Available from: https://www.geltamo.com/images/PDF/Recomendaciones%20linfomas%20Covid-19%2019%20MARZO%202020.pdf
- Terpos E, Engelhardt M, Cook G, et al. Management of patients with multiple myeloma in the era of COVID-19 pandemic: a consensus paper from the European Myeloma Network (EMN). Leukemia. 2020;34(8):2000–2011. .
- Sahu KK, Cerny J. Managing patients with hematological malignancies during COVID-19 pandemic [published online ahead of print, 2020 Jul 12]. Expert Rev Hematol. 2020;13(8):787–793.
- Xu Z‐L, Huang X‐J. COVID‐19 & allogeneic transplant: activity and preventive measures for best outcomes in China. Adv Cell Gene Ther. 2020; [cited 2020 Oct 9]: [4p.];3. DOI:10.1002/acg2.94.
- Balduzzi A, Brivio E, Rovelli A, et al. Lessons after the early management of the COVID-19 outbreak in a pediatric transplant and hemato-oncology center embedded within a COVID-19 dedicated hospital in Lombardia, Italy. Bone Marrow Transplant. 2020;55:1900–1905.
- Algwaiz G, Aljurf M, Koh M, et al. WBMT and the CIBMTR Health Services and International Studies Committee. Real-world issues and potential solutions in hematopoietic cell transplantation during the COVID-19 pandemic: perspectives from the worldwide network for blood and marrow transplantation and center for international blood and marrow transplant research health services and international studies committee. Biol Blood Marrow Transplant. 2020 [cited 2020 Oct 9]: [9p.]. DOI:10.1016/j.bbmt.2020.07.021.
- Organización Nacional de Trasplantes (ONT), Ministerio de Sanidad. Spanish recommendations to manage organ donation and transplantation regarding the infection associated with the new coronavirus (SARS-CoV-2) producer of COVID-19 (extract from the biovigilance alert reference BV-ES-20200122- last update 13 April 2020) [Internet]. [cited Oct 5]. Available from: http://www.ont.es/infesp/RecomendacionesParaProfesionales/Spanish%20Recommendations%20on%20Organ%20Donation%20and%20Transplantation%20COVID-19%20%20ONT.pdf
- World Marrow Donor Association. COVID-19 impact on registry operations. [Internet]. [cited Oct 9]. Available from: https://share.wmda.info/display/LP/COVID-19+-+Impact+on+Registry+Operations#/
- Kissler SM, Tedijanto C, Goldstein E, et al. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period.Science. 2020;368(6493):860–868.
- Di Ciaccio P, McCaughan G, Trotman J, et al. Australian and New Zealand consensus statement on the management of lymphoma, chronic lymphocytic leukaemia and myeloma during the COVID-19 pandemic. Intern Med J. 2020;50(6):667–679. .
- Perini GF, Fischer T, Gaiolla RD, et al. How to manage lymphoid malignancies during novel 2019 coronavirus (CoVid-19) outbreak: a Brazilian task force recommendation. Hematol Transfus Cell Ther. 2020;42(2):103–110. .
- Varma A, Kosuri S, Ustun C, et al. COVID-19 infection in hematopoietic cell transplantation: age, time from transplant and steroids matter. Leukemia. 2020;34(10):2809–2812.
- Niu A, McDougal A, Ning B, et al. COVID-19 in allogeneic stem cell transplant: high false-negative probability and role of CRISPR and convalescent plasma. Bone Marrow Transplant. 2020 [cited Oct 5]: [3p.];55:2354–2356.
