ABSTRACT
Background
Relapsed/refractory (R/R) classical HL (cHL) and systemic anaplastic large-cell lymphoma (sALCL) treatment options are limited in China. There is a need for new therapies.
Research design and methods
This single-arm, open-label, multicenter, Phase II study assessed efficacy, safety, and pharmacokinetics of single-agent brentuximab vedotin in Chinese patients with R/R cHL or sALCL. Patients received brentuximab vedotin 1.8 mg/kg by intravenous infusion on Day 1 of 3-week cycles (maximum 16 cycles).
Results
Patients (N = 39) received a median of 10 cycles (range: 2–16) of brentuximab vedotin. The objective response rate was 69% (95% CI: 52–83%), with 27 patients achieving objective responses (complete response: n = 11 [28%]; partial response: n = 16 [41%]). Median duration of response, progression-free survival and overall survival were 12.1 months, 13.5 months (95% CI: 6.8 months–not estimable) and not reached after a median follow-up of 16.6 months. Brentuximab vedotin was well tolerated with no on-study deaths. AEs were generally manageable and reversible. No new safety signals were identified. Pharmacokinetics were consistent with those previously described in Western populations.
Conclusion
Brentuximab vedotin had a positive benefit–risk profile for Chinese patients with R/R cHL or sALCL, confirming it as a potential treatment option.
Clinical trial registration
www.clinicaltrials.gov identifier is NCT02939014.
1. Introduction
Approximately 30–40% of patients with advanced-stage classical Hodgkin lymphoma (cHL) and 40–65% of patients with systemic anaplastic large cell lymphoma (sALCL) will be refractory to frontline therapy or will relapse [Citation1,Citation2]. In China, the standard management for relapsed/refractory (R/R) cHL is salvage chemotherapy followed by autologous stem cell transplant (ASCT) in chemosensitive disease [Citation3]. Approximately, 50% of these patients are expected to achieve a long-term cure [Citation4]. A standard therapy has not yet been established for R/R sALCL [Citation3].
R/R cHL and R/R sALCL are associated with high patient morbidity and mortality [Citation2,Citation5,Citation6]. This is, in part, due to poor response rates to second-line chemotherapy with or without stem cell transplant (SCT) [Citation7]. Standard second-line chemotherapy in patients with R/R cHL results in rates of freedom from treatment failure of 55% with SCT and 34% without SCT [Citation8]. For sALCL, the 5-year overall survival (OS) rate for anaplastic lymphoma kinase (ALK)-positive or negative patients is 70% and 49%, respectively [Citation2].
In end-of-study results from the pivotal Phase II trial of brentuximab vedotin in patients with R/R cHL after failed ASCT, the overall response rate (ORR) was 75% and the estimated 5-year OS rate in the overall patient population was 41% [Citation9]. Patients who achieved complete response (CR) with single-agent brentuximab vedotin achieved long-term disease control. A total of 38% of patients who achieved CR with brentuximab vedotin remained in remission for greater than 5 years and may be cured [Citation9]. In a Phase II trial of patients with ALCL, patients who achieved CR with brentuximab vedotin had an estimated OS of 79% at 5 years [Citation10].
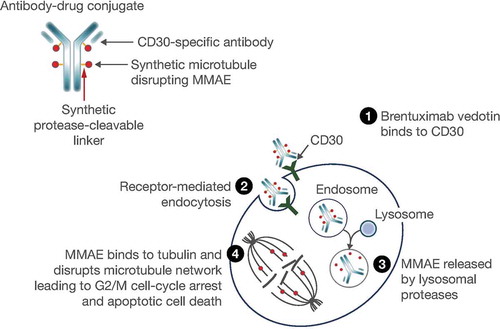
CD30 antigen is expressed universally on the surface of cancer cells in cHL and sALCL, and at a much lower level on normal activated T cells and other hematopoietic cells [Citation11], making it a useful diagnostic marker and therapeutic target for CD30-positive malignancies, such as cHL and sALCL. Brentuximab vedotin is an antibody–drug conjugate (ADC) comprising an anti-CD30 antibody linked to the tubulin-disrupting agent monomethyl auristatin E (MMAE) [Citation12] that is CD30 directed resulting in cell cycle arrest and apoptosis () [Citation13].
Figure 1. Mechanism of action of brentuximab vedotin. Reproduced with permission from Suri Citation12. © 2018 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc., on behalf of American Society for Clinical Pharmacology and Therapeutics.
Abbreviation: MMAE, monomethyl auristatin E
Treatment options for R/R cHL and sALCL are limited in China and there is a need for new therapies in these settings. Brentuximab vedotin has recently been added to the Chinese Society of Clinical Oncology guidelines as the recommended treatment option for patients with R/R cHL failing ASCT or in patients not eligible because of age/comorbidity or insufficient response to conventional salvage treatments [Citation3]. For sALCL, Chinese Guidelines include brentuximab vedotin as an alternative single drug [Citation3]. Inclusion in these guidelines is based on trials conducted in largely Western patients. Here, we have evaluated the efficacy and safety of brentuximab vedotin in Chinese patients with R/R cHL and sALCL.
2. Patients and methods
This single-arm, open-label, multicenter, Phase II study (NCT02939014) was designed to assess the efficacy, safety, and pharmacokinetics (PK) of single-agent brentuximab vedotin in Chinese patients with R/R cHL or sALCL.
2.1. Patients
Eligible patients aged ≥18 years with measurable, histologically confirmed R/R cHL or sALCL (after ≥1 prior therapy) were enrolled from seven study sites in China. Full inclusion and exclusion criteria are provided in the supplementary material.
2.2. Study design
Brentuximab vedotin was administered as a single 1.8 mg/kg intravenous (IV) infusion, over 30 minutes, on Day 1 of a 3-week cycle up to a maximum of 16 cycles. Sixteen cycles were chosen as the maximum exposure based on the approved posology of brentuximab vedotin in the relapsed cHL and sALCL settings at the time of study design. Criteria for discontinuation from study drug included disease progression, unacceptable toxicity, initiation of subsequent SCT, or withdrawal of consent.
Dedicated computed tomography (CT) scans (neck, chest, abdomen, and pelvis) were performed at baseline and at Cycles 2, 4, 7, 10, 13, and 16. Positron emission tomography (PET) scans were performed at baseline and at Cycles 4 and 7. No additional PET scanning was required beyond Cycle 7 unless clinically indicated. B symptoms were assessed at baseline and on Day 1 of each cycle. Serial blood samples for determination of the serum/plasma concentration and immunogenicity of brentuximab vedotin were obtained at prespecified time points (Supplementary Table 1).
Except for those who withdrew consent, patients had safety follow-up assessments 30 days after the last dose of brentuximab vedotin. Patients who discontinued study treatment with stable disease or better were followed for progression-free survival (PFS) until disease progression, death, withdrawal of consent, or initiation of a new treatment, whichever occurred first. During follow-up, a CT scan was performed every 12 weeks for the first 12 months and at 18 months after end of treatment (EOT). Patients were followed for OS every 12 weeks until death, withdrawal of consent, or study closure, whichever occurred first.
2.3. Endpoints
The primary efficacy endpoint was ORR (defined as complete response [CR] or partial response [PR] by EOT) per investigator (Revised Response Criteria for Malignant Lymphoma 2007) [Citation14]. Safety was also considered a primary endpoint and included monitoring of adverse events (AEs) per National Cancer Institute Common Terminology Criteria for Adverse Events v4.03 [Citation15], assessments of clinical laboratory values, and vital sign measurements by the investigators.
Secondary endpoints included additional efficacy measures (CR rate, duration of response [DOR], PFS, OS), B symptom resolution rate, PK (maximum concentration observed [Cmax; measured value], time of first occurrence of Cmax [observed, tmax], area under the concentration–time curve from time 0 to infinity [AUC0-inf]), and the presence of unwanted immunogenicity against brentuximab vedotin, i.e. anti-drug antibodies (ADA) and neutralizing anti-drug antibodies (NAb) which inhibit brentuximab vedotin from having an effect on its target.
2.4. Statistical analyses
Efficacy was analyzed using the safety/modified intent to treat (mITT) population for patients with R/R cHL and sALCL separately. The safety/mITT population differs from conventional ITT analyses in that ITT populations include all randomized patients regardless of the actual treatment received, whereas the safety/mITT population consisted of all patients who had measurable lesions at baseline and who received ≥1 dose of brentuximab vedotin. The planned sample size was approximately 30 evaluable patients, including 22 patients with HL and 8 patients with sALCL. Based on the exact binomial CI calculations, a minimum of nine responses observed from 22 evaluable patients with HL (ORR of 40.9%) would provide a 95% CI of 20.7–63.6%; five responses observed from 8 evaluable patients with sALCL (ORR of 62.5%) would yield a 95% CI of 24.5–91.5%. In both cases, the lower limits of the 95% CIs are greater than the threshold response rate of 20% obtained from the results of alternative therapies for the same indications [Citation9,Citation16–20]. Assuming similar ORR results to those in the Japan Study (65% for HL and 80% for sALCL) [Citation16], with 22 patients with HL and 8 patients with sALCL, the study would have >99% and >94% probability, respectively, to observe an ORR such that the lower limit of its 95% exact CI is still greater than the 20% threshold [Citation16]. Two-sided exact 95% confidence intervals (CIs) were computed for ORR and CR. When estimable, time-to-event variables were summarized by the Kaplan–Meier (K–M) method alongside 95% CIs. Safety was summarized descriptively.
PK parameters for ADC, MMAE, and total antibody (Tab, the sum of ADC and CD30-directed antibody) were estimated where applicable. The following parameters were included: Cmax (measured value), tmax, AUC from time 0 to 21 days (AUC0-21), the terminal elimination half-life (t1/2z), AUC0-inf, total clearance after IV administration calculated using the observed value of the last quantifiable concentration, and volume of distribution at steady state after IV administration calculated using the observed value of the last quantifiable concentration. In addition, the arithmetic means of the plasma or serum concentration of MMAE, ADC, and TAb were plotted over time.
2.5. Ethics approval and consent to participate
This study was conducted in compliance with the institutional review board/independent ethics committee regulations stated in the Good Clinical Practice regulations and guidelines, and all applicable local regulations. The study protocol, investigator’s brochure, sample informed consent form, and other study-related documents were reviewed and approved by an institutional review board at each study site. There were no protocol amendments for this study. Patients provided written informed consent prior to participation.
Trial registration: ClinicalTrials.gov, NCT02939014.
3. Results
3.1. Patients
A total of 39 Chinese patients, 30 with R/R cHL, and 9 with sALCL, 4 of whom were ALK-positive and 5 who were ALK-negative), were enrolled, all of whom had measurable disease at baseline and received ≥1 dose of study drug; therefore, the mITT population was the same as the safety population (n = 39). Patients had received a median of three (range: 1–7) and one (range: 1–4) prior systemic therapies and 20% and 44% had received a prior SCT or bone marrow transplantation (BMT) in the cHL and sALCL groups, respectively (). Patients with cHL had received first-line adriamycin bleomycin vinblastine and dacarbazine (ABVD) or bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisolone (BEACOPP) and those with sALCL had received frontline CHOP or CHOEP regimen per Chinese treatment guidelines. In both disease subgroups, 67% of patients were refractory to frontline therapy.
Table 1. Demographics and baseline disease characteristics (safety/mITT population)
3.2. Efficacy
The ORR for the mITT population was 69% (95% CI: 52–83%). No patients were excluded from the efficacy analysis (i.e. total N = 39). Overall responses were reported for 27 patients, which included 11 patients (28%) with a CR and 16 patients (41%) with a PR (). For the 30 patients with R/R cHL, the ORR was 70% (95% CI: 51–85%), with a CR reported for 6 patients (20%) and a PR for 15 patients (50%). For the 9 patients with R/R sALCL, the ORR was 67% (95% CI: 30–93%), with a CR reported for 5 patients (56%) and a PR for 1 patient (11%).
Table 2. Summary of best overall response (mITT population)
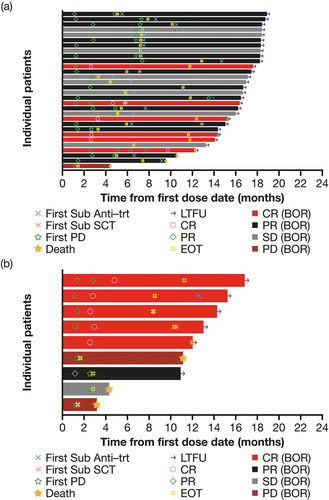
Sixteen patients (41%) attained a response within 2 months after the first dose, 7 patients (18%) had improved responses throughout the treatment and 5 patients (13%; 3 with cHL and 2 with sALCL) received subsequent ASCT (). Six patients with cHL achieved a CR, one of whom went on to receive an ASCT (). Five patients with sALCL achieved a CR, two of whom received a subsequent ASCT ().
Figure 2. Swimmer plot of response to treatment from date of first dose by disease type (mITT population). (a) cHL and (b) sALCL.
Abbreviations: BOR, best overall response; CR, complete response; EOT, end of treatment; cHL, classical Hodgkin lymphoma; LTFU, long-term follow-up; mITT, modified intent to treat; PD, progressive disease; PR, partial response; sALCL, systemic anaplastic large cell lymphoma; SCT, stem cell transplant; SD, stable disease; sub anti-trt, subsequent antineoplastic therapy
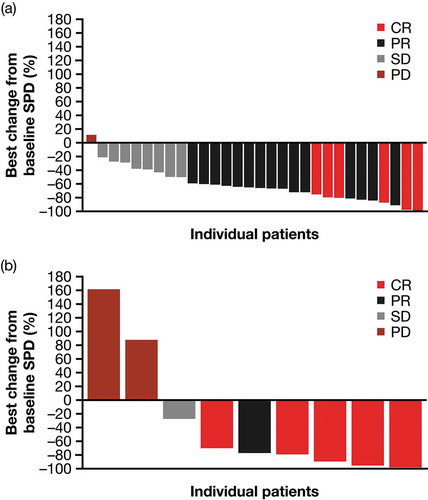
In the mITT population, 36/39 evaluable patients (92%; cHL: 29/30 patients [97%]; sALCL: 7/9 patients [78%]) assessed for disease response experienced reductions in the size of their target lesions (). The median maximum tumor reduction from baseline was 66% (cHL: 65%; sALCL: 77%).
Figure 3. Best percentage change in the sum of product diameters by disease type (mITT population; all patients). (a) cHL and (b) sALCL.
Abbreviations: CR, complete response; cHL, classical Hodgkin lymphoma; mITT, modified intent to treat; PD, progressive disease; PR, partial response; sALCL, systemic anaplastic large cell lymphoma; SD, stable disease; SPD, sum of product diameters
At a median follow-up of 12.9 months, median DOR for the patients with an overall response (CR or PR) was 12.1 months. At 12 months, the K–M estimated DOR was 50% (95% CI: 26–70%) for the responders in the safety/mITT population, 42% (95% CI: 17–65%) for patients with R/R cHL (), and 83% (95% CI: 27–98%) for patients with R/R sALCL ().
Figure 4. Kaplan–Meier curves for patients with cHL (left panels) and sALCL (right panels). Illustrating (a) and (b) duration of response (mITT population with CR or PR). (c) and (d) progression-free survival (mITT population). (e) and (f) overall survival (mITT population).
Abbreviations: CI, confidence interval; cHL, classical Hodgkin lymphoma; CR, complete response; mITT, modified intent to treat; NE, not estimable; ORR, overall response rate; sALCL, systemic anaplastic large cell lymphoma
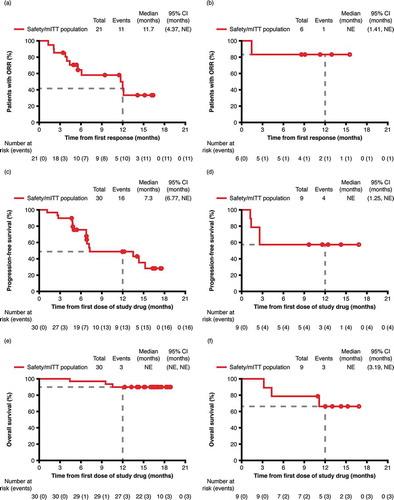
At a median PFS follow-up of 12.4 months, the median PFS for the safety/mITT population was 13.5 months (95% CI: 6.8–not estimable), with observed PFS durations ranging from 1.1 months to ≥17.6 months (censored). At 12 months, the K–M estimated PFS rate was 52% (95% CI: 34–67%) for the safety/mITT population, 49% (95% CI: 28–67%) for patients with R/R cHL () and 56% (95% CI: 20–81%) for patients with R/R sALCL ().
At a median OS follow-up of 16.6 months, median OS was not reached among the safety/mITT population and the individual lymphoma subtypes. At 12 months, the K–M estimate of OS rate was 85% (95% CI: 69–93%) for the safety/mITT population, 90% (95% CI: 72–97%) for patients with R/R cHL (), 65% (95% CI: 25–87%) for patients with R/R sALCL ().
Four patients (10%) in the safety/mITT population reported ≥1 B symptom at baseline. Of these, 3 patients experienced B symptom resolution at a median of 3.13 weeks (range: 3.1–6.3 weeks). Two patients were reported to have ≥1 B symptom in each lymphoma subtype at baseline. One patient with R/R cHL and 2 patients with R/R sALCL experienced B symptom resolution at 6.26 weeks and 3.13 weeks, respectively.
3.3. Safety
The safety/mITT population received a median of 10 cycles (range: 2–16 cycles) of brentuximab vedotin over a median of 30.3 weeks. Patients with R/R cHL received a median of 10 cycles (range: 2–16 cycles) and patients with R/R sALCL received a median of 12 cycles (range: 2–16 cycles). The median relative dose intensity (RDI) of brentuximab vedotin was 98% (range: 89–101%). Median RDIs for patients with R/R cHL and sALCL were comparable (98% [range: 89–101%] and 98%, [range: 91–100%], respectively). Ten patients (26%) completed the maximum 16 cycles of therapy (R/R cHL: n = 8; R/R sALCL: n = 2). The primary reasons for study drug discontinuation were as follows: progressive disease (R/R cHL: n = 13; R/R sALCL: n = 4), patient withdrawal (R/R cHL: n = 2), AEs (R/R cHL: n = 3; R/R sALCL: n = 1), subsequent stem cell transplantation (R/R cHL: n = 2; R/R sALCL: n = 2), and other (R/R cHL: n = 2).
Thirty-nine patients (100%) reported ≥1 treatment-emergent AE (TEAE) of any grade and 38 patients (97%) reported ≥1 drug-related TEAE of any grade (). The most commonly reported TEAEs were hematologic toxicity, serum chemistry abnormalities, and neuropathy. These were generally manageable and reversible. The rate of drug-related any grade (Grade ≥3) neutropenia was 62% (18%), leukopenia was 44% (5%), and anemia was 36% (0%). All cases of neutropenia, and most cases of leukopenia and anemia, resolved. Serum chemistry abnormalities were mild or moderate and consisted of Grade ≤2 increased alanine aminotransferase (ALT; 62%) and aspartate aminotransferase (AST; 59%); no clinical interventions were reported, and most cases of increased ALT and AST resolved.
Table 3. Summary of AEs (safety population)
Twelve patients (31%) reported at least one Grade ≥3 TEAE and 10 patients (26%) reported at least one Grade ≥3 drug-related TEAE (). The most commonly reported Grade 3 or higher TEAEs were hematologic abnormalities (neutropenia, lymphocyte count decreased, and leukopenia). There were two patients (5%) who reported at least one serious AE (SAE), each patient had one SAE: Grade 3 lung infection (considered drug-related) and Grade 2 large intestine polyp (not drug-related; removed by concomitant procedure). Both SAEs resolved. Three patients (8%) reported AE-related drug discontinuation; due to Grade 2 peripheral sensory neuropathy, Grade 2 neuropathy peripheral and Grade 2 hypoesthesia. No on-study deaths occurred.
Peripheral neuropathy (PN) events were mild or moderate in severity, with the highest Grade of 2 occurring in 3 patients (8%); Grade 1 PN was reported for 17 patients (44%). Onset of any grade PN was reported at a median of 10.6 weeks (range: 0.3–45.4); onset of Grade 2 PN events was 11.9 weeks (range: 0.3–45.4). At EOT, PN had resolved or resolved with sequelae for 11 patients (55%). Among the 9 patients (45%) with ongoing PN, Grade 1 PN was reported for 7 patients (35%) and Grade 2 PN for 2 patients (10%; ).
Table 4. Summary of resolution and improvement of PN at EOT (safety population)
In addition to the laboratory abnormalities reported as AEs, one patient (3%) experienced Grade 4 phosphate; 2 patients (5%) reported Grade 3 corrected calcium; and Grade 3 glucose, phosphate, and potassium were reported in one patient (3%), respectively. No patients experienced Grade ≥3 ALT or AST. One patient (3%) experienced Grade 4 decreased neutrophil count. Grade 3 decreased lymphocyte, neutrophil, and leukocyte counts were reported in 5 (13%), 6 (15%), and 2 (5%) patients, respectively.
No clinically significant findings were reported in physical examination, trends in vital signs or changes in Eastern Cooperative Oncology Group performance status between baseline and EOT. Clinically significant electrocardiogram findings were reported in three patients in the safety population at one point in time during the treatment; however, at the EOT electrocardiogram findings were clinically significant for 1 patient only.
Following IV infusion of brentuximab vedotin 1.8 mg/kg once every 3 weeks, the geometric mean AUC0-21, Cmax, and t1/2z of ADC on Cycle 1 Day 1 were 72.4 day*μg/mL, 35.6 μg/mL, and 5.3 days, respectively (Supplementary Figure 1 and Supplementary Table 2). There was no accumulation from Cycle 1–2. PK variability in Cmax and AUC0-21 was between 18% and 32%.
The t1/2z of MMAE was approximately 3–4 days. PK variability, as assessed by percentage coefficient of variation, of plasma MMAE exposure in Cmax and AUC0-21 was between 62% and 80%. There was no accumulation in MMAE PK from Cycle 1–2. Consistent with findings from previous studies, the exposure of MMAE appeared to decrease with time (as observed in this study between Cycle 1 and Cycle 2). The PK of ADC, TAb, and MMAE observed in this study are consistent with those previously described in Western patient populations.
Twenty-nine patients (74%) remained ADA-negative after receiving brentuximab vedotin, while 10 patients (26%) (transiently ADA-positive; 8 patients [21%], persistently ADA-positive; 2 patients [5%]) were confirmed ADA-positive after receiving brentuximab vedotin. There were no associations between patient’s ADA, NAb status, and antitumor response. ORR in the NAb-positive group was 70% and was consistent with the observed ORR of 69% in the safety/mITT population. The safety profile of the ADA-positive group was consistent with the safety/mITT population.
4. Discussion
The patients’ characteristics in this study reflect the clinical features of patients diagnosed with R/R cHL and R/R sALCL in China. At study entry, most of the patients had advanced stage disease and had received up to seven prior systemic therapies, including BMT or SCT, which reflects current clinical practice in China. Patients were treated with the approved posology of brentuximab vedotin for R/R cHL and sALCL in the US and EU [Citation21,Citation22], and received a median of 10 cycles of therapy (median RDI was 98%), indicating that most patients received the desired dose intensity.
Brentuximab vedotin showed antitumor activity in Chinese patients with R/R cHL and R/R sALCL: ORR (CR + PR) was 69%, median DOR was 12.1 months, median PFS was 13.5 months, and median OS was not reached at a median follow-up of 16.6 months. All measures of efficacy were consistent with those seen in the pivotal studies that supported regulatory approval of brentuximab vedotin the R/R cHL and sALCL settings [Citation9,Citation10]. The clinical efficacy of brentuximab vedotin in Chinese patients could potentially permit bridging of patients to subsequent SCT therapy with aim of achieving a cure.
Hematologic TEAEs, serum chemistry abnormalities, and neuropathy were the most commonly reported safety issues; however, these appeared generally manageable and reversible. At EOT, all cases of neutropenia had resolved, PN had resolved or resolved with sequelae for 11 patients (55%) and most hematologic TEAEs and serum chemistry abnormalities had resolved. These results demonstrate a favorable safety profile of brentuximab vedotin in Chinese patients compared with the pivotal monotherapy studies [Citation9,Citation10]. A high proportion of patients (n = 20: 51%) experienced treatment-related PN, mostly of Grade 1; Grade 2 PN, the highest severity reported in the study, was only reported for 3 patients (8%). Peripheral motor neuropathy was not reported for this population. PN occurred a median of 10.6 weeks after initiating brentuximab vedotin, and a later onset was reported for Grade 2 PN events (median 11.9 weeks). At the last EOT, 55% of treatment-emergent PN events had resolved.
Increased ALT of any grade was reported in 24 patients (62%) and increased AST in 23 patients (59%); most ALT/AST events were Grade 1. Grade 2 increased ALT was reported in 6 patients and Grade 2 increased AST in 2 patients. Grade 2 was the highest severity reported for this population. Most events resolved without study drug discontinuations or dose modifications. Results demonstrate an acceptable safety profile and support a positive benefit–risk ratio for brentuximab vedotin in R/R cHL or sALCL. No new safety signals were identified, and the safety profile among Chinese patients in this study was similar to that previously described for other patient populations with R/R cHL or sALCL [Citation9,Citation10,Citation23,Citation24].
The PK of ADC, TAb, and MMAE observed in this study were consistent with those previously described in Western patient populations [Citation9,Citation10,Citation12,Citation25]. The major limitations of our analyses were the small patient population and short median follow-up.
The efficacy of brentuximab vedotin monotherapy in the R/R setting has paved the way for its use in earlier stage of disease and in combination with chemotherapy. For example, a Phase III clinical trial is planned in combination with chemotherapy in patients with newly diagnosed cHL (NCT03907488).
5. Conclusions
Brentuximab vedotin had a positive benefit–risk profile for Chinese patients with R/R cHL and sALCL confirming it as a potential treatment option for this patient group. This conclusion is consistent with the positive benefit–risk profile of brentuximab vedotin in the global R/R cHL and sALCL population.
Previous presentation
Previously presented as an oral presentation at the Chinese Society of Clinical Oncology conference, Xiamen, China, September 18–22, 2019.
Author contributions
Y Guo, H Huang, W Li, X Ke, J Feng, W Xu, and G Song contributed to the conception of the work; design of the work; acquisition and analysis of the data; interpretation of the data; and provided approval of the final submitted version of the manuscript. Y Song, J Kinley, Y Dai, H Wang, and J Zhu contributed to the design of the work; interpretation of the data; and provided approval of the final submitted version of the manuscript. H Miao contributed to the acquisition and analysis of the data; interpretation of the data; and provided approval of the final submitted version of the manuscript. H Miao and Y Dai also drafted the work or substantially revised it. All authors agree to be accountable for all aspects of the work, which includes ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated, resolved, and the resolution documented in the literature.
Declaration of interest
H Miao, J Kinley, G Song, Y Dai and H Wang are employees of Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Supplemental Material
Download MS Word (141.9 KB)Acknowledgments
The authors would like to acknowledge all patients who participated in the trial and their families, as well as all the investigators and site staff who made the study possible. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Rebecca Vickers, BSc, and Laura Webb, PhD, of Ashfield MedComms, an Ashfield Health company, funded by Millennium Pharmaceuticals, Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and complied with the Good Publication Practice-3 (GPP3) guidelines (Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med 2015;163:461–4).
Data availability statement
The datasets, including the redacted study protocol, redacted statistical analysis plan, and individual participants’ data supporting the results reported in this article, will be made available within 3 months from initial request, to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Mina AA, Vakkalagadda C, Pro B. Novel therapies and approaches to relapsed/refractory HL beyond chemotherapy. Cancers (Basel). 2019;11(3):421.
- Savage KJ, Harris NL, Vose JM, et al. ALK- anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International Peripheral T-Cell Lymphoma Project. Blood. 2008;111(12):5496–5504.
- National Health Commission of the People’s Republic of China. Chinese guidelines for diagnosis and treatment of malignant lymphoma. 2018 (English version). Chin J Cancer Res. 2019;31(4):557–577.
- Shanbhag S, Ambinder RF. Hodgkin lymphoma: a review and update on recent progress. CA Cancer J Clin. 2018;68(2):116–132.
- Horning SJ, Chao NJ, Negrin RS, et al. High-dose therapy and autologous hematopoietic progenitor cell transplantation for recurrent or refractory Hodgkin’s disease: analysis of the Stanford University results and prognostic indices. Blood. 1997;89(3):801–813.
- Kleiner S, Kirsch A, Schwaner I, et al. High-dose chemotherapy with carboplatin, etoposide and ifosfamide followed by autologous stem cell rescue in patients with relapsed or refractory malignant lymphomas: a phase I/II study. Bone Marrow Transplant. 1997;20(11):953–959.
- Bennani-Baiti N, Ansell S, Feldman AL. Adult systemic anaplastic large-cell lymphoma: recommendations for diagnosis and management. Expert Rev Hematol. 2016;9(2):137–150.
- Schmitz N, Pfistner B, Sextro M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359(9323):2065–2071.
- Chen R, Gopal AK, Smith SE, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566.
- Pro B, Advani R, Brice P, et al., Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709–2717.
- Van Der Weyden CA, Pileri SA, Feldman AL, et al. Understanding CD30 biology and therapeutic targeting: a historical perspective providing insight into future directions. Blood Cancer J. 2017;7(9):e603.
- Suri A, Mould DR, Liu Y, et al. Population PK and exposure-response relationships for the antibody-drug conjugate brentuximab vedotin in CTCL patients in the Phase III ALCANZA study. Clin Pharmacol Ther. 2018;104(5):989–999.
- Bradley AM, Devine M, DeRemer D. Brentuximab vedotin: an anti-CD30 antibody-drug conjugate. Am J Health Syst Pharm. 2013;70(7):589–597.
- Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586.
- US Department of Health and Human Services, National Institutes of Health, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03 [Internet] 2010 [cited 2020 Oct 14]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- Ogura M, Tobinai K, Hatake K, et al. Phase I/II study of brentuximab vedotin in Japanese patients with relapsed or refractory CD30-positive Hodgkin’s lymphoma or systemic anaplastic large-cell lymphoma. Cancer Sci. 2014;105(7):840–846.
- Santoro A, Bredenfeld H, Devizzi L, et al. Gemcitabine in the treatment of refractory Hodgkin’s disease: results of a multicenter phase II study. J Clin Oncol. 2000;18(13):2615–2619.
- Zinzani PL, Bendandi M, Stefoni V, et al. Value of gemcitabine treatment in heavily pretreated Hodgkin’s disease patients. Haematologica. 2000;85(9):926–929.
- Venkatesh H, Di Bella N, Flynn TP, et al. Results of a phase II multicenter trial of single-agent gemcitabine in patients with relapsed or chemotherapy-refractory Hodgkin’s lymphoma. Clin Lymphoma. 2004;5(2):110–115.
- Oki Y, Pro B, Fayad LE, et al. Phase 2 study of gemcitabine in combination with rituximab in patients with recurrent or refractory Hodgkin lymphoma. Cancer. 2008;112(4):831–836.
- European Medicines Agency. ADCETRIS® (brentuximab vedotin) summary of product characteristics [Internet] 2018 [cited 2020 Oct 14]. Available from: https://www.ema.europa.eu/en/documents/product-information/adcetris-epar-product-information_en.pdf.
- ADCETRIS® (brentuximab vedotin) for injection, for intravenous use [prescribing information]. Bothell, WA: Seattle Genetics Inc. 2019.
- Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–1294.
- Pro B, Advani R, Brice P, et al., Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196.
- Suri A, Mould DR, Song G, et al. Population pharmacokinetic modeling and exposure-response assessment for the antibody-drug conjugate brentuximab vedotin in Hodgkin’s lymphoma in the Phase III ECHELON-1 study. Clin Pharmacol Ther. 2019;106(6):1268–1279.
