1. Introduction
Since the first patients received autologous hematopoietic stem cell transplantation (auto-HSCT) more than 60 years ago, therapeutic developments in the field have been substantial. Mobilized peripheral blood cells has replaced bone marrow as stem cell source, considerable improvements of high dose preparatory regimens have taken place, and the indication for auto-HSCT is extended to include a wide range of malignant and autoimmune conditions. However, the autologous peripheral hematopoietic stem cell (HSC) graft itself remain relatively unchanged from the start and is to a small extent adapted individually dependent on clinical factors.
2. Effects of different mobilization regimens on graft composition and clinical outcome
Published literature is contradictory regarding detailed associations between the dose of CD34+ hematopoietic stem cells in autologous grafts and time to neutrophil and platelet engraftment. In clinical practice, it is common to identify ‘unsafe,’ ‘minimum’ and ‘ideal’ CD34+ cell doses; however, the scientific basis for establishing consensus recommendations regarding CD34+ cell dosage is currently rather weak. Moreover, the higher yield of CD34+ cells in multiple myeloma (MM) patients after chemo-mobilization with cyclophosphamide and granulocyte colony-stimulating factor (G-CSF) compared to G-CSF alone does not lead to significant improvement of engraftment or overall survival [Citation1]. Furthermore, the graft composition with respect to both progenitor and mature immune cells is affected by the mobilization regimen [Citation2]. However, despite more efficient HSC collection and increased mobilization of the most primitive CD34+ CD133+ CD38- HSC subset as well as T-, NK-, and B-cells, there is no evidence for enhanced engraftment or overall survival benefit after mobilization with G-CSF and plerixafor compared to G-CSF alone [Citation3,Citation4].
Even though meta-analyses of the effect on hard endpoints do not justify universal use of CXCR4 inhibitors in auto-HSCT mobilization, their advantageousness used on demand in poor mobilizers after failure with standard mobilization, usually with G-CSF with or without chemotherapy mobilization regimen (), is beyond doubt. Disease and therapy-related factors leading to poor HSC mobilization will indeed also influence clinical outcome, and it is at present not clarified to which degree enhanced mobilization regimens and changed HSC graft characteristics contributes to different clinical outcome in this subset of patients.
Table 1. Examples of patient and autologous graft manufacturing process heterogeneity. The multi-step variability will potentially be reflected in graft composition and infused immune cell doses
3. Does auto-HSCT graft composition affect clinical outcomes?
In clinical routine auto-HSCT, the only target value is the dose of hematopoietic progenitor cells. However, differentiated immune cells make up more than 95% of the HSC leukapheresis product, and their presence in the graft and their potential effects are virtually neglected. The graft doses of various immune cell subsets will be influenced by numerous factors including disease and stage of disease, previous treatment, age, comorbidity, mobilization regimen, and technical aspects of leukapheresis and graft processing () (). A huge degree of heterogeneity in all these conditions is reflected in the graft, with extensive variability in infused doses of non-progenitor subsets [Citation4]. This lack of standardization complicates the studies of the clinical impact of graft composition. As the graft should be considered an interactive immune-regulatory network of cellular and soluble immune components, the balance between different factors may be more important than absolute concentrations and doses. Consequently, depiction of the clinical impact of a single immune cell subset is usually of little value without rooting it in a broader context.
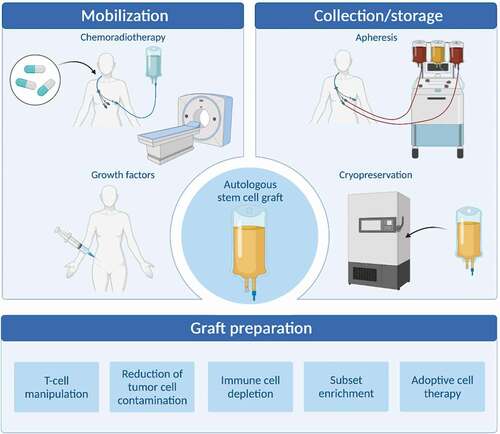
Figure 1. Methods for optimizing autologous hematopoietic stem cell grafts. Autologous hematopoietic stem cell grafts are influenced by several factors and the graft may be affected qualitatively and quantitatively by several factors including stem cell mobilization approaches, collection and storages techniques, and direct methods for preparation and improvement of the graft (Figure made by BioRender).
Despite these complex and relatively unexplored conditions, a few promising studies of graft composition clinical effects have been published. Russell and coworkers recently reported an inverse association between HSC yield and CD8+ cytotoxic T-cells in poor mobilizers, suggesting a mobilization inhibition and engraftment facilitating effect of this subset [Citation5]. Several studies have also explored the association between infused doses and ratios of various lymphoid and monocytoid subsets with engraftment, relapse, and survival. For example, the graft content of various lymphoid and monocytoid subsets does not only predict the post-transplant recovery of the corresponding populations, which further is a predictor of progression-free survival, but the graft ratio between lymphoid and monocytoid cells seems to be a determinant for engraftment and post-transplant immune function in terms of transplant-related infection, disease progression, and survival [Citation6,Citation7]. A high lymphocyte to monocyte ratio seems beneficial and may lead to changed graft specifications, although to date this knowledge has to a small degree been confirmed by other groups or translated into graft engineering. In our opinion, a scientific basis exists for introduction of target doses for lymphocyte collection as well as HSC collection in the setting of auto-HSCT.
4. Manipulation of autologous hematopoietic stem cell grafts
Compared to allogeneic hematopoietic stem cell transplantation (allo-HSCT), the experience with auto-HSCT graft manipulation is scarce and mainly limited to reduction of contaminating tumor cells in the graft, which can potentially lead to relapse. Both negative selection, so-called tumor cell purging and transplants with positively selected CD34+ cells have been tried in multiple studies. However, reduction of the infused tumor cell load is not documented to translate into improvement of progression-free or overall survival, and infusion of CD34+ selected grafts is associated with significantly increased rate of serious post-transplant infections [Citation6,Citation8,Citation9]. Apparently, the benefits of tumor reduction are outweighed by the reduced post-transplant immunity resulting from removal of important constituents of the graft. This decreased resistance to infection is probably accompanied by reduced post-transplant tumor surveillance and may contribute to the failure of relapse prevention.
5. Combining autologous HSCT with adoptive cell therapy
High-dose chemotherapy leads to severe immunosuppression, and the reconstitution of adaptive immune cell subsets is slow and incomplete after auto-HSCT. Regeneration of full-scale T-cell functionality may take a year or more, and the absolute lymphocyte count early after auto-HSCT is an independent prognostic indicator for relapse rate and overall survival in MM and non-Hodgkin lymphoma [Citation10,Citation11]. Enhancement of auto-HSCT with infusion of autologous activated T cells accelerates reconstitution and shortening of the early post-transplant phase where immunosuppression seems to be a critical factor [Citation12]. Combination of adoptive T-cell transfer with pre-transplant vaccination further improves host infection resistance, and genetic engineering of adoptively transferred T cells will additionally amplify the anti-tumor armamentarium [Citation12]. In two small pilot studies, auto-HSCT has been combined safely with infusion of autologous T cells transduced with a chimere antigen receptor (CAR) against MM-positive CD19 cells and lymphoma-positive CD19/22 cells [Citation13,Citation14]. The preliminary results are promising and indicate prolonged response compared to standard therapy as an effect of the enhanced secondary immune response [Citation13,Citation14].
6. Moving forward – time to personalize auto-HSCT grafts?
In allo-HSCT, the quantitative dominating differentiated immune cells accompanying the scarce HSCs in the graft pay far more recognition for their beneficial and harmful clinical effects. Large amounts of research have explored plentiful graft manipulation and adoptive transfer techniques in order to separate successfully the deeply interwoven graft versus leukemia and graft versus host effects. Nevertheless, allo-HSCT remains a high-risk therapy encumbered with unacceptable rates of serious complications and fatality. We see a hitherto unexploited potential to transfer the knowledge from allogeneic graft manipulation techniques to the autologous setting to improve the immunological potential of the graft at minimal risk.
Improved autografts, leading to a swifter and more sustained post-transplant immune competence, may reduce the incidence of infections and relapse, shorten hospital stay, and prolong survival, thus representing a safer alternative to allo-HSCT. Further, the effects of the various cellular and soluble contents in autologous HSC grafts still represents a substantial knowledge gap. In particular, disease-dependent categorization of autologous graft composition with respect to clinical outcome are blank spots on the map. A composition of the graft that appears to be beneficial for tumor cell eradication in cancer patients may not unlikely demonstrate to increase de novo autoimmunity in patients transplanted to cure disease, originating from an overactive immune system. On the converse, a tolerable graft causing fewer complications in transplant recipients with autoimmune disease may fall short in combating infections and relapse in cancer patients.
The development of a broad range of graft modifying techniques including cellular subset selection and enhancement, depletion and dampening, differentiation, or reversal and genetic modification pave the way for the engineering of autologous HSC grafts to reflect the immunological status and therapeutic need of the recipient. Thus, the immunological potential of the graft can potentially be fine-tuned to cover the full spectrum of immunotherapeutic scenarios from alert cancer surveillance to induction of tolerance in autoimmune disease. Moreover, engineering techniques give possibilities to customize the graft to individual risk factors like disease stage, comorbidity, and age. In interaction with pre- and post-transplant adjustable factors including induction and conditioning chemo- and radiotherapy and medicinal immunosuppression and enhancement, autologous HSC graft engineering and adoptive immune therapy will make auto-HSCT a more complete personalized therapy.
Improvements of auto-HSCT is not without risk. CAR-T therapy has serious complications including cytokine release syndrome (CRS), autologous graft versus host disease (GVHD) has been reported after adoptive T-cell infusion [Citation15], and the economical expenses of customized auto-HSCT and adoptive cell transfer probably represent a limiting factor. However, compared to the extreme human and societal costs associated with allo-HSCT, we believe that development of a safer autologous alternative can turn out feasible in terms of both reduced transplant morbidity and mortality and reduced economical burdens.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Acknowledgments
Research related to HSCT was funded by Helse Vest, The Norwegian Cancer Society, Rakel and Otto Kristian Bruun’s fund, Øyvinn Mølbach-Pedersens fund, and Norwegian Society of Internal Medicine.
Additional information
Funding
References
- Wang L, Xiang H, Yan Y, et al. Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G-CSF or G-CSF alone in multiple myeloma: a meta-analysis. Ann Hematol. 2021 Feb;100(2):563–573.
- Fruehauf S, Veldwijk MR, Seeger T, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11(8):992–1001.
- Hartmann T, Hubel K, Monsef I, et al. Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst Rev. 2015 Oct 20;(10):CD010615. DOI:https://doi.org/10.1002/14651858.CD010615.pub2.
- Gaugler B, Arbez J, Legouill S, et al. Characterization of peripheral blood stem cell grafts mobilized by granulocyte colony-stimulating factor and plerixafor compared with granulocyte colony-stimulating factor alone [Research Support, Non-U.S. Gov’t]. Cytotherapy. 2013 Jul;15(7):861–868.
- Russell A, Malik S, Litzow M, et al. Dual roles of autologous CD8+ T cells in hematopoietic progenitor cell mobilization and engraftment. Transfusion. 2015 Jul;557:1758–1765; quiz 1757.
- Rutella S, Rumi C, Laurenti L, et al. Immune reconstitution after transplantation of autologous peripheral CD34+ cells: analysis of predictive factors and comparison with unselected progenitor transplants. Br J Haematol. 2000 Jan;108(1):105–115.
- Porrata LF, Inwards DJ, Ansell SM, et al. Autograft immune content and survival in non-Hodgkin’s lymphoma: a post hoc analysis. Leuk Res. 2019 Jun;81:1–9.
- Alvarnas JC, Forman SJ. Graft purging in autologous bone marrow transplantation: a promise not quite fulfilled. Oncology. 2004 Jun;18(7):867–876; discussion 876-8, 881, 884.
- Bourhis JH, Bouko Y, Koscielny S, et al. Relapse risk after autologous transplantation in patients with newly diagnosed myeloma is not related with infused tumor cell load and the outcome is not improved by CD34+ cell selection: long term follow-up of an EBMT phase III randomized study. Haematologica. 2007 Aug;92(8):1083–1090.
- Hiwase DK, Hiwase S, Bailey M, et al. Higher infused lymphocyte dose predicts higher lymphocyte recovery, which in turn, predicts superior overall survival following autologous hematopoietic stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2008 Jan;14(1):116–124.
- Porrata LF, Gertz MA, Inwards DJ, et al. Early lymphocyte recovery predicts superior survival after autologous hematopoietic stem cell transplantation in multiple myeloma or non-Hodgkin lymphoma. Blood. 2001 Aug 1;98(3):579–585.
- Rapoport AP, Stadtmauer EA, Aqui N, et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nat Med. 2005 Nov;11(11):1230–1237.
- Garfall AL, Stadtmauer EA, Hwang WT, et al. Anti-CD19 CAR T cells with high-dose melphalan and autologous stem cell transplantation for refractory multiple myeloma. JCI Insight. 2018 Apr 19;3(8). DOI:https://doi.org/10.1172/jci.insight.120505.
- Wu J, Meng F, Cao Y, et al. Sequential CD19/22 CAR T-cell immunotherapy following autologous stem cell transplantation for central nervous system lymphoma. Blood Cancer J. 2021 Jul 15;11(7):131.
- Rapoport AP, Stadtmauer EA, Aqui N, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of costimulated autologous T cells. Clin Cancer Res. 2009 Jul 1;15(13):4499–4507.
