Positron emission tomography/computed tomography with [18F]fluorodeoxyglucose, or [18F]FDG PET/CT, represents one of the key modalities for imaging Hodgkin lymphoma (HL). In the last decade, the implementation of volumetric analysis on [18F]FDG PET/CT has provided additional tools for disease prognostication [Citation1–3], although not yet fully established in the management of pediatric HL patients.
Recently, Milgrom et al. have published their research on the British Journal of Hematology and entitled ‘Baseline metabolic tumor burden improves risk stratification in Hodgkin lymphoma: A Children’s Oncology Group study’ [Citation4]. In particular, the authors have conducted a retrospective evaluation of the patients enrolled from December 2009 to January 2012 in the COG AHOD0831 trial [Citation5], by selecting those cases with available high-quality, baseline [18F]FDG PET/CT scan images (N = 94) in the study repository at the Imaging and Radiation Oncology Core-Rhode Island (IROC-RI). Previously, the same group had published some subgroup analyses from COG AHOD0031 in children and adolescents with intermediate-risk HL [Citation6]
As clearly stated from the title of the present article [Citation4], the authors aimed to define the role of baseline metabolic tumor burden, i.e. MTV (metabolic tumor volume) and TLG (total lesion glycolysis), in the outcome prediction of patients under 21 years of age with high-risk Hodgkin lymphoma. For this purpose, two threshold methods were applied for the computation of the tumor burden: I) an absolute SUV of 2.5 (MTV2.5 and TLG2.5); II) a relative threshold of 40% of the tumor SUVmax (MTV40% and TLG40%) within the VOI (volume of interest).
The results of the study reveal that in stage III-IV HL, a high upfront tumor burden (TLG cutoff >1841) was associated with event-free survival (EFS) in the entire cohort and in the rapid early responders (RERs), but not in slow early responders (SERs). To furthermore investigate the prognostic role, the authors tried to stratify patients according to TLG cutoff and response-adapted approach (RER/SER), concluding that a high upfront tumor burden may benefit from intensified therapy, even if they achieve a RER.
Despite the implicit promises derived from the results of this study, we should not forget that the original trial was not designed to respond to the specific aim addressed in the present retrospective/exploratory analyses. Firstly, the sample size is not computed based on the statistical demand, as only part of the cohort could be evaluated for the volumetric analyses. Also, the timeline of the study, starting from 2009 to 2012 is not much in favor of the quantitative measurements on PET, although the authors stated that the high-quality of the baseline [18F]FDG PET/CT scan was a criterion for inclusion. Nevertheless, the variability among systems/tomographs in a multi-institutional study cannot be removed retrospectively, unless a dedicated trial design focused on quantitative measurements is outlined.
Secondarily, it is difficult to state how reliable the conclusions of the study can be. RER and SER have different associations with tumor burden (one correlates, the other not), and yet, the authors proceed in stratifying patients according to the early response and TLG. Notwithstanding, the original trial design was based on response-adapted treatment, which includes an additional bias in the analyses. That is why, in our opinion, to set the line on the real role of metabolic tumor burden in pediatric HL, large prospective trials properly developed to respond to this specific question must be designed.
Currently, the AIEOP (Associazione Italiana di Ematologia e Oncologia Pediatrica) Hodgkin Lymphoma Study Group has designed a prospective study of the Italian cohort enrolled in the EuroNet-PHL-C2 trial [Citation7–9]. The primary objective of this study is to compare volumetric assessment with standard visual and semiquantitative analyses in patients with pediatric HL at baseline and during the course of therapy. The estimated cohort is round 500 patients, with approximately half presenting with bulky masses.
To guarantee the reliability of the quantitative computations on [18F]FDG PET/CT, a preliminary validation study has been conducted to select the optimal threshold method for the volumetric assessment [Citation10], by comparing the four most widely used ones: 1) fixed 41% threshold of the maximum standardized uptake value (SUVmax) within the respective lymphoma site (V41%), (2) fixed absolute SUV threshold of 2.5 (V2.5); (3) SUVmax(lesion)/SUVmean liver >1.5 (Vliver); (4) adaptive method (AM) (). The major differences in MTV and TLG among different methods were validated by logistic regression with respect to early (ERA) and later response assessment (LRA), proving that the volumetric analyses obtained from V2.5 and Vliver significantly correlated in response to therapy and should be preferred in pediatric HL.
Figure 1. MIP (maximal intensity projection) image of the four segmentation techniques, from left to right: fixed 41% threshold (V41%), fixed absolute SUV threshold of 2.5 (V2.5), SUVmax(lesion)/SUVmean liver > 1.5 (Vliver) and adaptive method (AM). Corresponding MTV (metabolic tumor volumes) at baseline are shown beneath each MIP image. The areas highlighted in red in the figures indicate the contours of the malignant lesions delineated by each segmentation technique. Reproduced from Lopci et al. [Citation10] under the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
![Figure 1. MIP (maximal intensity projection) image of the four segmentation techniques, from left to right: fixed 41% threshold (V41%), fixed absolute SUV threshold of 2.5 (V2.5), SUVmax(lesion)/SUVmean liver > 1.5 (Vliver) and adaptive method (AM). Corresponding MTV (metabolic tumor volumes) at baseline are shown beneath each MIP image. The areas highlighted in red in the figures indicate the contours of the malignant lesions delineated by each segmentation technique. Reproduced from Lopci et al. [Citation10] under the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).](/cms/asset/88a4895d-c003-48fa-b72b-f1ee896307a9/ierr_a_2238125_f0001_oc.jpg)
At present, we are able to report the preliminary analyses on the first 150 patients of the prospective Italian cohort enrolled in the EuroNet-PHL-C2 trial (). Herein, semi-automatically delineated contours of the lesions were performed by using as reference a fixed absolute SUV threshold of 2.5, previously validated [Citation10]. A statistically significant difference of median values was depicted for all baseline parameters and treatment evaluation at ERA PET (p < 0.05). The significant correlation of volumetric parameters was also confirmed at logistic regression for MTV (p = 0.008) and TLG (p = 0.009).
Figure 2. Box-plots of baseline MTV and TLG of the first 150 patients of the Italian cohort classified according to ERA PET: IR (inadequate response), AR (adequate response).
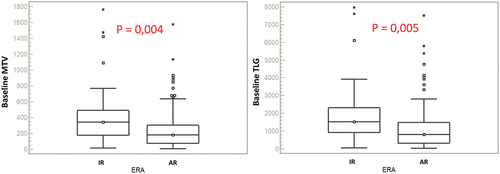
These preliminary reports comply with the expectations on the predictive role of MTV and TLG, as parameters of the metabolic tumor burden, on early response in pediatric HL. As previously clarified, no definite conclusions can be drawn yet, until the complete cohort of patients (currently n = 564) already enrolled in this multicentric prospective trial has been analyzed. This is particularly true considering the implicit limitations to the analysis of MTV and TLG in Hodgkin lymphoma. These include:
-Technical limitations: The accuracy and reproducibility of MTV and TLG measurements can be affected by various technical factors, such as image resolution, reconstruction algorithms, and image noise. As such, careful attention must be paid to the technical parameters of the imaging studies to ensure accurate and reliable measurements. This is particularly evident in case of multicentric trials, where specific imaging recommendations and accreditation certificates can help solve the inter- and intracenter variability [Citation11].
-Biological heterogeneity: Tumors are often characterized by biological heterogeneity, which can lead to variations in metabolic activity within different regions of the tumor. As a result, MTV and TLG measurements may not fully capture the complexity of the tumor biology and may not accurately reflect the true extent of the disease. In this context, a significant role can be played by radiomics and texture analyses in HL [Citation12], designed to capture those imaging features hidden to the naked eye and yet relevant for disease characterization and prognostication [Citation13].
-Interpretation: The interpretation of MTV and TLG values must be done in the context of other clinical and imaging findings, as well as the individual patient’s clinical history and risk factors. In some cases, high values of MTV and TLG may not necessarily indicate a poor prognosis, and other factors must be considered in the overall management of the disease. Not forgetting, that for an adequate implementation in clinical routine, the computation of volumetric analyses has to take into account time consumption, and the implicit dependence on the availability and characteristics of the software used for these measurements.
In summary, volumetric analysis on [18F]FDG PET/CT is a valuable tool, although not yet fully assessed in the management of pediatric HL patients. Once validated, it might allow for a more accurate measurement of tumor burden and response to therapy, which can help guide treatment decisions and improve outcomes.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgments
The authors would like to thank A.G.M.E.N. FVG ONLUS, Fondazione AIRC and Fondazione Umberto Veronesi for the support in the research. The AIEOP (Associazione Italiana Ematologia Oncologia Pediatrica) Centers involved in the Hodgkin Lymphoma Study and the referring local investigators are acknowledged for their support in the study. The authors would like to thank the entire EuroNet-PHL-C2 consortium for support in the trial conduction.
Additional information
Funding
References
- Akhtari M, Milgrom SA, Pinnix CC, et al. Reclassifying patients with early-stage Hodgkin lymphoma based on functional radiographic markers at presentation. Blood. 2018;131(1):84–94. doi: 10.1182/blood-2017-04-773838
- Cottereau AS, Versari A, Loft A, et al. Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood. 2018;131(13):1456–1463. doi: 10.1182/blood-2017-07-795476
- van Heek L, Stuka C, Kaul H, et al. Predictive value of baseline metabolic tumor volume in early-stage favorable Hodgkin Lymphoma - Data from the prospective, multicenter phase III HD16 trial. BMC Cancer. 2022;22(1):672. doi: 10.1186/s12885-022-09758-z
- Milgrom SA, Kim J, Pei Q, et al. Baseline metabolic tumour burden improves risk stratification in Hodgkin lymphoma: A children’s oncology group study. Br J Haematol. 2023;201(6):1192–1199. doi: 10.1111/bjh.18734
- Kelly KM, Cole PD, Pei Q, et al. Response-adapted therapy for the treatment of children with newly diagnosed high risk Hodgkin lymphoma (AHOD0831): a report from the children’s oncology group. Br J Haematol. 2019;187(1):39–48. doi: 10.1111/bjh.16014
- Milgrom SA, Kim J, Chirindel A, et al. Prognostic value of baseline metabolic tumor volume in children and adolescents with intermediate-risk Hodgkin lymphoma treated with chemo-radiation therapy: FDG-PET parameter analysis in a subgroup from COG AHOD0031. Pediatr Blood Cancer. 2021;68(9):e29212. doi: 10.1002/pbc.29212
- Lopci E, Burnelli R, Elia C, AIEOP Hodgkin Lymphoma Study Group. et al. Additional value of volumetric and texture analysis on FDG PET assessment in paediatric Hodgkin lymphoma: an Italian multicentric study protocol. BMJ Open. 2021;11(3):e041252. doi: 10.1136/bmjopen-2020-041252
- Mauz-Körholz C, Landman-Parker J, Balwierz W, et al. Response-adapted omission of radiotherapy and comparison of consolidation chemotherapy in children and adolescents with intermediate-stage and advanced-stage classical Hodgkin lymphoma (EuroNet-PHL-C1): A titration study with an open-label, embedded, multinational, non-inferiority, randomised controlled trial. Lancet Oncol. 2022;23(1):125–137. doi: 10.1016/S1470-2045(21)00470-8
- Second International Inter-Group Study for Classical Hodgkin Lymphoma in Children and Adolescents. [cited 2022 29 Aug]. Available from: https://clinicaltrials.gov/ct2/show/NCT02684708
- Lopci E, Elia C, Catalfamo B, et al. Prospective evaluation of different methods for volumetric analysis on [18F]FDG PET/CT in pediatric Hodgkin lymphoma. J Clin Med. 2022;11(20):6223. doi: 10.3390/jcm11206223
- Aide N, Lasnon C, Veit-Haibach P, et al. EANM/EARL harmonization strategies in PET quantification: from daily practice to multicentre oncological studies. Eur J Nucl Med Mol Imaging. 2017;44(Suppl 1):17–31. doi: 10.1007/s00259-017-3740-2
- Milgrom SA, Elhalawani H, Lee J, et al. A PET radiomics model to predict refractory mediastinal Hodgkin lymphoma. Sci Rep. 2019;9(1):1322. doi: 10.1038/s41598-018-37197-z
- Ortega C, Eshet Y, Prica A, et al. Combination of FDG PET/CT radiomics and clinical parameters for outcome prediction in patients with Hodgkin’s lymphoma. Cancers (Basel). 2023;15(7):2056. doi: 10.3390/cancers15072056
