ABSTRACT
Introduction
The treatment of multiple myeloma (MM) is evolving rapidly. Quadruplet regimens incorporating proteasome inhibitors, immunomodulatory drugs (IMiDs), and CD38 monoclonal antibodies have emerged as standard-of-care options for newly diagnosed MM, and numerous novel therapies have been approved for relapsed/refractory MM. However, there remains a need for novel options in multiple settings, including refractoriness to frontline standards of care.
Areas covered
Targeting degradation of IKZF1 and IKZF3 – Ikaros and Aiolos – through modulation of cereblon, an E3 ligase substrate recruiter/receptor, is a key mechanism of action of the IMiDs and the CELMoD agents. Two CELMoD agents, iberdomide and mezigdomide, have demonstrated substantial preclinical and clinical activity in MM and have entered phase 3 investigation. Using a literature search methodology comprising searches of PubMed (unlimited time-frame) and international hematology/oncology conference abstracts (2019–2023), this paper reviews the importance of Ikaros and Aiolos in MM, the mechanism of action of the IMiDs and CELMoD agents and their relative potency for targeting Ikaros and Aiolos, and preclinical and clinical data on iberdomide and mezigdomide.
Expert opinion
Emerging data suggest that iberdomide and mezigdomide have promising activity, including in IMiD-resistant settings and, pending phase 3 findings, may provide additional treatment options for patients with MM.
1. Introduction
Multiple myeloma (MM) is the third most common hematologic malignancy globally [Citation1], as well as in the United States (US) [Citation2] and Europe [Citation3], after pooled incidences of non-Hodgkin’s lymphomas and leukemias. MM is typically a disease of the elderly, with the median age at diagnosis in the US being 69 years [Citation4]. MM is a plasma cell neoplasm that affects multiple organs/systems [Citation5] and can be considered a disease of the immune system, associated with an increased risk of infection, with MM plasma cells and their interactions with the bone marrow niche resulting in a highly immunosuppressive tumor microenvironment that promotes MM cell proliferation, immune evasion, effector cell dysfunction, and upregulation of immunosuppressive cell populations [Citation6]. Mechanisms by which this can occur include impaired T cell response, activation of T regulatory cells (Tregs) and myeloid-derived suppressor cells (MDSCs), and decreased presentation of tumor antigens [Citation6]. The median overall survival (OS) for patients with MM has increased over the past 4 decades and is >10 years in younger, fitter patients [Citation7]; the 5-year OS rate in the US is 59.8% [Citation4]. However, MM remains generally incurable, and patient outcome differs substantially associated with multiple patient- and disease-related characteristics [Citation5,Citation8–10]. Thus, MM is a highly complex disease with a highly heterogeneous patient population [Citation9].
1.1. Standard-of-care treatment for MM
The standard-of-care treatment approaches for MM are traditionally based on three classes of agents: proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), and CD38 monoclonal antibodies (mAbs) [Citation11]. For patients with newly diagnosed MM (NDMM) who are eligible for transplant, induction with a triplet (PI+IMiD+dexamethasone) or quadruplet (CD38 mAb+PI+IMiD+dexamethasone) regimen is followed by high-dose melphalan with autologous stem cell transplantation (ASCT), plus consolidation and single-agent lenalidomide or lenalidomide-based doublet maintenance, for example, lenalidomide plus CD38 mAb [Citation12]. Transplant-ineligible patients with NDMM may also receive triplets (PI+IMiD+dexamethasone; CD38 mAb+IMiD+dexamethasone) or quadruplets as initial therapy until disease progression or followed by single-agent maintenance. MM patients will typically require multiple lines of therapy [Citation8], with common subsequent treatment options including doublet or triplet combinations of a CD38 mAb (in patients naïve to CD38 mAb therapy) or anti-SLAMF7 mAb plus an IMiD, PI, or cyclophosphamide, and/or dexamethasone [Citation11,Citation13–15].
Additional novel therapies have recently been approved or have been under investigation for the treatment of relapsed/refractory MM (RRMM). These include selinexor [Citation16], a selective inhibitor of the nuclear export protein XPO1, the antibody – drug conjugate belantamab mafodotin [Citation17], which targets B-cell maturation antigen (BCMA), and which no longer carries US Food and Drug Administration (FDA) or European Union approvals, and melphalan flufenamide (melflufen) [Citation18], a peptide – drug conjugate, which no longer carries a US FDA approval but remains approved in the European Union. Furthermore, multiple immune effector cell therapies have been approved in recent years, including the chimeric antigen receptor (CAR) T cell therapies idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel) and the bispecific antibodies/T cell engagers teclistamab and elranatamab, which both target BCMA, and talquetamab, which targets G protein-coupled receptor, class C, group 5, member D (GPRC5D) [Citation8,Citation15,Citation19,Citation20]. Nevertheless, there remains an ongoing need for novel therapies. With the rise of quadruplet therapies as induction or initial treatment, and usage of upfront CD38 mAb therapies, patients who progress after one to two lines of treatment often have triple-class-refractory or penta-drug-refractory disease, in which they are refractory to at least one IMiD, one PI, and one CD38 mAb [Citation15]. Additionally, given the specialized infrastructure, apheresis, and long wait times needed for certain immune effector therapies, as well as patient-specific preferences or requirements, there is a need for more accessible therapies with limited treatment burden, i.e. oral therapies with real-world utility [Citation9,Citation21–23].
1.2. The evolution of CELMoD agents for the treatment of MM
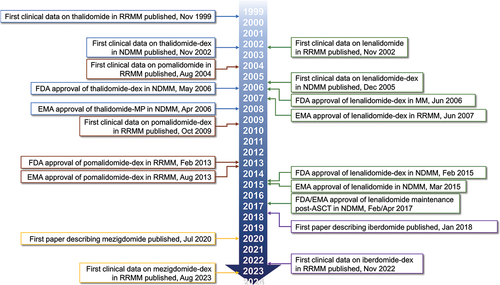
The IMiDs – thalidomide, lenalidomide, and pomalidomide – are among the backbones of therapy throughout the MM treatment algorithm [Citation11]; lenalidomide is used extensively for the treatment of patients with NDMM as a component of triplet or quadruplet induction/upfront regimens and as long-term maintenance therapy, and pomalidomide is a component of multiple standard-of-care triplet regimens in the RRMM setting. However, since the elucidation of the mechanism of action of the IMiDs, following initial investigations based on their anti-tumor necrosis factor-alpha activity [Citation24], this class of drugs has been expanded upon through the development of a novel group of agents with a similar mechanism of action but enhanced potency in targeting cereblon modulation – a group referred to as CELMoD agents. A number of these next-generation agents are currently under investigation for the treatment of MM [Citation25]. The evolution of these therapeutic classes – the IMiDs and CELMoD agents – for the treatment of MM, including initial clinical data publications and approvals by the US FDA and European Medicines Agency, is shown in [Citation26–33].
Figure 1. Timeline of the development of the IMiD/CELMoD compounds in MM.
As noted above, the name ‘CELMoD agents’ derives from the common critical mechanism of action shared by all the IMiDs and CELMoD agents, that of cereblon modulation [Citation34]. Prior studies demonstrated that thalidomide, lenalidomide, and pomalidomide all require cereblon for their activity in MM [Citation35–37], which was shown to be mediated through selective degradation of two critical lymphoid transcription factors, IKZF1 and IKZF3, also known as Ikaros and Aiolos [Citation38–40]. The next-generation CELMoD family of agents – iberdomide (formerly CC-220) [Citation41] and mezigdomide (formerly CC-92480) [Citation25] – have been developed to specifically enhance this mechanism and thereby potentially offer improved antimyeloma activity. Additional CELMoD agents have also been developed [Citation42,Citation43], including avadomide (CC-122), which is structurally and functionally similar to pomalidomide [Citation34,Citation44], plus golcadomide (CC-99282) [Citation45] and eragidomide (CC-90009) [Citation46], which are a different kind of ‘molecular glue’ that preferentially targets degradation of G1 to S phase transition protein 1 (GSPT1) rather than Ikaros and Aiolos [Citation46]. Avadomide has been investigated and golcadomide is under investigation for the treatment of B-cell non-Hodgkin’s lymphoma, and eragidomide has been studied in acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), but neither have been evaluated in MM and are therefore not discussed further in this review.
Here, we review the clinical significance of Ikaros and Aiolos in MM, the rationale for targeting these proteins for degradation, the improved potency of the CELMoD agents compared with the IMiDs for degrading Ikaros and Aiolos, and the clinical efficacy and safety of these agents in the treatment of MM. We used a literature search methodology comprising searches of PubMed (unlimited time-frame) and abstracts presented at international hematology/oncology conferences (2019–2023, including the meetings of the American Society of Hematology (ASH), the American Society of Clinical Oncology, the American Association for Cancer Research, the International Myeloma Society (IMS), and the European Hematology Association) using relevant search terms including IKZF1, IKZF3, Ikaros, Aiolos, CELMoD, IMiD, iberdomide, mezigdomide, and MM. We also present an expert opinion on how iberdomide and mezigdomide may be integrated into the MM treatment algorithm over the next 5-year period.
2. Ikaros and Aiolos: critical targets in MM
Ikaros and Aiolos are critical targets for the treatment of MM as their roles are of key importance to some of the cellular processes and signaling contributing to the proliferation and survival of MM cells. Ikaros and Aiolos are two transcription factors in the Ikaros family zinc finger (IKZF) family of proteins [Citation47] and are critical to the development and differentiation of hematopoietic cells via a network of interactions that control gene expression through chromatin remodeling and other mechanisms [Citation48,Citation49]. For example, it has been shown that Ikaros and Aiolos support the positive-feedback relationship with the oncogenes c-MYC and interferon regulatory factor 4 (IRF4) [Citation50]. Interestingly, the IKZF family of transcription factors have been shown to have differing roles in repressing or promoting gene transcription and to have different patterns of expression in different cells; they also have differing roles between hematologic malignancies [Citation51]. For example, Ikaros is a tumor suppressor that is functionally inhibited in B-cell precursor acute lymphoblastic leukemia (ALL), blocking differentiation and supporting the early B-cell phenotype [Citation52], and in B-cell and early T-cell precursor ALL [Citation53,Citation54], with this impairment associated with leukemogenesis [Citation51,Citation52]. Furthermore, loss of Ikaros has also been suggested as a determinant in AML oncogenesis [Citation55]. This leads to the hypothesis that treatment-induced modulation of Ikaros could be a possible reason for the observation of an increased risk of hematologic second primary malignancies, including ALL and AML, following ASCT and/or lenalidomide maintenance seen in MM [Citation51]. However, preclinical studies also suggest antitumor activity through Ikaros degradation specifically in lysine methyltransferase 2A rearranged (KMT2A-r) and nucleophosmin mutant (NPM1c) AML, alone or in combination with Menin inhibition [Citation56].
Ikaros and Aiolos are also involved in determining cell fate in lymphoid precursors and thus can impact the adaptive immune system [Citation49]. For example, Ikaros has been shown to be involved in regulation of multiple genes and signaling pathways associated with lymphocyte growth, reproduction, differentiation [Citation57], and function (e.g. mediating T-cell exhaustion) [Citation58], and have multiple effects across innate and adaptive immune cells including pre-B cells and mature B cells, T helper cells, NK cells, and CD4+ CD8+ thymocytes [Citation49,Citation59]. Indeed, the IKZF transcription factors more broadly have been specifically associated with regulation of CD4+ T helper cell subsets [Citation60], with differential expression across different CD4+ T cell subsets affecting differentiation and function. Further, it has been demonstrated that Ikaros is critical to early neutrophil differentiation [Citation61].
2.1. Roles of and rationale for targeting ikaros and Aiolos in MM
In MM, Ikaros, Aiolos, and the c-Myc and IRF4 proteins are highly expressed and function as anti-apoptotic transcription factors [Citation38,Citation39,Citation50,Citation62], with c-MYC upregulation shown to be important to MM development and progression via inhibition of microRNA-34, a tumor suppressor [Citation63]. Aiolos specifically has been shown to enhance transcriptional repression by B lymphocyte-induced maturation protein-1 (Blimp-1), an interaction that enhances MM cell survival [Citation62]. Indeed, both Ikaros and Aiolos are key components of a molecular transcriptional network that promotes and sustains MM and that incorporates the positive-feedback relationship with the MM oncogenes c-Myc and IRF4 [Citation50]. C-Myc, IRF4, Ikaros, and Aiolos proteins are all expressed at increased levels in MM versus normal plasma cells, contributing to both the pathogenesis of the disease and the proliferative and antiapoptotic activity in MM cells [Citation50]. Such disruption of Ikaros and Aiolos function has been associated with the pathobiology of MM and other hematologic malignancies [Citation39,Citation48]. Ikaros and Aiolos suppress the expression of interferon-stimulating genes (ISGs), including CD38 [Citation64], and suppress T cell stimulation [Citation65], and are also responsible for the repression of gene expression involved in NK cell activation [Citation66]. Notably, while Ikaros and Aiolos are essential to neoplastic plasma cells, they are less critical in normal plasma cells and, consequently, IMiDs do not deplete normal plasma cells.
Collectively, these findings provide a rationale for targeting the degradation of Ikaros and Aiolos in MM. In MM cells, the positive feedback loop between Ikaros and Aiolos and IRF4 and c-Myc supports cell proliferation and survival [Citation50]; however, expression of IRF4 and MYC is downregulated upon Ikaros and Aiolos degradation, removing these pro-survival mechanisms [Citation50,Citation67,Citation68]. Additionally, degradation of Ikaros and Aiolos enables activation of the innate and adaptive immune system via T lymphocyte stimulation [Citation65], enhanced cytokine expression including TNFα, IL-6, and interferon-γ [Citation65], increased NK cell activity and cell-mediated immune surveillance [Citation66,Citation69], and promotion of CD4+ and CD8+ T cell responses through Treg inhibition [Citation65], all of which contribute to subsequent antimyeloma activity [Citation47]. More comprehensive reviews focused specifically on the mechanistic roles of Ikaros and Aiolos in MM and other tumors have been published in 2021 by Cippitelli et al. [Citation51] and Xia et al. [Citation47].
2.2. CELMoD agents for targeting ikaros and Aiolos
2.2.1. Mechanism of action
As noted above, all IMiDs and CELMoD agents target cereblon, a substrate recruiter/receptor in the cullin-RING ligase-4 E3 ligase complex CRL4CRBN. CRL4CRBN is one of the multiple E3 ligases within the ubiquitin – proteasome system (UPS) that plays an important role in degrading intracellular proteins () [Citation70]. The IMiDs and CELMoD agents require cereblon for their antimyeloma activity [Citation35,Citation71] because the drugs act as a molecular glue that binds to cereblon to neo-substrate proteins including Ikaros and Aiolos, leading CRL4CRBN to promote their proteasomal degradation () [Citation38,Citation72]; IMiDs and CELMoD agents alter the conformation of cereblon [Citation34,Citation73] from ‘open’ to ‘closed’ [Citation74], with the latter conformation being highly active in recruiting Ikaros and Aiolos as substrates [Citation74].
Figure 2. The cereblon substrate receptor on the CRL4CRBN E3 ligase is conformationally altered through IMiD/CELMoD binding from an open conformation (top) to a closed conformation (bottom), resulting in the complex ubiquitinating alternative substrates (‘neo-substrates’) such as ikaros and Aiolos for degradation by the proteasome. Cereblon binding affinities differ between the IMiDs and CELMoD agents [Citation25,Citation41].
![Figure 2. The cereblon substrate receptor on the CRL4CRBN E3 ligase is conformationally altered through IMiD/CELMoD binding from an open conformation (top) to a closed conformation (bottom), resulting in the complex ubiquitinating alternative substrates (‘neo-substrates’) such as ikaros and Aiolos for degradation by the proteasome. Cereblon binding affinities differ between the IMiDs and CELMoD agents [Citation25,Citation41].](/cms/asset/d36a5a3f-7e4d-401d-bff2-91cc82a69de7/ierr_a_2382897_f0002_oc.jpg)
Clinical analyses have suggested that Ikaros and Aiolos expression on immunohistochemistry is associated with improved outcomes with lenalidomide-dexamethasone (Rd) therapy in patients with RRMM [Citation75]. High expression of cereblon is also associated with improved response to IMiDs – except in cases with a high proportion of alternatively spliced variants of cereblon, including isoforms lacking the IMiD-binding domain, which are associated with non-response [Citation76]. Indeed, the expression of Ikaros in bone marrow cell populations has been suggested as a biomarker for the activity of Rd, although Ikaros protein level in MM cells was not associated with OS [Citation77]. Similarly, malignant plasma cells from MM patients have been shown to express cereblon, Ikaros, and Aiolos, regardless of lenalidomide sensitivity [Citation78,Citation79]. Another analysis has suggested that relative baseline expression of Ikaros to cereblon in MM cells might be associated with outcome following Rd therapy, with low ratios of Ikaros/cereblon associated with poor progression-free survival (PFS) and OS [Citation80]. Conversely, suppression of Ikaros and Aiolos mRNA expression following treatment with lenalidomide has been shown to be significantly associated with longer PFS and OS in an analysis of NDMM following treatment [Citation81,Citation82], as well as in NDMM patients receiving lenalidomide-bortezomib-dexamethasone (RVd) triplet therapy [Citation83]. Further, Aiolos has also been suggested as a pharmacodynamic marker for lenalidomide and pomalidomide based on its demonstrated degradation in peripheral T cells [Citation65]. Interestingly, in an analysis of > 200 NDMM patients, Ikaros and Aiolos expression was similar between groups of patients with high-risk or standard-risk cytogenetics [Citation84]. However, higher expression was seen in patients with hyperdiploidy [Citation84], which has been associated with better prognosis in some instances [Citation85]. Multiple studies suggest that MM cells with greater Ikaros/Aiolos dependence might be more susceptible to IMiD-based treatment [Citation84].
2.2.2. Potency and substrate specificity
The IMiDs and CELMoD agents differ in their cereblon-binding potency, with the latter exhibiting enhanced potency () [Citation25,Citation41]. While the IMiDs exist as a mix of S and R enantiomers, CELMoD agents exist solely as S enantiomers, and their structures result in greater interaction with cereblon outside of the thalidomide binding pocket compared with the IMiDs; consequently, due to these binding differences, CELMoD agents have much lower IC50 values (0.03–0.06 µM) and, at saturating concentrations, result in a higher proportion of cereblon in the closed conformation (50% with iberdomide, 100% with mezigdomide) compared with the IMiDs (20% with pomalidomide) [Citation25,Citation41]. Thus, CELMoD agents – and mezigdomide in particular – result in more potent degradation of Ikaros and Aiolos and greater associated downstream antimyeloma activity [Citation25,Citation86].
Furthermore, the IMiDs and CELMoD agents can exhibit different substrate specificity beyond Ikaros and Aiolos upon binding cereblon [Citation44], albeit that full substrate specificity for CELMoD agents remains to be published. For example, lenalidomide specifically promotes degradation of casein kinase 1α (CK1α, encoded by CSNK1A1), inducing p53-mediated apoptosis, which is key to its activity in del(5q) MDS [Citation87]; however, pomalidomide does not degrade CK1α and thus shows no activity in this disease setting [Citation87]. This specific degradation of CK1α by lenalidomide may also play a role in promoting secondary leukemias, as suggested by work in preclinical models of TP53-mutated MDS [Citation87]. Importantly, it should be noted that the additional degradation of various other substrates beyond Ikaros and Aiolos by the IMiDs and CELMoD agents can have multiple effects, including the potential to result in activity and/or toxicity and to impair drug activity through substrates competing for access to cereblon [Citation44].
2.2.3. Preclinical antimyeloma activity and immune effects of CELMoD agents
The antimyeloma activity and immune effects of the IMiDs have been comprehensively characterized previously [Citation88–92]. These agents are known to have direct apoptotic and anti-proliferative effects on MM cells through reductions in cytokine secretion and activation of tumor-suppressor genes, and these direct cytotoxic effects are likely their primary mechanism of action. IMiDs are also known to disrupt MM cell – bone marrow microenvironment interactions that otherwise provide a supportive milieu for MM cell growth. One mechanism by which the latter effect has been suggested to occur is via the degradation of Ikaros; in the absence of Ikaros, myeloma-associated macrophage polarization in the bone marrow microenvironment is switched away from a nurturing, immune-evasive M2-like phenotype toward a tumoricidal M1-like phenotype promoting T cell stimulation [Citation93]. Indeed, the IMiDs have been shown to promote an antitumor immune response through T lymphocyte stimulation more broadly, including through dendritic cell differentiation and T cell proliferation [Citation94], increased interleukin-2 and interferon-γ secretion, NK lymphocyte activation, and a reduction in Tregs [Citation65], resulting in extensive antimyeloma effects, notably in combination with mAbs [Citation88]. Of note, the antimyeloma activity of lenalidomide has been shown to be potentiated by arsenic trioxide through upregulation of cereblon expression, supporting the central role of cereblon binding in the mechanism of action of the IMiDs [Citation95], although it should be emphasized that these are entirely preclinical findings.
The CELMoD agents iberdomide and mezigdomide have been shown to confer antimyeloma activity through similar mechanisms to the IMiDs, but with greater potency, reflecting their enhanced degradation of Ikaros and Aiolos [Citation41,Citation96,Citation97]. Consequently, both agents demonstrate enhanced preclinical profiles relative to lenalidomide and pomalidomide in terms of tumor antiproliferation, tumor apoptosis, and immune stimulation [Citation25,Citation41,Citation89,Citation96,Citation97]. Both iberdomide and mezigdomide have been shown to have superior apoptotic and antiproliferative effects compared with the IMiDs in MM cells and models, including in lenalidomide-resistant and pomalidomide-resistant models [Citation97,Citation98]. Further, they have demonstrated enhanced immunomodulatory effects, including activation of innate and adaptive immunity; in an immunophenotyping analysis of patients with RRMM using mass cytometry, iberdomide was shown to substantially disrupt the tumor immune microenvironment, driving reductions in naïve and regulatory B cells, increases in cytotoxic CD8+ T cells and reductions in inhibitory T cells, and similar changes in the populations of NK cells, as well as increases in NKT cells [Citation99]. Dose-dependent immune-stimulatory activity has also been demonstrated in healthy subjects in an early pharmacokinetic and pharmacodynamic study of iberdomide [Citation100]. Similarly, enhanced immune effects have been demonstrated in preclinical studies of mezigdomide, with enhanced cytokine production and T-cell activation resulting in potent immune-mediated MM cell killing in vitro [Citation96]. Like iberdomide, mezigdomide has shown activation of innate and adaptive immunity, with a reduction in exhausted or senescent T and NK cell populations, and a shift to an activated phenotype resulting from Ikaros/Aiolos degradation [Citation101,Citation102]. Furthermore, mezigdomide has demonstrated immune sensitization effects in MM cells, shifting the profile of adhesion molecules involved in T-cell-to-target interactions to prime for T-cell-mediated killing [Citation103].
CELMoD-based combinations have been evaluated in multiple preclinical studies, with both iberdomide and mezigdomide demonstrating synergy with dexamethasone, PIs, and CD38 mAbs [Citation97,Citation104,Citation105] (, [Citation97,Citation98,Citation105–111]). The substantial activity of combinations of IMiDs and CELMoD agents with PIs may appear counterintuitive given that IMiDs/CELMoD agents promote proteasomal degradation of neosubstrates, whereas PIs inhibit this mechanism; it is likely that enhanced antimyeloma activity arises through targeting different cell populations or due to switching mechanisms over the course of differing dosing regimens [Citation34]. In combination with PIs, iberdomide and mezigdomide have demonstrated potent immunomodulation with bortezomib-dexamethasone (Vd), with no adverse impact on the immunostimulatory effects of CELMoD agents from combination with bortezomib [Citation97,Citation110,Citation111]. Of note, substantially greater cytotoxic effects and tumor regressions in a lenalidomide-resistant MM mouse model were seen with mezigdomide-Vd compared with pomalidomide-Vd [Citation98,Citation111]. Preclinical studies have also demonstrated synergistic effects with iberdomide and mezigdomide in combination with CD38 mAbs [Citation97,Citation105] (). This synergy may arise due to potent degradation of Ikaros and Aiolos, as both proteins suppress CD38 expression [Citation64,Citation106]. Through this mechanism, a synergistic increase in daratumumab-mediated complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC) has been demonstrated with iberdomide and daratumumab in MM cells [Citation106], as well as greater tumor growth inhibition than with either agent alone in a lenalidomide-resistant mouse xenograft model. Similarly, synergistic activity has been observed with iberdomide plus daratumumab in pomalidomide-resistant cells [Citation97].
Table 1. Preclinical studies of iberdomide and mezigdomide in combination with other antimyeloma agents providing the rationale for synergy in combination regimens in the clinic.
Iberdomide and mezigdomide have been evaluated in preclinical studies in combination with bispecific antibodies (), a therapeutic class that is growing rapidly in importance in RRMM [Citation20]. Specifically, both CELMoD agents were studied in combination with alnuctamab, a BCMA-targeted T cell engager, and were shown to increase alnuctamab-mediated antitumor activity across multiple MM cell lines in an in vitro co-culture model [Citation107]. As mezigdomide resulted in greater cereblon modulation than iberdomide, it was evaluated in combination with alnuctamab in a humanized mouse MM xenograft model, demonstrating enhanced T cell activation and tumor tissue T cell infiltration effects, as well as enhanced alnuctamab-induced tumor clearance [Citation107]. Similarly, both iberdomide and mezigdomide enhanced tumor regressions and improved PFS in a mouse-xenograft model when combined with forimtamig, a GPRC5DxCD3 bispecific antibody, compared to forimtamig alone [Citation108]. These findings provide the rationale for evaluating a CELMoD – bispecific combination in the clinic. Moreover, and of potential clinical relevance, pretreatment with iberdomide and, in particular, mezigdomide strongly reduced secretion of proinflammatory cytokines by monocytes/macrophages and peripheral blood mononuclear cells, with mezigdomide pretreatment prior to alnuctamab exposure also resulting in suppression of alnuctamab-induced cytokine secretion [Citation107]. These findings suggest a potential role of CELMoD agents in mitigating cytokine release syndrome (CRS) with bispecific antibodies [Citation112].
The effects of iberdomide on CAR T cells have also been evaluated in preclinical studies [Citation109]. The immune-stimulatory effects of iberdomide were shown to have a positive impact on anti-BMCA CAR T cell functionality, counteracting exhaustion; Ikaros and Aiolos levels in CAR T cells were reduced and increased activation was seen, with increased cytokine production against BCMA-expressing MM cells and greater antigen-specific toxicity.
2.2.4. Other agents targeting ikaros and Aiolos
In addition to CELMoD agents, CFT7455, a monofunctional degradation activating compound (MonoDAC) targeting Ikaros and Aiolos is under development for the treatment of MM [Citation113,Citation114]. CFT7455 has been shown to selectively bind to cereblon with high affinity, resulting in deep and durable Ikaros and Aiolos degradation [Citation113]. This selective binding with CFT7455 is also anticipated to potentially reduce its off-target effects. Potent antiproliferative activity has been reported in MM cell lines, and in vivo antitumor activity has been seen in MM xenograft models, including IMiD-resistant or IMiD-insensitive models [Citation113]. As with iberdomide and mezigdomide, the cereblon binding affinity and degradation potency of CFT7455 appears to be substantially greater than for lenalidomide and pomalidomide [Citation113].
Furthermore, initial development has been reported of photoswitchable IMiDs (PHOIMiDs) that can degrade Ikaros and Aiolos in a light-dependent manner [Citation115]. Additional Ikaros-targeted protein degraders have been elucidated using arrayed small-molecule chemical screens, including Spautin-1, which degrades Ikaros in a cereblon-independent manner [Citation116], while baicalein has also been shown to enhance cereblon expression and Ikaros and Aiolos degradation in MM cells [Citation117]. Moreover, the effective degradation of Ikaros and Aiolos has been demonstrated with a proteolysis targeting chimera (PROTAC) degrader of nuclear receptor binding SET domain protein 2 (NSD2), which is frequently overexpressed in MM [Citation118], likely due to the PROTAC retaining IKZF substrate domains in its chemical structure. However, it should be noted that these agents are either in the very early stages of clinical development or are yet to enter the clinic.
2.2.5. Resistance mechanisms
Preclinical work has established resistance mechanisms against IMiDs and CELMoD agents [Citation119], including CRBN mutations, copy number loss, epigenetic modifications, and copy loss of COP9 signalosome genes involved in recycling the CRL4CRBN E3 ligase [Citation120]. Additionally, changes in lipid synthesis pathways, such as SREBP [Citation121], can result in reduced cereblon protein expression and thus reduced activity. In one analysis of patients with RRMM who were IMiD exposed and pomalidomide-refractory, nearly 30% had genetic alterations in CRBN [Citation122]. A specific analysis of factors that may mediate resistance to mezigdomide identified CRBN mutations, mRNA splice variants lacking exon 10, and monoallelic 3p26 loss [Citation123]. Moreover, specific mutations in cereblon and Ikaros/Aiolos that affect the binding site within the CRL4CRBN E3 ligase have been demonstrated to give rise to IMiD/CELMoD resistance [Citation36,Citation38,Citation124]; additional point mutations in CRL4 that arise during treatment may also confer resistance, while some mutations in IKZF3 are seen prior to treatment and may thus be more related to MM pathobiology than therapy-induced IMiD/CELMoD resistance [Citation125]. Similarly, lenalidomide resistance may be driven by Runt-related transcription factor 1 and 3 (RUNX1 and RUNX3), which inhibit the cereblon binding and degradation of Ikaros and Aiolos [Citation126]. IMiD resistance has also been associated with overexpression of USP15, which antagonizes ubiquitination of substrate proteins on CRL4CRBN E3 ligase [Citation127].
IMiD/CELMoD resistance has also been suggested to arise through a decoupling of the positive feedback loop between IRF4 and Ikaros/Aiolos, with IRF4 expression remaining high despite Ikaros/Aiolos degradation; however, cereblon-dependent synergistic activity has been demonstrated with EZH2 inhibition, possibly due to a recoupling of Ikaros/Aiolos with IRF4 expression [Citation128]. Similarly, c-FOS, a member of the activator protein-1 family, has been suggested as a driver of IMiD/CELMoD resistance through maintaining IRF expression despite Ikaros degradation [Citation129]. Meanwhile, the poor prognosis associated with the presence of specific genetic translocations in the immunoglobulin lambda light-chain locus may be due to IMiD/CELMoD resistance; this has been suggested as arising from the high levels of Ikaros binding of the immunoglobulin lambda enhancer in MM cells with such translocations, rendering them resistant to the Ikaros-degrading mechanism of action of the IMiDs/CELMoD agents [Citation130].
3. Clinical efficacy of targeting Ikaros and Aiolos in MM
3.1. IMiDs: established standard-of-care regimens
As outlined in the introduction, the IMiDs are an established pillar of standard-of-care treatment for both NDMM and RRMM based on their substantial antimyeloma activity and synergistic effects with other key classes of agents. In the setting of NDMM, the triplet of RVd is an important and highly active induction/upfront regimen for both transplant-eligible and non-transplant patients [Citation131], with the DETERMINATION phase 3 trial recently reporting a median PFS of >5.5 years in patients receiving RVd as induction and consolidation with ASCT and nearly 4 years in patients receiving RVd alone [Citation132]. Quadruplet therapy with RVd plus daratumumab is an emerging standard of care in the transplant-eligible setting based on impressive efficacy in the randomized GRIFFIN phase 2 trial [Citation133] and the randomized PERSEUS phase 3 trial [Citation12], which demonstrated significantly improved PFS (4-year rate: 84.3% vs 67.7%, hazard ratio [HR] 0.42), rate of complete response or better (87.9% vs 70.1%), and rate of minimal residual disease (MRD)-negative status (75.2% vs. 47.5%) with daratumumab-RVd versus RVd alone as induction and consolidation therapy pre- and post-ASCT. Lenalidomide maintenance is a standard of care following ASCT and was used in all three of the above studies, either as a single agent until disease progression or in combination with daratumumab maintenance for 2 years or longer [Citation12,Citation132,Citation133]. Additionally, in transplant-ineligible patients, lenalidomide in combination with daratumumab and dexamethasone (Dara-Rd) is a standard-of-care regimen based on the findings from the MAIA phase 3 trial [Citation134], which demonstrated improved PFS (5-year rate: 52.5% vs 28.7%, hazard ratio [HR] 0.53) and OS (5-year rate: 66.3% vs 53.1%, HR 0.68) compared to Rd alone, a former standard of care in this setting.
In patients with RRMM, pomalidomide is a backbone of various standard-of-care doublet and triplet regimens. Pomalidomide plus dexamethasone (Pom-dex) has demonstrated notable efficacy in this setting, including specifically in patients refractory to lenalidomide following prior exposure [Citation135]. Pomalidomide in combination with bortezomib (Pom-Vd) in the OPTIMISMM phase 3 trial [Citation136], daratumumab (Dara-Pom-dex) in APOLLO phase 3 trial [Citation137], or isatuximab (Isa-Pom-dex) in the ICARIA-MM phase 3 trial [Citation138] has demonstrated significant increased efficacy. In APOLLO, Dara-Pom-dex improved both PFS (median 12.4 vs 6.9 months, HR 0.63 [Citation139]) and OS (median 34.4 vs 23.7 months, HR 0.82 [Citation137] compared with Pom-dex alone in lenalidomide-exposed/refractory patients with RRMM after ≥ 1 prior line, and similar benefit was seen in ICARIA-MM with Isa-Pom-dex, with improved PFS (median 11.5 vs 6.5 months, HR 0.60 [Citation140]) and OS (median 24.6 vs 17.7 months, HR 0.76 [Citation138]) compared with Pom-dex in predominantly lenalidomide-refractory patients with RRMM after 2–4 prior lines.
3.2. Emerging clinical efficacy of iberdomide and mezigdomide
The clinical efficacy data reported to date from studies of iberdomide- and mezigdomide-based regimens in MM are summarized in [Citation32,Citation33,Citation114,Citation141–148]. In the first-in-human CC-220-MM-01 [Citation32] and CC-92480-MM-001 [Citation33] phase 1/2 studies of iberdomide and mezigdomide, respectively, each agent was investigated in combination with dexamethasone in dose-escalation and expansion cohorts to determine the maximum tolerated dose and explore the safety profile in heavily pretreated patients with RRMM. The CC-220-MM-01 study enrolled 90 patients in the dose-escalation phase to receive iberdomide doses from 0.3 mg to 1.6 mg, including 13 who received the recommended phase 2 dose (RP2D) of 1.6 mg [Citation32]. These patients were heavily pretreated, having received a median of 5 prior lines, which included PIs and IMiDs in all patients and CD38 mAbs in 76%; overall, 59% were triple-class refractory (refractory to a PI, an IMiD, and a CD38 mAb). Despite this, the overall response rate (ORR) was promising, at 32% across all dose levels, including 10% with very good partial response or better (≥VGPR), and the median duration of response (DOR) was 10.4 months [Citation32]. A similar patient population was enrolled in the CC-92480-MM-001 phase 1/2 study of mezigdomide plus dexamethasone; 77 patients, who had received a median of six prior lines, 56% of whom were triple-class refractory, and 30% of whom had high-risk cytogenetics [any of del17p, t(4;14), t(14;16), or amp1q21 (≥4 copies)], received mezigdomide 0.1–2.0 mg on a range of dosing schedules, including 11 who received the RP2D of mezigdomide 1.0 mg once-daily for 21 days of every 28 days [Citation33]. The ORR was 25% across all doses and 55% in the patients treated at the RP2D, including an overall rate of ≥VGPR of 13%. Responses were durable, with a median DOR of 6.0 months [Citation33]. These encouraging findings were followed by an expansion of each study to include a larger cohort treated at the RP2D.
Table 2. Clinical efficacy in NDMM and RRMM reported with the CELMoD agents iberdomide and mezigdomide and the MonoDAC CFT7455.
In the CC-220-MM-01 study of iberdomide-dexamethasone, 107 patients with RRMM, who had received a median of six prior lines and of whom 97% were triple-class refractory, were enrolled to the expansion cohort to receive iberdomide 1.6 mg [Citation32]. The ORR was 26%, including 8% ≥VGPR, similar to that seen during dose escalation, with a median DOR of 7.0 months in the context of a median PFS of 3.0 months [Citation32]. Likewise, 101 patients with a median of six prior lines, all of whom were triple-class refractory, were enrolled to the expansion cohort in the CC-92480-MM-001 study to receive mezigdomide 1.0 mg [Citation33] – the ORR in this difficult-to-treat population was 41%, including 25% ≥VGPR, and, as in the dose-escalation cohort, responses were durable, with a median DOR of 7.6 months (and five responses lasting for ≥12 months) indicating prolonged benefit in responders in the context of an overall median PFS of 4.4 months. Notable activity was seen in patients with poor prognosis, with an ORR of 30% in those with plasmacytomas and 32% in patients with high-risk cytogenetics, including a median DOR of 10.0 months in the latter, while the ORR was 50%, with a median DOR of 6.9 months, in patients who had previously been treated with anti-BCMA therapy [Citation33]. Supporting these findings, data from a specific cohort of the CC-220-MM-001 study in 38 BCMA-exposed patients with RRMM, who had received a median of seven prior lines, showed an ORR of 37% with iberdomide-dexamethasone, with a median DOR of 7.5 months [Citation143]. These data demonstrate that the mechanism of action of potent Ikaros and Aiolos degradation remained active in these heavily pretreated patients, including those who were refractory to prior IMiDs and who had been exposed to immune-based therapies.
More extensive triplet regimens with iberdomide and mezigdomide have also been investigated in patients with RRMM, based on the demonstrated preclinical synergies and mechanistic rationales for combination with other agents. Paralleling standard-of-care triplet regimens based on an IMiD, a PI, and dexamethasone, iberdomide has been evaluated in combination with Vd and with carfilzomib-dexamethasone (Kd) [Citation144], with ORRs of 56% for both regimens and respective ≥VGPR rates of 28% and 38%. As with the iberdomide-dexamethasone doublet, responses were durable, with the median DOR with iberdomide-Vd being approximately 8.3 months. Substantial efficacy has been demonstrated with the similar mezigdomide-based triplets in less heavily treated patient populations with RRMM. With mezigdomide-Vd, ORRs of 75–91%, including ≥VGPR rates of 39–82% and ≥CR rates of 18–27%, have been seen in preliminary analyses [Citation145], with similar findings with mezigdomide-Kd (). Responses were again durable, with median DORs of 10.9 months with mezigdomide-Vd and 12.3 months with mezigdomide-Kd in dose-escalation cohorts, which included some highly prolonged responses of >2.5 years [Citation145]. Importantly, in the context of emerging PI-IMiD-mAb-based quadruplet regimens as induction or upfront therapy [Citation12] and pomalidomide-based triplets as standard-of-care regimens for first or second relapses, both mezigdomide-based triplets were active in lenalidomide-/CD38 mAb-dual-refractory patients (ORRs 69–82%) and pomalidomide-refractory patients (ORRs 77–83%) [Citation145].
Combinations of iberdomide or mezigdomide and a mAb have also been investigated and demonstrated preliminary activity in patients with RRMM (). In Cohort E of the CC-220-MM-01 trial, 43 patients with RRMM and median of four prior therapies (range 2–13), 33% of whom were triple-class refractory, received iberdomide 1.0–1.6 mg plus daratumumab and dexamethasone (Dara-dex) [Citation144]. The triplet resulted in an ORR of 46%, including 24% ≥VGPR, and the median DOR had not been reached at data cutoff [Citation144]. Similarly, mezigdomide 0.3–0.6 mg plus Dara-dex has been evaluated in 56 patients in the CC-92480-MM-002 trial, of whom 83% were IMiD-refractory and 61% were PI-refractory but only 9% had received a prior CD38 mAb [Citation146]. In this relatively less heavily pretreated population (compared with those who received iberdomide-Dara-dex), the ORR was 75%, including 46% ≥VGPR. Promising early efficacy has also been seen with the combination of mezigdomide, elotuzumab, and dexamethasone in this study ().
Preliminary efficacy data with iberdomide-based combinations in patients with NDMM have also been reported at the 2023 Annual Meetings of the IMS and ASH. At IMS 2023, initial findings were reported from the phase 2 expansion cohort of the CC-220-MM-001 trial in which 18 transplant-ineligible patients with NDMM received iberdomide-Vd [Citation148]; substantial efficacy was reported, including a ≥VGPR rate of 78% (). Similarly, at ASH 2023, early findings from the KID study of iberdomide-Kd as induction therapy in transplant-eligible patients with NDMM showed responses in all nine evaluable patients, including five with ≥VGPR [Citation142]. The use of iberdomide as post-ASCT maintenance therapy in the EMN26 study was also reported at ASH 2023 [Citation141], with early findings showing that 45%–48% of patients had an improvement in their depth of response after 6 months of maintenance.
3.3. Pharmacodynamic effects of iberdomide and mezigdomide
Reflecting the clinical efficacy demonstrated to date with iberdomide-based and mezigdomide-based regimens, multiple studies have shown the anticipated pharmacodynamic effects of these therapies, including substantial reductions from baseline in the concentrations of Ikaros and Aiolos in MM cells in the bone marrow [Citation32,Citation33,Citation148,Citation149]. With iberdomide, a dose of 1.1 mg resulted in a > 90% decrease in Aiolos [Citation32], while a mezigdomide dose of 1.0 mg produced a ≥ 80% decrease in Aiolos that was sustained for 24 h [Citation33]. These data provided support for the use of daily rather than twice-daily dosing and the need for prolonged dosing periods (i.e. 14 or 21 days on, 7 days off), rather than intermittent dosing, in order to sustain suppression of Aiolos levels [Citation33]. Importantly, target protein reductions were seen in patients refractory to pomalidomide, including a median ~ 70% decrease with mezigdomide-dexamethasone [Citation33], indicating that the greater potency of CELMoD agents for cereblon binding maintained this pharmacodynamic effect despite prior IMiD resistance. Effects were also reported with iberdomide-Vd in patients with NDMM, including a median decrease of > 50% in substrate levels [Citation148].
These changes in target protein levels corresponded with various immune pharmacodynamic effects with iberdomide and mezigdomide, including the expansion of proliferating, activated CD4+, activated CD8+, and effector memory T cells, although the clinical consequences of these immune modulatory effects are not clear [Citation32,Citation33,Citation148,Citation149]. With iberdomide-dexamethasone, mature B cell numbers were reduced and T-cell and NK-cell proliferation increased in an exposure-related manner, with an apparent saturation of effect at higher doses of iberdomide in the dose-escalation component of the CC-220-MM-001 trial [Citation32], while iberdomide-Vd stimulated a median increase in T-cell proliferation of 177% in patients with NDMM [Citation148]. As well as the activated effector memory phenotype seen with mezigdomide-dexamethasone [Citation33], mezigdomide plus Vd or Kd increased proliferating T cell levels and mezigdomide-Dara-dex promoted immune stimulation of T cells and NK cells [Citation105,Citation146]. Notably, similar immune effects have been reported in a longitudinal analysis of patients with RRMM treated with pomalidomide-Vd in the OPTIMISMM phase 3 trial, which also showed that a number of markers of immune activation were prognostic for enhanced PFS with pomalidomide-Vd [Citation150], although it remains unclear if this is a causal association or a pharmacodynamic marker.
4. Clinical safety profile associated with targeting ikaros and Aiolos in MM
The common toxicities reported with lenalidomide [Citation132,Citation134,Citation151,Citation152], pomalidomide [Citation135–139], iberdomide [Citation141–144,Citation148,Citation153], and mezigdomide [Citation33,Citation145–147] are summarized in . As shown, the most common grade ≥ 3 toxicities are hematologic, including neutropenia, anemia, and thrombocytopenia, and these – in particular neutropenia – are a class effect of IMiDs and CELMoD agents associated with the impact of Ikaros and Aiolos degradation on normal hematopoietic differentiation, including granulocyte maturation arrest [Citation74,Citation154] and inhibition of megakaryocyte maturation [Citation155]. In key phase 3 trials of lenalidomide-based regimens in NDMM and RRMM, rates of grade ≥ 3 neutropenia were 35%–54%, while rates with similar pomalidomide-based regimens in RRMM appeared slightly higher at 42%–69% (). Of note, the rates of grade ≥ 3 neutropenia were higher in regimens containing an CD38 mAb and lenalidomide or pomalidomide [Citation134,Citation137–139]. Rates of toxicities in early-phase studies of iberdomide and mezigdomide should be interpreted in the context of some data being derived from dose-escalation cohorts and of the relatively smaller sample sizes, as well as some of the patient populations being more heavily pretreated. Nevertheless, data from studies of iberdomide-based regimens in RRMM showed that rates of grade ≥ 3 neutropenia (28%–67%) broadly appeared to be in a similar range to those with pomalidomide-based therapy, and the higher rate was seen with the combination of iberdomide plus daratumumab and dexamethasone, reflecting findings with lenalidomide and pomalidomide triplet regimens [Citation144]; importantly, while 71%–76% of patients receiving mezigdomide-dexamethasone in the CC-92480-MM-001 phase 1/2 study experienced grade ≥ 3 neutropenia, the rate of mezigdomide discontinuation due to adverse events (AEs) was low (5%–6%) in both the dose-escalation and expansion parts of the study [Citation33]. Indeed, the grade ≥ 3 neutropenia seen with iberdomide-dexamethasone [Citation153] and mezigdomide-dexamethasone [Citation33] appeared generally manageable with dose interruptions and supportive or prophylactic use of granulocyte colony-stimulating factor (G-CSF), which was reported in 61%–77% of patients.
Table 3. Safety data from key studies in NDMM and RRMM with the IMiDs, the CELMoD agents, and the MonoDAC CFT7455.
Across the clinical studies of IMiDs and CELMoD agents, the second most common grade ≥ 3 toxicity was generally that of infections, with rates of 28%–34%, 15%–33%, and 16%–40% reported with pomalidomide-based, iberdomide-based, and mezigdomide-based regimens, respectively, in patients with RRMM (). Indeed, the two dose-limiting toxicities observed in the dose-escalation part of the CC-220-MM-001 study of iberdomide-dexamethasone were both infections that occurred at dose levels below the recommended phase 2 dose of iberdomide [Citation32].
With regard to other nonhematologic toxicities, common all-grade AEs in the key studies of lenalidomide-based regimens shown in included neuropathy (73% in DETERMINATION [Citation132], likely primarily attributable to the bortezomib component of the regimen), fatigue (31%–57%), and gastrointestinal AEs (diarrhea 47%–66%, constipation 38%–43%, nausea 24%–36%) [Citation132,Citation134,Citation151]; similarly, in key studies pomalidomide-based therapy, common all-grade nonhematologic AEs included neuropathy (48% in OPTIMISMM [Citation136], again likely attributable to bortezomib) fatigue (20%–37%), pyrexia (14%–27%), and gastrointestinal AEs (diarrhea 14%–34%, constipation 16%–37%, nausea 9%–18%) [Citation135,Citation136,Citation138,Citation139]. These common toxicities were reflected in data from early-phase studies of iberdomide and mezigdomide. With iberdomide-dexamethasone, the most common nonhematologic AEs of any grade were fatigue (37%), insomnia (32%), and diarrhea (23%) in the dose-escalation cohort and fatigue, diarrhea (each 23%), constipation (22%), rash, and dyspnea (each 20%) in the expansion phase [Citation144,Citation153], while with mezigdomide-dexamethasone, the most common nonhematologic AEs were fatigue (40%), nausea (27%), decreased appetite, diarrhea, and pyrexia (each 26%) in the dose-escalation cohort and fatigue (36%) and diarrhea (31%) in the expansion cohort [Citation33]. Broadly similar safety profiles were reported in early-phase studies of other iberdomide- and mezigdomide-based regimens. Importantly, the rates of grade ≥ 3 nonhematologic toxicities were generally low across all IMiD- and CELMoD-based regimens (). Thus, data to date suggest that CELMoD agents exhibit safety profiles consistent with those established for lenalidomide and pomalidomide.
Notably, however, while the IMiDs – particularly lenalidomide in combination with dexamethasone – have been associated with an elevated risk of thromboembolic events, with rates of all thromboembolic events of 2.8–4.1% with RVd±ASCT in DETERMINATION [Citation132], grade ≥ 3 pulmonary embolism (PE) of 5.5–7.1% with Dara±Rd in MAIA [Citation134], grade ≥ 3 deep vein thrombosis (DVT)/PE of 13% with Rd in MM-009/MM-010 [Citation152], and DVT/PE of 1%/4% with Pom-Vd in OPTIMISMM [Citation136], the incidence of thromboembolic events appears limited to date in studies of iberdomide (no thromboembolic events with iberdomide-dexamethasone in the CC-220-MM-001 study [Citation32]) and mezigdomide (CC-92480-MM-001 study, mezigdomide-dexamethasone, DVT: 3%, 1% grade ≥ 3 [Citation33]). However, interpretation of these findings is confounded by differences between studies in patient populations and potentially in thromboprophylaxis used; additional data from larger studies of CELMoD-based regimens in earlier treatment settings will be needed to elucidate any differences in thromboembolic risk compared with the IMiDs.
5. Conclusions
Targeting Ikaros and Aiolos has proven highly effective as a mechanism of action for the treatment of NDMM and RRMM, with the IMiDs lenalidomide and pomalidomide established as components of multiple standard-of-care regimens and approaches [Citation11]. Following the identification of this mechanism of action of the IMiDs and the critical role of cereblon modulation in mediating Ikaros and Aiolos degradation, CELMoD agents have been developed as a specific class of targeted agent with improved cereblon binding affinity that offer more potent Ikaros and Aiolos degradation compared with their IMiD ‘ancestors.’ Preclinical studies have shown these new agents to have substantial antimyeloma activity, including in lenalidomide-refractory and pomalidomide-refractory cell lines and in vivo models. CELMoD agents may potentially therefore provide additional therapeutic options for patients with RRMM beyond the development of initial resistance to the IMiDs, an area of clinical need in the context of the established roles of lenalidomide in NDMM and RRMM treatment algorithms and pomalidomide in RRMM. Importantly, CELMoD agents, like the IMiDs, are oral drugs and thus also offer the associated feasibility and accessibility benefits seen with lenalidomide and pomalidomide.
Early-phase clinical studies of iberdomide- and mezigdomide-based regimens have shown highly promising efficacy, including in hard-to-treat populations such as IMiD-refractory, heavily pretreated patients, those previously treated with anti-BCMA therapies, and patients with high-risk cytogenetics. Additionally, reflecting preclinical findings of synergistic activity with other MM agents, the feasibility and activity of combining iberdomide and mezigdomide with standard-of-care and emerging drug classes, including PIs, mAbs, and bispecific antibodies has been seen in preliminary data from ongoing studies. Across these studies and regimens, a generally manageable toxicity profile is emerging for CELMoD agents that is consistent with the well-established safety profiles of lenalidomide and pomalidomide, including frequent grade ≥ 3 neutropenia as a mechanism-based class effect of both the IMiDs and CELMoD agents. Thus, there is the potential for these emerging CELMoD agents, with their improved targeting of Ikaros and Aiolos, to expand upon current IMiD-based therapies and provide additional treatment options based on this mechanism of action within the evolving MM algorithm, with further novel agents targeting degradation of these and other key substrate proteins also beginning to be developed.
6. Expert opinion
The MM treatment algorithm continues to evolve rapidly, reflecting the emergence of novel agents, therapeutic classes, and novel triplet and quadruplet combinations in the past 5 years [Citation11,Citation156]. The standards of care for NDMM are evolving to incorporate quadruplet regimens comprising a PI, an IMiD, and an CD38 mAb [Citation12], with long-term single-agent or doublet maintenance [Citation11] or potentially triplet maintenance for patients with high-risk disease [Citation157–159]. Novel mAbs, antibody – drug conjugates, bispecifics, CAR T cell therapies, and other therapies have been approved for RRMM in the past few years, including ide-cel, cilta-cel, teclistamab, elranatamab, talquetamab, selinexor, belantamab mafodotin, and melflufen. Currently, these novel agents were studied and are approved for use in heavily pretreated patient populations. However, ongoing studies are investigating these new/recently approved agents in earlier lines of treatment. Current and future studies will need to tackle several areas of unmet need, including patients with treatment failure following quadruplet therapy, in whom subsequent outcomes may be poor [Citation160]; triple-class refractory patients; penta-exposed/refractory patients; patients who have progressed following immune effector cell therapies targeting BCMA or GPCR5D; and, associated with this, patients with an exhausted T cell phenotype. Additionally, there is the ongoing goal of further improving outcomes in the NDMM setting to ‘make the most’ of this point at which the disease is most sensitive to therapy.
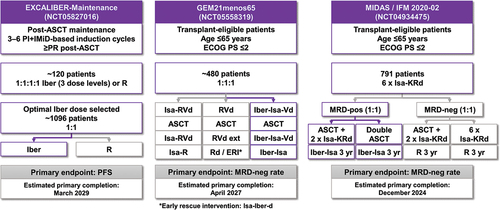
The new CELMoD compounds are anticipated to emerge within these multiple areas of unmet need over the next 5 years, based on current and ongoing clinical studies. At the time of manuscript development (January 2024), there are six ongoing phase 3 studies of iberdomide and mezigdomide in various MM treatment settings. Three phase 3 studies are evaluating iberdomide for the treatment of NDMM (); the EXCALIBER-Maintenance trial [Citation161] is a head-to-head evaluation of iberdomide versus lenalidomide as post-ASCT maintenance to determine if the greater potency for targeting Ikaros and Aiolos degradation translates into better PFS, whereas the GEM21menos65 and MIDAS (IFM 2020–02) trials are investigating iberdomide as a replacement for lenalidomide in some or all components of treatment for transplant-eligible NDMM patients, with the aim of increasing the MRD-negative rate following treatment, given its positive prognostic impact on long-term outcome. Notably, iberdomide is a component of the escalated therapy planned in MIDAS for patients who remain MRD-positive after six cycles of quadruplet induction therapy. In the RRMM setting, the large phase 3, randomized, EXCALIBER-RRMM [Citation162] trial () is evaluating iberdomide in combination with daratumumab and dexamethasone as an alternative to the CASTOR regimen of Dara-Vd. Meanwhile, there are two phase 3 randomized trials of mezigdomide ongoing in RRMM (); SUCCESSOR-1 [Citation163] and SUCCESSOR 2 [Citation164] are both evaluating mezigdomide-PI-dex triplet regimens against existing standards of care in the lenalidomide/daratumumab-exposed early relapse setting.
Figure 3. Ongoing phase 3 studies of iberdomide and mezigdomide in (a) NDMM [Citation161] and (b) RRMM [Citation162–164].
![Figure 3. Ongoing phase 3 studies of iberdomide and mezigdomide in (a) NDMM [Citation161] and (b) RRMM [Citation162–164].](/cms/asset/e25a2421-23fd-4417-a008-ce1e6b01077d/ierr_a_2382897_f0003b_oc.jpg)
Beyond the phase 3 trials, there are also numerous ongoing early-phase studies of iberdomide- and mezigdomide-based combination regimens in different treatment settings (). Triplets incorporating iberdomide and daratumumab or bortezomib plus dexamethasone are being evaluated as initial therapy in non-transplant patients with NDMM, and studies are exploring similar regimens as post-ASCT maintenance therapy. In patients with RRMM, studies are exploring triplet regimens that reflect lenalidomide- or pomalidomide-based combinations in this setting, including iberdomide plus ixazomib-dexamethasone or elotuzumab-dexamethasone, as well as novel combinations with emerging immune therapies. Mezigdomide is primarily being studied in RRMM, with ongoing studies evaluating similar regimens to those being explored with iberdomide ().
Table 4. Ongoing studies of iberdomide and mezigdomide in novel combinations in NDMM and RRMM (ClinicalTrials.Gov, March 21, 2024).
The potential future roles of iberdomide and mezigdomide in the treatment algorithm, and possible future combinations, including, for example, with immune effector cell therapies based on the rationale of immune priming with CELMoD agents, will obviously be dependent on the results of these ongoing studies, as well as on other developments in the MM treatment algorithm. For example, depending on the findings from the head-to-head phase 3 EXCALIBER-maintenance trial, iberdomide may emerge as a potential additional option for post-ASCT maintenance therapy, whereas it is expected that, based on SUCCESSOR-1 and SUCCESSOR 2, mezigdomide will be utilized in the RRMM setting, with its potency as a CELMoD agent potentially offering continued clinical activity in IMiD-refractory settings. Further clinical data are eagerly awaited, especially from these randomized phase 3 trials, that will elucidate the activity of iberdomide and mezigdomide in MM and provide guidance to clinicians about the roles these agents will play in our continuing efforts to improve MM patient outcome.
Article highlights
Novel treatment options are needed for patients with multiple myeloma (MM) in the context of the evolving treatment algorithm and the development of disease that is refractory to established standards of care.
The key mechanism of action of the immunomodulatory drugs (IMiDs) in MM is that of cereblon modulation mediating selective degradation of the neo-substrates IKZF1 and IKZF3, also known as Ikaros and Aiolos; the CELMoD compounds iberdomide and mezigdomide are next-generation agents with enhanced potency for targeting Ikaros and Aiolos degradation.
Ikaros and Aiolos are overexpressed in MM cells versus normal plasma cells, contributing to MM pathogenesis and to proliferative and antiapoptotic activity via a positive-feedback relationship with MM oncogenes c-Myc and IRF4.
Through their mechanism of action, IMiDs and CELMoD agents have direct apoptotic and anti-proliferative effects on MM cells, disrupt supportive MM cell – bone marrow microenvironment interactions, and also promote an antitumor immune response, with superior antimyeloma and immunomodulatory effects demonstrated with CELMoD agents compared with the IMiDs.
As seen with the IMiDs, iberdomide and mezigdomide have demonstrated enhanced activity in preclinical studies in combination with standard-of-care agents including the CD38 monoclonal antibody daratumumab, the proteasome inhibitor bortezomib, and bispecific antibodies, with notable activity seen in lenalidomide- and pomalidomide-resistant cells and models.
The efficacy of IMiD-based regimens in patients with newly diagnosed (NDMM) or relapsed/refractory MM (RRMM) is well established from multiple phase 3 trials, and promising clinical activity has been seen in early-phase studies of iberdomide- and mezigdomide-based therapy in the RRMM setting, including in triple-class refractory patients.
The common grade ≥ 3 toxicities of the IMiDs and CELMoD agents are hematologic, with the high rates of grade ≥ 3 neutropenia reflecting a class effect associated with the impact of Ikaros and Aiolos degradation on normal hematopoietic differentiation; infections are generally the second most common grade ≥ 3 toxicity with the IMiDs and CELMoD agents.
Six phase 3 trials of iberdomide and mezigdomide are currently ongoing in the NDMM and RRMM settings to help elucidate their future roles in the MM treatment algorithm.
Declaration of interest
CC Mo has acted on the advisory boards for AbbVie, BMS, GSK, Janssen, Karyopharm, Sanofi, and Takeda, has acted as a consultant for AbbVie, Janssen, Karyopharm, and Sanofi. MA Hartley-Brown has acted on the advisory boards for Abbvie, BMS, Celgene, GSK, Janssen, Karyopharm, and Sanofi, and has received research funding from BMS/Celgene, GSK, and Sanofi. AS Sperling has acted as a consultant for Novartis and Roche. S Midha has acted as a consultant for Pfizer. AJ Yee has acted as a consultant for AbbVie, Adaptive Biotechnologies, Amgen, BMS, Celgene, GSK, Janssen, Karyopharm, Oncopeptides, Pfizer, Prothena, Regeneron, Sanofi, Sebia, Takeda, and has received research funding from Amgen, BMS, Janssen. O Nadeem has acted on the advisory boards for BMS, Janssen, GSK, Sanofi, and GPCR Therapeutics, and has received research funding from Takeda and Janssen. PG Richardson has received grants to their institution for clinical trials from Karyopharm and Oncopeptides, and has acted on the advisory committees for Bristol Myers Squibb/Celgene, GSK, Karyopharm, Oncopeptides, Regeneron, Sanofi, and Takeda. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A peer reviewer has received honoraria and travel support from Janssen.
Acknowledgments
The authors gratefully acknowledge Steve Hill of Ashfield MedComms, an Inizio company, for medical writing and editing support, funded by the Dana-Farber Cancer Institute and the RJ Corman Multiple Myeloma Research Fund.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi: 10.3322/caac.21763
- Dyba T, Randi G, Bray F, et al. The European cancer burden in 2020: incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308–347. doi: 10.1016/j.ejca.2021.07.039
- NIH National Cancer Institute Surveillance E and end results program. Cancer stat facts: myeloma. [cited 2023 Aug 22]. Available from: https://seer.cancer.gov/statfacts/html/mulmy.html
- van de Donk N, Pawlyn C, Yong KL. Multiple myeloma. Lancet. 2021;397(10272):410–427. doi: 10.1016/S0140-6736(21)00135-5
- Leone P, Solimando AG, Malerba E, et al. Actors on the scene: immune cells in the myeloma niche. Front Oncol. 2020;10:599098. doi: 10.3389/fonc.2020.599098
- Joseph NS, Kaufman JL, Dhodapkar MV, et al. Long-term follow-up results of lenalidomide, bortezomib, and dexamethasone induction therapy and risk-adapted maintenance approach in newly diagnosed multiple myeloma. J Clin Oncol. 2020;38(17):1928–1937. doi: 10.1200/JCO.19.02515
- Raje N, Mateos MV, Iida S, et al. Clinical evidence for immune-based strategies in early-line multiple myeloma: current challenges in decision-making for subsequent therapy. Blood Cancer J. 2023;13(1):41. doi: 10.1038/s41408-023-00804-y
- Richardson PG, San Miguel JF, Moreau P, et al. Interpreting clinical trial data in multiple myeloma: translating findings to the real-world setting. Blood Cancer J. 2018;8(11):109. doi: 10.1038/s41408-018-0141-0
- Gandolfi S, Vekstein C, Laubach JP, et al. The evolving role of transplantation in multiple myeloma: the need for a heterogeneous approach to a heterogeneous disease. Clin Adv Hematol Oncol. 2018;16(8):564–574.
- Kumar SK, Callander NS, Adekola K, et al. Multiple myeloma, version 2.2024, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(12):1281–1301. doi: 10.6004/jnccn.2023.0061
- Sonneveld P, Dimopoulos MA, Boccadoro M, et al. Daratumumab, Bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2024;390(3):301–313. doi: 10.1056/NEJMoa2312054
- Dimopoulos MA, Moreau P, Terpos E, et al. Multiple myeloma: EHA-ESMO clinical practice guidelines for diagnosis, treatment and follow-up(dagger). Ann Oncol. 2021;32(3):309–322. doi: 10.1016/j.annonc.2020.11.014
- Rajkumar SV. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am J Hematol. 2022;97(8):1086–1107. doi: 10.1002/ajh.26590
- Stalker ME, Mark TM. Clinical management of triple-class refractory multiple myeloma: a review of current strategies and emerging therapies. Curr Oncol. 2022;29(7):4464–4477. doi: 10.3390/curroncol29070355
- Mo CC, Yee AJ, Midha S, et al. Selinexor: targeting a novel pathway in multiple myeloma. EJHaem. 2023;4(3):792–810. doi: 10.1002/jha2.709
- More S, Offidani M, Corvatta L, et al. Belantamab Mafodotin: from clinical trials data to real-life experiences. Cancers (Basel). 2023;15(11):2948. doi: 10.3390/cancers15112948
- Nadeem O, Mateos MV, Efebera YA, et al. Melphalan flufenamide for relapsed/refractory multiple myeloma. Drugs Today (Barc). 2022;58(8):407–423. doi: 10.1358/dot.2022.58.8.3367680
- Parikh RH, Lonial S. Chimeric antigen receptor T-cell therapy in multiple myeloma: a comprehensive review of current data and implications for clinical practice. CA Cancer J Clin. 2023;73(3):275–285. doi: 10.3322/caac.21771
- Cho SF, Yeh TJ, Anderson KC, et al. Bispecific antibodies in multiple myeloma treatment: a journey in progress. Front Oncol. 2022;12:1032775. doi: 10.3389/fonc.2022.1032775
- Gengenbach L, Graziani G, Reinhardt H, et al. Choosing the right therapy for patients with relapsed/refractory multiple myeloma (RRMM) in consideration of patient-, disease- and treatment-related factors. Cancers (Basel). 2021;13(17):4320. doi: 10.3390/cancers13174320
- Ramasamy K, Gay F, Weisel K, et al. Improving outcomes for patients with relapsed multiple myeloma: challenges and considerations of current and emerging treatment options. Blood Rev. 2021;49:100808. doi: 10.1016/j.blre.2021.100808
- Snowden JA, Greenfield DM, Bird JM, et al. Guidelines for screening and management of late and long-term consequences of myeloma and its treatment. Br J Haematol. 2017;176(6):888–907. doi: 10.1111/bjh.14514
- Marriott JB, Clarke IA, Dredge K, et al. Thalidomide and its analogues have distinct and opposing effects on TNF-alpha and TNFR2 during co-stimulation of both CD4(+) and CD8(+) T cells. Clin Exp Immunol. 2002;130(1):75–84. doi: 10.1046/j.1365-2249.2002.01954.x
- Hansen JD, Correa M, Nagy MA, et al. Discovery of CRBN E3 ligase modulator CC-92480 for the treatment of relapsed and refractory multiple myeloma. J Med Chem. 2020;63(13):6648–6676. doi: 10.1021/acs.jmedchem.9b01928
- Singhal S, Mehta J, Desikan R, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102
- Rajkumar SV, Hayman S, Gertz MA, et al. Combination therapy with thalidomide plus dexamethasone for newly diagnosed myeloma. J Clin Oncol. 2002;20(21):4319–4323. doi: 10.1200/JCO.2002.02.116
- Richardson PG, Schlossman RL, Weller E, et al. Immunomodulatory drug CC-5013 overcomes drug resistance and is well tolerated in patients with relapsed multiple myeloma. Blood. 2002;100(9):3063–3067. doi: 10.1182/blood-2002-03-0996
- Rajkumar SV, Hayman SR, Lacy MQ, et al. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. doi: 10.1182/blood-2005-07-2817
- Schey SA, Fields P, Bartlett JB, et al. Phase I study of an immunomodulatory thalidomide analog, CC-4047, in relapsed or refractory multiple myeloma. J Clin Oncol. 2004;22(16):3269–3276. doi: 10.1200/JCO.2004.10.052
- Lacy MQ, Hayman SR, Gertz MA, et al. Pomalidomide (CC4047) plus low-dose dexamethasone as therapy for relapsed multiple myeloma. J Clin Oncol. 2009;27(30):5008–5014. doi: 10.1200/JCO.2009.23.6802
- Lonial S, Popat R, Hulin C, et al. Iberdomide plus dexamethasone in heavily pretreated late-line relapsed or refractory multiple myeloma (CC-220-MM-001): a multicentre, multicohort, open-label, phase 1/2 trial. Lancet Haematol. 2022;9(11):e822–e32. doi: 10.1016/S2352-3026(22)00290-3
- Richardson PG, Trudel S, Popat R, et al. Mezigdomide plus dexamethasone in relapsed and refractory multiple myeloma. N Engl J Med. 2023;389(11):1009–1022. doi: 10.1056/NEJMoa2303194
- Jan M, Sperling AS, Ebert BL. Cancer therapies based on targeted protein degradation - lessons learned with lenalidomide. Nat Rev Clin Oncol. 2021;18(7):401–417. doi: 10.1038/s41571-021-00479-z
- Zhu YX, Braggio E, Shi CX, et al. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–4779. doi: 10.1182/blood-2011-05-356063
- Zhu YX, Braggio E, Shi CX, et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood. 2014;124(4):536–545. doi: 10.1182/blood-2014-02-557819
- Ito T, Ando H, Handa H. Discovery of the target for immunomodulatory drugs (IMiDs). Rinsho Ketsueki. 2016;57(5):556–562. doi: 10.11406/rinketsu.57.556
- Kronke J, Udeshi ND, Narla A, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science. 2014;343(6168):301–305. doi: 10.1126/science.1244851
- Lu G, Middleton RE, Sun H, et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science. 2014;343(6168):305–309. doi: 10.1126/science.1244917
- Kronke J, Hurst SN, Ebert BL. Lenalidomide induces degradation of IKZF1 and IKZF3. Oncoimmunology. 2014;3(7):e941742. doi: 10.4161/21624011.2014.941742
- Matyskiela ME, Zhang W, Man HW, et al. A cereblon modulator (CC-220) with improved degradation of ikaros and Aiolos. J Med Chem. 2018;61(2):535–542. doi: 10.1021/acs.jmedchem.6b01921
- Rasco DW, Papadopoulos KP, Pourdehnad M, et al. A first-in-human study of novel cereblon modulator avadomide (CC-122) in advanced malignancies. Clin Cancer Res. 2019;25(1):90–98. doi: 10.1158/1078-0432.CCR-18-1203
- Costacurta M, He J, Thompson PE, et al. Molecular mechanisms of Cereblon-interacting small molecules in multiple myeloma therapy. J Pers Med. 2021;11(11):1185. doi: 10.3390/jpm11111185
- Sperling AS, Burgess M, Keshishian H, et al. Patterns of substrate affinity, competition, and degradation kinetics underlie biological activity of thalidomide analogs. Blood. 2019;134(2):160–170. doi: 10.1182/blood.2019000789
- Michot J-N, Chavez JC, Carpio C, et al. Clinical activity of CC-99282, a novel, oral small molecule cereblon E3 ligase modulator (CELMoD) agent, in patients (pts) with relapsed or refractory non-hodgkin lymphoma (R/R NHL) - first results from a phase 1, open-label study. Blood. 2021;138(Supplement 1):3574. doi: 10.1182/blood-2021-147333
- Surka C, Jin L, Mbong N, et al. CC-90009, a novel cereblon E3 ligase modulator, targets acute myeloid leukemia blasts and leukemia stem cells. Blood. 2021;137(5):661–677. doi: 10.1182/blood.2020008676
- Xia R, Cheng Y, Han X, et al. Ikaros proteins in tumor: Current perspectives and new developments. Front Mol Biosci. 2021;8:788440. doi: 10.3389/fmolb.2021.788440
- Affar M, Bottardi S, Quansah N, et al. IKAROS: from chromatin organization to transcriptional elongation control. Cell Death Differ. 2023. doi: 10.1038/s41418-023-01212-2
- John LB, Ward AC. The ikaros gene family: transcriptional regulators of hematopoiesis and immunity. Mol Immunol. 2011;48(9–10):1272–1278. doi: 10.1016/j.molimm.2011.03.006
- Bjorklund CC, Lu L, Kang J, et al. Rate of CRL4(CRBN) substrate ikaros and aiolos degradation underlies differential activity of lenalidomide and pomalidomide in multiple myeloma cells by regulation of c-myc and IRF4. Blood Cancer J. 2015;5(10):e354. doi: 10.1038/bcj.2015.66
- Cippitelli M, Stabile H, Kosta A, et al. Role of Aiolos and ikaros in the antitumor and immunomodulatory activity of IMiDs in multiple myeloma: better to lose than to find them. Int J Mol Sci. 2021;22(3):1103. doi: 10.3390/ijms22031103
- Marke R, van Leeuwen FN, Scheijen B. The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia. Haematologica. 2018;103(4):565–574. doi: 10.3324/haematol.2017.185603
- Song C, Gowda C, Pan X, et al. Targeting casein kinase II restores ikaros tumor suppressor activity and demonstrates therapeutic efficacy in high-risk leukemia. Blood. 2015;126(15):1813–1822. doi: 10.1182/blood-2015-06-651505
- Zhang J, Ding L, Holmfeldt L, et al. The genetic basis of early T-cell precursor acute lymphoblastic leukaemia. Nature. 2012;481(7380):157–163. doi: 10.1038/nature10725
- Conserva MR, Redavid I, Anelli L, et al. IKAROS in acute leukemia: a positive influencer or a mean hater? Int J Mol Sci. 2023;24(4):3282. doi: 10.3390/ijms24043282
- Bourgeois W, Cutler JA, Aubrey BJ, et al. Mezigdomide is effective alone and in combination with Menin inhibition in pre-clinical models of KMT2A-r and NPM1c AML. Blood. 2024;143(15):1513–1527. doi: 10.1182/blood.2023021105
- Mulet-Lazaro R, Delwel R. From genotype to Phenotype: how enhancers control gene expression and cell identity in hematopoiesis. Hemasphere. 2023;7(11):e969. doi: 10.1097/HS9.0000000000000969
- Geng CL, Chen JY, Song TY, et al. Lenalidomide bypasses CD28 co-stimulation to reinstate PD-1 immunotherapy by activating notch signaling. Cell Chem Biol. 2022;29(8):1260–72 e8. doi: 10.1016/j.chembiol.2022.05.012
- Kirstetter P, Thomas M, Dierich A, et al. Ikaros is critical for B cell differentiation and function. Eur J Immunol. 2002;32(3):720–730. doi: 10.1002/1521-4141(200203)32:3<720:AID-IMMU720>3.0.CO;2-P
- Powell MD, Read KA, Sreekumar BK, et al. Ikaros zinc finger transcription factors: regulators of cytokine signaling pathways and CD4(+) T helper cell differentiation. Front Immunol. 2019;10:1299. doi: 10.3389/fimmu.2019.01299
- Dumortier A, Kirstetter P, Kastner P, et al. Ikaros regulates neutrophil differentiation. Blood. 2003;101(6):2219–2226. doi: 10.1182/blood-2002-05-1336
- Hung KH, Su ST, Chen CY, et al. Aiolos collaborates with blimp-1 to regulate the survival of multiple myeloma cells. Cell Death Differ. 2016;23(7):1175–1184. doi: 10.1038/cdd.2015.167
- Murakami Y, Kimura-Masuda K, Oda T, et al. MYC causes multiple myeloma progression via attenuating TP53-induced MicroRNA-34 expression. Genes (Basel). 2022;14(1):100. doi: 10.3390/genes14010100
- Fedele PL, Willis SN, Liao Y, et al. IMiDs prime myeloma cells for daratumumab-mediated cytotoxicity through loss of Ikaros and Aiolos. Blood. 2018;132(20):2166–2178. doi: 10.1182/blood-2018-05-850727
- Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164(6):811–821. doi: 10.1111/bjh.12708
- Fionda C, Abruzzese MP, Zingoni A, et al. The IMiDs targets IKZF-1/3 and IRF4 as novel negative regulators of NK cell-activating ligands expression in multiple myeloma. Oncotarget. 2015;6(27):23609–23630. doi: 10.18632/oncotarget.4603
- Shaffer AL, Emre NC, Lamy L, et al. IRF4 addiction in multiple myeloma. Nature. 2008;454(7201):226–231. doi: 10.1038/nature07064
- Lopez-Girona A, Heintel D, Zhang LH, et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol. 2011;154(3):325–336. doi: 10.1111/j.1365-2141.2011.08689.x
- Hideshima T, Ogiya D, Liu J, et al. Immunomodulatory drugs activate NK cells via both zap-70 and cereblon-dependent pathways. Leukemia. 2021;35(1):177–188. doi: 10.1038/s41375-020-0809-x
- Richardson PG, Mateos MV, Vangsted AJ, et al. The role of E3 ubiquitin ligase in multiple myeloma: potential for cereblon E3 ligase modulators in the treatment of relapsed/refractory disease. Expert Rev Proteomics. 2022;19(4–6):235–246. doi: 10.1080/14789450.2022.2142564
- Errico A. Haematological cancer: ikaros–not a myth for myeloma. Nat Rev Clin Oncol. 2014;11(2):65. doi: 10.1038/nrclinonc.2013.237
- Chamberlain PP, Lopez-Girona A, Miller K, et al. Structure of the human cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nat Struct Mol Biol. 2014;21(9):803–809. doi: 10.1038/nsmb.2874
- Fischer ES, Bohm K, Lydeard JR, et al. Structure of the DDB1-CRBN E3 ubiquitin ligase in complex with thalidomide. Nature. 2014;512(7512):49–53. doi: 10.1038/nature13527
- Shortt J, Phimister EG. Mezigdomide and multiple myeloma. N Engl J Med. 2023;389(11):1046–1050. doi: 10.1056/NEJMe2307370
- Pourabdollah M, Bahmanyar M, Atenafu EG, et al. High IKZF1/3 protein expression is a favorable prognostic factor for survival of relapsed/refractory multiple myeloma patients treated with lenalidomide. J Hematol Oncol. 2016;9(1):123. doi: 10.1186/s13045-016-0354-2
- Borsi E, Mazzocchetti G, Dico AF, et al. High levels of CRBN isoform lacking IMiDs binding domain predicts for a worse response to IMiDs-based upfront therapy in newly diagnosed myeloma patients. Clin Exp Med. 2023;23(8):5227–5239. doi: 10.1007/s10238-023-01205-y
- Bolomsky A, Hubl W, Spada S, et al. IKAROS expression in distinct bone marrow cell populations as a candidate biomarker for outcome with lenalidomide-dexamethasone therapy in multiple myeloma. Am J Hematol. 2017;92(3):269–278. doi: 10.1002/ajh.24634
- Dimopoulos K, Fibiger Munch-Petersen H, Winther Eskelund C, et al. Expression of CRBN, IKZF1, and IKZF3 does not predict lenalidomide sensitivity and mutations in the cereblon pathway are infrequent in multiple myeloma. Leuk Lymphoma. 2019;60(1):180–188. doi: 10.1080/10428194.2018.1466290
- Ng YLD, Ramberger E, Bohl SR, et al. Proteomic profiling reveals CDK6 upregulation as a targetable resistance mechanism for lenalidomide in multiple myeloma. Nat Commun. 2022;13(1):1009. doi: 10.1038/s41467-022-28515-1
- Tachita T, Kinoshita S, Ri M, et al. Expression, mutation, and methylation of cereblon-pathway genes at pre- and post-lenalidomide treatment in multiple myeloma. Cancer Sci. 2020;111(4):1333–1343. doi: 10.1111/cas.14352
- Kronke J, Kuchenbauer F, Kull M, et al. IKZF1 expression is a prognostic marker in newly diagnosed standard-risk multiple myeloma treated with lenalidomide and intensive chemotherapy: a study of the German myeloma study group (DSMM). Leukemia. 2017;31(6):1363–1367. doi: 10.1038/leu.2016.384
- Kronke J, Knop S, Langer C. Prognostic impact of ikaros expression in lenalidomide-treated multiple myeloma. Oncotarget. 2017;8(63):106163–106164. doi: 10.18632/oncotarget.22572
- Misiewicz-Krzeminska I, de Ramon C, Corchete LA, et al. Quantitative expression of ikaros, IRF4, and PSMD10 proteins predicts survival in VRD-treated patients with multiple myeloma. Blood Adv. 2020;4(23):6023–6033. doi: 10.1182/bloodadvances.2020002711
- Kriegsmann K, Baertsch MA, Awwad MHS, et al. Cereblon-binding proteins expression levels correlate with hyperdiploidy in newly diagnosed multiple myeloma patients. Blood Cancer J. 2019;9(2):13. doi: 10.1038/s41408-019-0174-z
- Chretien ML, Corre J, Lauwers-Cances V, et al. Understanding the role of hyperdiploidy in myeloma prognosis: which trisomies really matter? Blood. 2015;126(25):2713–2719. doi: 10.1182/blood-2015-06-650242
- Watson ER, Novick S, Matyskiela ME, et al. Molecular glue CELMoD compounds are regulators of cereblon conformation. Science. 2022;378(6619):549–553. doi: 10.1126/science.add7574
- Sperling AS, Guerra VA, Kennedy JA, et al. Lenalidomide promotes the development of TP53-mutated therapy-related myeloid neoplasms. Blood. 2022;140(16):1753–1763. doi: 10.1182/blood.2021014956
- D’Souza C, Prince HM, Neeson PJ. Understanding the role of T-Cells in the antimyeloma effect of immunomodulatory drugs. Front Immunol. 2021;12:632399. doi: 10.3389/fimmu.2021.632399
- Thakurta A, Pierceall WE, Amatangelo MD, et al. Developing next generation immunomodulatory drugs and their combinations in multiple myeloma. Oncotarget. 2021;12(15):1555–1563. doi: 10.18632/oncotarget.27973
- Quach H, Ritchie D, Stewart AK, et al. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24(1):22–32. doi: 10.1038/leu.2009.236
- Hayashi T, Hideshima T, Akiyama M, et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: clinical application. Br J Haematol. 2005;128(2):192–203. doi: 10.1111/j.1365-2141.2004.05286.x
- Galustian C, Meyer B, Labarthe MC, et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol Immunother. 2009;58(7):1033–1045. doi: 10.1007/s00262-008-0620-4
- Mougiakakos D, Bach C, Bottcher M, et al. The IKZF1-IRF4/IRF5 axis controls polarization of myeloma-associated macrophages. Cancer Immunol Res. 2021;9(3):265–278. doi: 10.1158/2326-6066.CIR-20-0555
- Costa F, Vescovini R, Bolzoni M, et al. Lenalidomide increases human dendritic cell maturation in multiple myeloma patients targeting monocyte differentiation and modulating mesenchymal stromal cell inhibitory properties. Oncotarget. 2017;8(32):53053–53067. doi: 10.18632/oncotarget.18085
- Jian Y, Gao W, Geng C, et al. Arsenic trioxide potentiates sensitivity of multiple myeloma cells to lenalidomide by upregulating cereblon expression levels. Oncol Lett. 2017;14(3):3243–3248. doi: 10.3892/ol.2017.6502
- Lopez-Girona A, Havens CG, Lu G, et al. CC-92480 is a novel cereblon E3 ligase modulator with enhanced tumoricidal and immunomodulatory activity against sensitive and resistant multiple myeloma cells. Blood. 2019;134(Supplement_1):1812. doi: 10.1182/blood-2019-124338
- Bjorklund CC, Kang J, Amatangelo M, et al. Iberdomide (CC-220) is a potent cereblon E3 ligase modulator with antitumor and immunostimulatory activities in lenalidomide- and pomalidomide-resistant multiple myeloma cells with dysregulated CRBN. Leukemia. 2020;34(4):1197–1201. doi: 10.1038/s41375-019-0620-8
- Amatangelo M, Bjorklund CC, Hagner P, et al. P-230: preclinical and translational biomarker analysis to support further clinical development and dose optimization of mezigdomide (MEZI; CC-92480) in combination with either bortezomib or carfilzomib. Clin Lymphoma Myeloma Leukemia. 2022;22(Supplement):S161–S2. doi: 10.1016/S2152-2650(22)00560-2
- Van Oekelen O, Amatangelo M, Guo M, et al. Large-scale mass cytometry reveals significant activation of innate and adaptive immunity in bone marrow tumor microenvironment of iberdomide-treated myeloma patients. Blood. 2021;138(Supplement 1):730. doi: 10.1182/blood-2021-151542
- Ye Y, Gaudy A, Schafer P, et al. First-in-human, single- and multiple-ascending-dose studies in healthy subjects to assess pharmacokinetics, pharmacodynamics, and safety/tolerability of iberdomide, a novel cereblon E3 ligase modulator. Clin Pharmacol Drug Dev. 2021;10(5):471–485. doi: 10.1002/cpdd.869
- Chen LY, Van Oekelen O, Amatangelo M, et al. Mezigdomide treatment in relapsed-refractory myeloma patients shifts bone marrow NK and T cell populations from exhaustion to activation. Blood. 2023;142(Supplement 1):4686. doi: 10.1182/blood-2023-187748
- Chiu H, Zhao J, Ortiz Estevez M, et al. Mezigdomide reverses T-Cell exhaustion through degradation of aiolos/ikaros and reinvigoration of cytokine production pathways. Blood. 2023;142(Supplement 1):335. doi: 10.1182/blood-2023-189445
- Gaffney B, Shi Y, de Jong P, et al. Mezigdomide (CC-92480), a novel cereblon E3 ligase modulator, induces vulnerability of multiple myeloma cells to T-Cell-mediated killing. Blood. 2022;140(Supplement 1):7108–7109. doi: 10.1182/blood-2022-157939
- Wong L, Krishna Narla R, Leisten J, et al. CC-92480, a novel cereblon E3 ligase modulator, is synergistic with dexamethasone, bortezomib, and daratumumab in multiple myeloma. Blood. 2019;134(Supplement_1):1815. doi: 10.1182/blood-2019-124345
- Chow TT, Amatangelo M, Ma P, et al. Preclinical and translational biomarker analyses to inform clinical development of mezigdomide (CC-92480) in combination with dexamethasone and daratumumab in multiple myeloma. Blood. 2023;142(Supplement 1):3318. doi: 10.1182/blood-2023-178881
- Ma P, Sridharan V, Wollerman K, et al. Iberdomide enhances dara mediated cytotoxicity through upregulation of CDC activity and elevated NK cell mediated ADCC. Blood. 2023;142(Supplement 1):3289. doi: 10.1182/blood-2023-189423
- Paiva B, Gaffney B, Burnett K, et al. Synergistic antitumor activity of alnuctamab (ALNUC; BMS-986349; CC-93269), a BCMA 2+1 T cell engager (TCE), and celmod agents in multiple myeloma (MM) preclinical models. Blood. 2022;140(Supplement 1):7054–7055. doi: 10.1182/blood-2022-157987
- Eckmann J, Hage C, Stefanie L, et al. Early intervention with Celmods, but not imids, prevents relapse to forimtamig driven by GPRC5D-Negative myeloma cells. Blood. 2023;142(Supplement 1):4659. doi: 10.1182/blood-2023-174253
- Aleman A, Kogan‑Zajdman A, Upadhyaya B, et al. Improving anti‑BCMA CAR‑T functionality with novel immunomodulatory agent iberdomide (CC220) in multiple myeloma. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S131–S2. doi: 10.1016/S2152-2650(23)01793-7
- Bjorklund CC, Amatangelo M, Chiu H, et al. Pre-clinical and clinical immunomodulatory effects of pomalidomide or CC-92480 in combination with bortezomib in multiple myeloma. Blood. 2021;138(Supplement 1):1613. doi: 10.1182/blood-2021-153994
- Bjorklund CC, Amatangelo M, Kang J, et al. CC-92480 enhances cell-autonomous cytotoxicity through blockade of G 2/M transition when combined with bortezomib/dexamethasone in pre-clinical multiple myeloma. Blood. 2021;138(Supplement 1):2669. doi: 10.1182/blood-2021-153907
- Jeyaraju DV, Alapa M, O’Donohue A, et al. Suppression of myeloid cell-derived proinflammatory cytokines with celmod agents: implications for CRS with T-Cell engagers (TCEs). Blood. 2022;140(Supplement 1):7070–7071. doi: 10.1182/blood-2022-157927
- Henderson JA, Eron SJ, Good A, et al. Abstract ND13: the discovery and characterization of CFT7455: a potent and selective degrader of IKZF1/3 for the treatment of relapsed/refractory multiple myeloma. Cancer Res. 2022;82(12_Supplement):ND13. doi: 10.1158/1538-7445.AM2022-ND13
- Lonial S, Richard S, Matous JV, et al. Pharmacokinetic (PK) profile of a novel IKZF1/3 degrader, CFT7455, enables significant potency advantage over other IKZF1/3 degraders in models of multiple myeloma (MM) and the results of the initial treatment cohort from a first-in-human (FIH) phase 1/2 study of CFT7455 in MM. Cancer Res. 2022;82(12_Supplement):CT186. doi: 10.1158/1538-7445.AM2022-CT186
- Arp CJ, Reynders M, Sreekanth V, et al. Photoswitchable molecular glues enable optical control of transcription factor degradation. bioRxiv. 2023. doi: 10.1101/2023.04.09.536172
- Koduri V, Duplaquet L, Lampson BL, et al. Targeting oncoproteins with a positive selection assay for protein degraders. Sci Adv. 2021;7(6):eabd6263. doi: 10.1126/sciadv.abd6263
- Liu XP, He L, Zhang QP, et al. Baicalein inhibits proliferation of myeloma U266 cells by downregulating IKZF1 and IKZF3. Med Sci Monit. 2018;24:2809–2817. doi: 10.12659/MSM.907058
- Meng F, Xu C, Park KS, et al. Discovery of a first-in-class degrader for nuclear receptor binding SET domain protein 2 (NSD2) and ikaros/aiolos. J Med Chem. 2022;65(15):10611–10625. doi: 10.1021/acs.jmedchem.2c00807
- Bird S, Pawlyn C. IMiD resistance in multiple myeloma: current understanding of the underpinning biology and clinical impact. Blood. 2023;142(2):131–140. doi: 10.1182/blood.2023019637
- Gooding S, Ansari-Pour N, Kazeroun M, et al. Loss of COP9 signalosome genes at 2q37 is associated with IMiD resistance in multiple myeloma. Blood. 2022;140(16):1816–1821. doi: 10.1182/blood.2022015909
- Bird S, Xu Y, Sialana F, et al. P-179 altered lipid metabolism in IMiD/CELMoD resistant multiple myeloma confers novel and targetable vulnerabilities. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S134. doi: 10.1016/S2152-2650(23)01797-4
- Gooding S, Ansari-Pour N, Towfic F, et al. Multiple cereblon genetic changes are associated with acquired resistance to lenalidomide or pomalidomide in multiple myeloma. Blood. 2021;137(2):232–237. doi: 10.1182/blood.2020007081
- Tilmont R, Maity R, Leblay N, et al. CRBN structural changes, copy number changes and COP9 signalosome subunits gene expression mediate sensitivity to new celmod compound CC-92480 in multiple myeloma patients. Blood. 2022;140(Supplement 1):9964–9965. doi: 10.1182/blood-2022-169550
- Chrisochoidou Y, LeBihan Y-V, Morales S, et al. Investigating the functional impact of CRBN mutations on response to IMiD/Celmod agents in myeloma. Blood. 2023;142(Supplement 1):753. doi: 10.1182/blood-2023-179986
- Barrio S, Munawar U, Zhu YX, et al. IKZF1/3 and CRL4(CRBN) E3 ubiquitin ligase mutations and resistance to immunomodulatory drugs in multiple myeloma. Haematologica. 2020;105(5):e237–e41. doi: 10.3324/haematol.2019.217943
- Zhou N, Gutierrez-Uzquiza A, Zheng XY, et al. RUNX proteins desensitize multiple myeloma to lenalidomide via protecting IKZFs from degradation. Leukemia. 2019;33(8):2006–2021. doi: 10.1038/s41375-019-0403-2
- Nguyen TV. USP15 antagonizes CRL4(CRBN)-mediated ubiquitylation of glutamine synthetase and neosubstrates. Proc Natl Acad Sci USA. 2021;118(40):e2111391118. doi: 10.1073/pnas.2111391118
- Li Y, Barber A, Martin S, et al. EZH2 inhibition overcomes immunomodulatory drug (IMiD) resistance in multiple myeloma cell lines in a cereblon pathway dependent manner. Blood. 2023;142(Supplement 1):4187. doi: 10.1182/blood-2023-186614
- Osada N, Kikuchi J, Iha H, et al. c-FOS is an integral component of the IKZF1 transactivator complex and mediates lenalidomide resistance in multiple myeloma. Clin Transl Med. 2023;13(8):e1364. doi: 10.1002/ctm2.1364
- Barwick BG, Neri P, Bahlis NJ, et al. Multiple myeloma immunoglobulin lambda translocations portend poor prognosis. Nat Commun. 2019;10(1):1911. doi: 10.1038/s41467-019-09555-6
- Durie BGM, Hoering A, Sexton R, et al. Longer term follow-up of the randomized phase III trial SWOG S0777: bortezomib, lenalidomide and dexamethasone vs. lenalidomide and dexamethasone in patients (pts) with previously untreated multiple myeloma without an intent for immediate autologous stem cell transplant (ASCT). Blood Cancer J. 2020;10(5):53. doi: 10.1038/s41408-020-0311-8
- Richardson PG, Jacobus SJ, Weller EA, et al. Triplet therapy, transplantation, and maintenance until progression in myeloma. N Engl J Med. 2022;387(2):132–147. doi: 10.1056/NEJMoa2204925
- Voorhees PM, Sborov DW, Laubach J, et al. Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol. 2023;10(10):e825–e37. doi: 10.1016/S2352-3026(23)00217-X
- Facon T, Kumar SK, Plesner T, et al. Daratumumab, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone alone in newly diagnosed multiple myeloma (MAIA): overall survival results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(11):1582–1596. doi: 10.1016/S1470-2045(21)00466-6
- Miguel JS, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(11):1055–1066. doi: 10.1016/S1470-2045(13)70380-2
- Richardson PG, Oriol A, Beksac M, et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): a randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(6):781–794. doi: 10.1016/S1470-2045(19)30152-4
- Dimopoulos MA, Terpos E, Boccadoro M, et al. Subcutaneous daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone in patients with relapsed or refractory multiple myeloma (APOLLO): extended follow up of an open-label, randomised, multicentre, phase 3 trial. Lancet Haematol. 2023;10(10):e813–e24. doi: 10.1016/S2352-3026(23)00218-1
- Richardson PG, Perrot A, San-Miguel J, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): follow-up analysis of a randomised, phase 3 study. Lancet Oncol. 2022;23(3):416–427. doi: 10.1016/S1470-2045(22)00019-5
- Dimopoulos MA, Terpos E, Boccadoro M, et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): an open-label, randomised, phase 3 trial. Lancet Oncol. 2021;22(6):801–812. doi: 10.1016/S1470-2045(21)00128-5
- Attal M, Richardson PG, Rajkumar SV, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394(10214):2096–2107. doi: 10.1016/S0140-6736(19)32556-5
- van de Donk NWCJ, Touzeau C, Terpos E, et al. Iberdomide maintenance after autologous stem-cell transplantation in newly diagnosed MM: first results of the phase 2 EMN26 study. Blood. 2023;142(Supplement 1):abstract 208. doi: 10.1182/blood-2023-177564
- Biran N, Vesole DH, Parmar H, et al. A phase 1/2 study of carfilzomib, Iberdomide and dexamethasone (KID) in patients with newly diagnosed transplant-eligible multiple myeloma. Blood. 2023;142(Supplement 1):abstract 2022. doi: 10.1182/blood-2023-1899072022-2022
- Lonial S, Abdallah A-O, Anwer F, et al. Iberdomide (IBER) in combination with dexamethasone (DEX) in relapsed/refractory multiple myeloma (RRMM): results from the anti-B-Cell maturation antigen (BCMA)-exposed cohort of the CC-220-MM-001 trial. Blood. 2022;140(Supplement 1):4398–4400. doi: 10.1182/blood-2022-158180
- Lonial S, Richardson PG, Popat R, et al. Iberdomide in combination with dexamethasone and daratumumab, bortezomib, or carfilzomib in patients with relapsed/refractory multiple myeloma. Hemasphere. 2021;5(suppl 2):S187. doi: 10.1097/HS9.0000000000000566
- Oriol A, Sandhu I, Raab M, et al. OA-49 mezigdomide (MEZI) plus dexamethasone (DEX) and bortezomib (BORT) or carfilzomib (CFZ) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): results from the CC-92480-MM-002 trial. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S31. doi: 10.1016/S2152-2650(23)01616-6
- Richardson PG, Sandhu I, Hofmeister CC, et al. Mezigdomide (MEZI) plus dexamethasone (DEX) and Daratumumab (DARA) or elotuzumab (ELO) in patients (pts) with relapsed/refractory multiple myeloma (RRMM): results from the CC-92480-MM-002 trial. Blood. 2023;142(Supplement 1):1013. doi: 10.1182/blood-2023-174443
- Goldsmith S, Oriol A, Anttila P, et al. P-265 mezigdomide (MEZI) monotherapy in relapsed/refractory multiple myeloma (RRMM): results from the CC-92480-MM-001 trial. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S181–S2. doi: 10.1016/S2152-2650(23)01883-9
- White D, Lipe B, Gironella Mesa M, et al. Iberdomide, bortezomib, and dexamethasone (IberVd) in transplant‑ineligible newly diagnosed multiple myeloma (NDMM): results from the CC‑220‑MM‑001 trial. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S25. doi: 10.1016/S2152-2650(23)01608-7
- Wong L, Lamba M, Jiménez Nuñez MD, et al. Dose- and schedule-dependent immunomodulatory effects of the novel celmod agent CC-92480 in patients with relapsed/refractory multiple myeloma. Blood. 2020;136(Supplement 1):47–48. doi: 10.1182/blood-2020-137161
- Prabhala R, Pierceall WE, Samur M, et al. Immunomodulation of NK, NKT and B/T cell subtypes in relapsed/refractory multiple myeloma patients treated with pomalidomide along with velcade and dexamethasone and its association with improved progression-free survival. Front Oncol. 2023;13:1271807. doi: 10.3389/fonc.2023.1271807
- Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23(11):2147–2152. doi: 10.1038/leu.2009.147
- Dimopoulos MA, Swern AS, Li JS, et al. Efficacy and safety of long-term treatment with lenalidomide and dexamethasone in patients with relapsed/refractory multiple myeloma. Blood Cancer J. 2014;4(11):e257. doi: 10.1038/bcj.2014.77
- van de Donk N, White D, Lipe B, et al. Iberdomide (IBER) plus dexamethasone (DEX) in patients with relapsed/refractory multiple myeloma (RRMM): a safety analysis from the CC‑220‑MM‑001 trial. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S214–S5. doi: 10.1016/S2152-2650(23)01938-9
- Pal R, Monaghan SA, Hassett AC, et al. Immunomodulatory derivatives induce PU.1 down-regulation, myeloid maturation arrest, and neutropenia. Blood. 2010;115(3):605–614. doi: 10.1182/blood-2009-05-221077
- Liu A, Li S, Donnenberg V, et al. Immunomodulatory drugs downregulate IKZF1 leading to expansion of hematopoietic progenitors with concomitant block of megakaryocytic maturation. Haematologica. 2018;103(10):1688–1697. doi: 10.3324/haematol.2018.188227
- Kumar SK, Callander NS, Alsina M, et al. NCCN guidelines insights: multiple myeloma, version 3.2018. J Natl Compr Canc Netw. 2018;16(1):11–20. doi: 10.6004/jnccn.2018.0002
- Dytfeld D, Wrobel T, Jamroziak K, et al. Carfilzomib, lenalidomide, and dexamethasone or lenalidomide alone as maintenance therapy after autologous stem-cell transplantation in patients with multiple myeloma (ATLAS): interim analysis of a randomised, open-label, phase 3 trial. Lancet Oncol. 2023;24(2):139–150. doi: 10.1016/S1470-2045(22)00738-0
- Nooka AK, Kaufman JL, Muppidi S, et al. Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia. 2014;28(3):690–693. doi: 10.1038/leu.2013.335
- Leypoldt LB, Tichy D, Besemer B, et al. Isatuximab, carfilzomib, Lenalidomide, and dexamethasone for the treatment of high-risk newly diagnosed multiple myeloma. J Clin Oncol. 2024;42(1):26–37. doi: 10.1200/JCO.23.01696
- Ravi G, Bal S, Joiner L, et al. Subsequent therapy and outcomes in patients with newly diagnosed multiple myeloma experiencing disease progression after quadruplet combinations. Br J Haematol. 2024;204(4):1300–1306. doi: 10.1111/bjh.19303
- Gay F, Dimopoulos M, Huang X, et al. A phase 3, two‑stage, randomized study of iberdomide maintenance versus lenalidomide maintenance post autologous stem cell transplantation in newly diagnosed multiple myeloma: EXCALIBER‑maintenance. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 2):S111. doi: 10.1016/S2152-2650(23)01755-X
- Lonial S, Quach H, Dimopoulos MA, et al. EXCALIBER-RRMM: a phase 3, two-stage study of iberdomide, daratumumab, and dexamethasone (IberDd) versus daratumumab, bortezomib, and dexamethasone (DVd) in patients (pts) with relapsed/refractory multiple myeloma (RRMM). J Clin Oncol. 2023;41(16_suppl):TPS8069. doi: 10.1200/JCO.2023.41.16_suppl.TPS8069
- Richardson PG, Badelita SN, Besemer B, et al. MM-372 a phase III, two-stage, randomized study of mezigdomide, bortezomib, and dexamethasone (MeziVd) versus pomalidomide, bortezomib, and dexamethasone (PVd) in relapsed/refractory multiple myeloma (RRMM): SUCCESSOR-1. Clin Lymphoma Myeloma Leukemia. 2023;23(Supplement 1):S495–S6. doi: 10.1016/S2152-2650(23)01446-5
- Richardson P, Amatangelo M, Berenson J, et al. A phase 3, two-stage, randomized study of mezigdomide, carfilzomib, and dexamethasone (MeziKd) versus carfilzomib and dexamethasone (kd) in relapsed/refractory multiple myeloma (RRMM): SUCCESSOR-2. J Clin Oncol. 2023;42(Supplement):TPS8070. doi: 10.1200/JCO.2023.41.16_suppl.TPS8070