ABSTRACT
Introduction: Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a complication that is typically associated with conditioning for hematopoietic stem cell transplantation (HSCT). In patients with concomitant multi-organ dysfunction, mortality may be >80%. Recently, the European Society for Blood and Marrow Transplantation established separate criteria for diagnosis and severity of VOD/SOS for adults and children, to better reflect current understanding of the disease.
Areas covered: This review provides an overview of post-HSCT hepatic VOD/SOS and defibrotide, including its pharmacological, clinical, and regulatory profile. In children and adults following HSCT, defibrotide is approved for the treatment of hepatic VOD/SOS with concomitant renal or pulmonary dysfunction in the United States and for the treatment of severe hepatic VOD/SOS in the European Union. Day +100 survival rates with defibrotide are superior to those of historical controls receiving best supportive care only, and safety profiles are similar.
Expert commentary: Defibrotide appears to act through multiple mechanisms to restore thrombo-fibrinolytic balance and protect endothelial cells, and there are promising data on the use of defibrotide for VOD/SOS prophylaxis in high-risk children undergoing HSCT. An ongoing randomized controlled trial in children and adults will better assess the clinical value of defibrotide as a preventive medication.
1. Introduction
Hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) is a serious complication of hematopoietic stem cell transplantation (HSCT) that causes significant morbidity and, outside of relapse, it is one of the principal causes of transplant-related mortality [Citation1]. For optimal diagnosis and treatment, it is of pivotal importance for physicians to be aware of the substantial and newly recognized differences in VOD/SOS between adult and pediatric patients. Adults with VOD/SOS typically present with fluid retention, ascites, jaundice, weight gain, and painful hepatomegaly, with no other identifiable cause for the hepatic disease [Citation2]. One of the most important features in pediatric patients with VOD/SOS is that up to one-third present without hyperbilirubinemia; this is also the case in severe VOD/SOS [Citation3].
Patients with severe VOD/SOS experience cerebral, renal, or respiratory comorbidities indicative of life-threatening MOD [Citation4]. A 2010 meta-analysis of 19 studies noted an overall mortality rate of >80% for patients with severe VOD/SOS [Citation5]. More recent data show that compared with patients without VOD/SOS, patients with severe VOD/SOS after HSCT have a 5.9-fold higher risk of inpatient mortality and significantly higher healthcare utilization and costs [Citation6]. Studies found a fourfold increased Day +100 mortality in children with the diagnosis VOD/SOS post-HSCT, independent of retroactively assessed severity [Citation7,Citation8].
A meta-analysis of 135 studies that included nearly 25,000 recipients of HSCT between 1979 and 2007 found a 14% mean incidence of VOD/SOS (range, 0–62%) [Citation5]. The incidence of VOD/SOS ranged from 10 to 60% following allogeneic HSCT with myeloablative conditioning regimens and from 5 to 30% following autologous HSCT [Citation3]. More recently, the use of reduced intensity conditioning (RIC) regimens has decreased the incidence of VOD/SOS [Citation3,Citation9], although VOD/SOS still occurs and has been reported in 9% of patients in an RIC setting [Citation10].
In contrast, the incidence of VOD/SOS in children averages to approximately 20% across various studies, with reported incidences as high as 60% in high-risk populations (e.g. infantile osteopetrosis) [Citation8,Citation11,Citation12], making VOD/SOS an important transplant complication of childhood. VOD/SOS has also been reported in children with Wilms tumor, rhabdomyosarcoma, and brain tumors treated with conventional chemotherapy, particularly combination therapies including actinomycin D [Citation3,Citation8,Citation13–Citation16].
In addition to differences between populations, another reason that reported prevalence of VOD/SOS varies in the literature is the use of different diagnostic criteria: the Baltimore criteria (bilirubin ≥2 mg/dL plus 2 or more of ascites, ≥5% weight gain, and hepatomegaly) and the modified Seattle criteria (2 or more of bilirubin >2 mg/dL, hepatomegaly or right upper quadrant pain, and weight gain >2% of baseline), both of which require diagnosis within the first 3 weeks post-HSCT [Citation3–Citation5,Citation17,Citation18]. These criteria are more than 2 decades old, and thus predate important changes in the understanding of the disease (e.g. onset after Day +21, which may occur in 15–20% of cases, and presentation without hyperbilirubinemia in pediatric patients) and in practice (e.g. the widespread use of RIC and the availability of effective therapy) [Citation2,Citation3,Citation19]. Therefore, the European Society for Blood and Marrow Transplantation (EBMT) has proposed updated diagnostic and severity grading criteria for adults [Citation2] and for pediatric patients [Citation3]. Of note, in response to the missing focus of the established criteria on pediatric characteristics, the EBMT has proposed criteria for diagnosis and severity grading specifically for children, which have been separated from the new criteria specifically for adults [Citation2,Citation3].
The new EBMT criteria for diagnosis of VOD/SOS in adults recognize two forms of the disease based on timing of onset () [Citation2]. Classical (early onset) VOD/SOS occurs during the first 21 days post-HSCT, as with the Baltimore criteria, and late-onset VOD/SOS occurs >21 days post-HSCT. The EBMT new classification criteria for grading of suspected VOD/SOS in adults are based on onset of first clinical symptoms, bilirubin levels and kinetics (doubling within 48 h is a stand-alone criterion for severe disease), transaminases, weight increase, and renal function () [Citation2]. The VOD/SOS EBMT grading system, which needs to be validated in prospective clinical studies, parallels the 5 categories used in the Common Terminology Criteria for Adverse Events (CTCAE): mild (grade 1), moderate (grade 2), severe (grade 3), very severe (grade 4), and death (grade 5). Important contributions of the new adult diagnostic criteria, including recognition of late-onset VOD/SOS beyond Day +21 and the standardized grading of VOD/SOS, will facilitate prospective assessment of these patients and the monitoring of their responses to treatment.
Table 1. New EBMT criteria for VOD/SOS diagnosis in children and adults.
Table 2. EBMT grading criteria for VOD/SOS in children and adults.
The EBMT criteria for diagnosis of VOD/SOS in pediatric patients have no limit for timing of VOD/SOS onset and require the presence of ≥2 of the criteria listed in [Citation3]. The EBMT criteria properly reflect, for the first time, the distinct clinical presentation of VOD/SOS in pediatric patients. Persistent refractory thrombocytopenia, an observation already made by McDonald et al. in their seminal paper [Citation18] and by many others [Citation17,Citation20,Citation21] is now recognized as a sensitive marker for early diagnosis in children. Persistence of platelet transfusion dependency is a marker for active disease and severe VOD/SOS. Of note, a fixed level of hyperbilirubinemia is not required, because up to a third of children do not present with this symptom even in severe disease. Also, weight gain is no longer fixed arbitrarily at 2 or 5% (modified Seattle and Baltimore criteria, respectively). The dynamics of the disease are reflected in a steady rise of bilirubin and/or a weight gain despite proper fluid intake and use of diuretics, both over 3 consecutive days. The specificity of the criteria is raised by introducing reproducibility via imaging (ultrasound suggested) of hepatomegaly and/or ascites.
Similar to the adult criteria, the pediatric VOD/SOS EBMT grading system parallels the 5 categories used in the CTCAE (). Indicators for severe disease include liver function tests (aspartate transaminase, alanine transaminase, glutamate dehydrogenase), bilirubin levels and kinetics (doubling within 48 h of bilirubin kinetics is an indicator of very severe VOD/SOS), severe and productive ascites necessitating paracentesis for relief of imminent respiratory insufficiency, and coagulopathy needing replacement. Renal deficit, pulmonary insufficiency, and new onset cognitive impairment reflect VOD/SOS-associated MOD [Citation3]. Thus, the newly established pediatric criteria for diagnosis best reflect the particularities in children and raise the sensitivity for early diagnosis and prompt therapeutic intervention.
In both adults and children, the pathophysiology of VOD/SOS is linked to endothelial cell (EC) damage and hepatocellular injury, believed to be caused by the toxic metabolites generated by the conditioning regimen used to prepare patients for HSCT [Citation4]. In addition to HSCT, VOD/SOS can also be a complication of conventional radiation therapy and high-dose chemotherapy, especially in children [Citation3], and has been described in the setting of exposure to pyrrolizidine alkaloids, or after solid-organ transplantation [Citation19,Citation22]. The VOD/SOS pathophysiologic cascade promotes a prothrombotic-hypofibrinolytic state, which involves increases in von Willebrand factor and plasminogen activator inhibitor, as well as decreases in thrombomodulin and tissue plasminogen activator [Citation23]. Injured ECs round up, creating gaps in the sinusoidal barrier, and allowing red blood cells, leukocytes, and cellular debris into the space of Disse, promoting sloughing of sinusoidal cells, which embolize downstream, causing obstruction of sinusoidal flow [Citation19]. Thickening of the subintimal zone, sclerosis, and narrowing of the venular lumen contribute to further deterioration of the vasculature [Citation23]. The pathophysiologic cascade may progress to portal hypertension, hepatorenal syndrome, obliteration of terminal hepatic venules, and multiorgan dysfunction (MOD; sometimes called multiorgan failure) [Citation18,Citation21].
The main risk factors for VOD/SOS can be grouped into four categories: transplant related, patient/disease related, hepatic related, and pediatric specific [Citation19]. Careful assessment and screening for risk factors such as obesity, alcoholism, preexisting liver function testing abnormalities, previous imaging abnormalities, and history of viral hepatitis are warranted prior to HSCT [Citation24].
1.1. Overview of treatments for VOD/SOS
1.1.1. Prevention
The two main approaches to prevent VOD/SOS due to patient-related and transplant-related risk factors are to address/reverse risk factors and to use pharmacologic prevention/prophylaxis [Citation1,Citation2,Citation22]. Patient-related risk factors include age extreme, Karnofsky status score below 90%, metabolic syndrome, advanced disease, thalassemia, genetic factors, and hepatic dysfunction [Citation2]. Measures to address patient-related reversible risk factors include reducing iron overload in patients with thalassemia [Citation22] and delaying HSCT until resolution of reversible conditions such as hepatitis and other active disease [Citation2]. Also, for patient-related risk factors involving age and comorbidities, measures to reduce the incidence of VOD/SOS include use of RIC regimens, especially in older adults and adults with comorbidities or who are heavily pretreated [Citation22].
Transplant-related risk factors include unrelated and/or mismatched donor, and use of pretransplant therapy or conditioning known to be toxic to the endothelium (e.g. busulfan) [Citation2]. Measures to address transplant-related risk factors include avoidance of cyclophosphamide with high-dose busulfan (and other high-risk drugs); monitoring of busulfan pharmacokinetics to target a specific therapeutic window; use of intravenous (IV) instead of oral busulfan dosing; and reduced dosing for children [Citation2,Citation9,Citation22]. However, although these measures may reduce the incidence of VOD/SOS, it may still occur after RIC or IV busulfan [Citation10,Citation16,Citation25] or in children with certain high-risk diseases [Citation26].
Pharmacologic prevention entails avoidance of hepatotoxic and nephrotoxic drugs, if possible [Citation2,Citation27], and aggressive fluid management [Citation1]. Currently, there are no drugs with regulatory-approved indications for VOD/SOS prophylaxis, and evidence for most therapies is limited [Citation28]. Use of heparin prophylaxis is controversial: a 2006 meta-analysis of 12 studies found no statistically significant reduction in VOD/SOS risk [Citation29]. However, 2 of 3 randomized controlled trials (RCTs) included in the meta-analysis reported a significant decrease in the incidence of VOD/SOS [Citation19,Citation30–Citation32]. Data on prophylaxis with ursodeoxycholic acid are inconclusive; some studies found a significant benefit, and others have not found a benefit in reducing the incidence of VOD/SOS [Citation19,Citation25,Citation33,Citation34]. Of note, ursodeoxycholic acid reduces bilirubin levels via stimulation of bile acid secretion, but this change in bilirubin may not be therapeutic [Citation35]. As discussed later in this manuscript, a pediatric phase III RCT of prophylaxis in high-risk pediatric patients found reduced incidence of VOD/SOS in patients undergoing HSCT receiving prophylactic defibrotide [Citation26].
1.1.2. Treatment
Defibrotide is the only medication with an approved indication for the treatment of post-HSCT severe hepatic VOD/SOS in the European Union and hepatic VOD/SOS with pulmonary or renal dysfunction in the USA [Citation19]. In particular, in children, it was demonstrated that early use of defibrotide was associated with a better outcome and overall survival [Citation36,Citation37]. The recommended dose is 6.25 mg/kg given every 6 h (25 mg/kg/day) as a 2-h IV infusion administered for ≥21 days and, if symptoms of VOD/SOS have not resolved, continue treatment until resolution of signs/symptoms of VOD/SOS [Citation38] (up to 60 days) [Citation39]. This evidence is reflected in the new pediatric criteria so that the use of this effective medication is moved to ‘almost first suspicion’ of post-HSCT VOD/SOS [Citation3].
In addition, patients are started on supportive care to minimize extracellular fluid accumulation without exacerbating renal damage, with the goal of maintaining baseline weight [Citation4]. Fluids and sodium balance is managed with cautious use of diuretics (e.g. spironolactone, furosemide); hepatotoxic medications (e.g. paracetamol for pain control) should be avoided, if possible [Citation19]. Symptomatic measures to reduce discomfort caused by sizable ascites or pleural effusions include oxygen therapy in conjunction with analgesia [Citation1,Citation19]. Particularly in children, paracentesis and rarely thoracentesis, as appropriate, are the most effective measures to avoid ventilator support due to mechanical obstruction. Patients with fluid accumulation and uncontrolled renal failure require hemodialysis/hemofiltration; MOD requires transfer to an intensive care unit [Citation1,Citation19]. In rare cases, supportive care with a transjugular intrahepatic portosystemic shunt may be considered for patients with less advanced VOD/SOS and there are a few case reports of hepatic transplantation in the most severe diseases [Citation19].
2. Introduction to defibrotide
2.1. Chemistry
Chemically, defibrotide is a sodium salt of polydeoxyribonucleotide. Produced by the controlled depolymerization of porcine intestinal mucosal DNA, defibrotide is composed of 90% single-stranded phosphodiester oligonucleotides (average length 50mer; range 9–80mer) and 10% double-stranded phosphodiester oligonucleotides [Citation40,Citation41]. Defibrotide contains two distinct aptamer sequences that confer its thrombin-antagonizing properties [Citation42].
2.2. Pharmacodynamics
Although not yet completely elucidated, results of preclinical studies indicate that defibrotide has multiple mechanisms of action, primarily involving EC protection, reduced activation, and enhancement of plasmin enzymatic activity [Citation41,Citation43–Citation46].
In preclinical studies, defibrotide prevents the activation of macrovascular and microvascular endothelia caused by soluble factors released to blood by autologous HSCT [Citation43]. Other studies have found that defibrotide binds to ECs, protecting the endothelium from chemotherapy, tumor necrosis factor-alpha, serum starvation, perfusion damage, oxidative stress, and inflammation [Citation41,Citation43,Citation47–Citation50]. Palomo et al. clearly showed that although defibrotide is actively taken up by ECs via macropinocytosis; inhibition of this mechanism did not affect its activity on the manipulation of the expression of various endothelial markers of activation. Therefore, it is thought that defibrotide exerts these protective effects primarily through interaction with EC membranes, but the internalization raises the possibility of other mechanisms of action that require further investigation [Citation47]. Defibrotide also increases tissue plasminogen activator and thrombomodulin expression, and decreases von Willebrand factor and plasminogen activator inhibitor-1 expression, supporting endothelial structure, tone, and function [Citation40,Citation45,Citation46,Citation50–Citation52]. Other researchers have found that defibrotide enhances enzymatic activity of plasmin, leading to the hydrolysis of fibrin clots [Citation45,Citation46,Citation53].
2.3. Pharmacokinetics and metabolism
In healthy volunteers, a single 6.25 mg/kg dose of defibrotide given as a 2-h infusion has a Tmax at 2 h, a half-life of approximately 43 min, and does not induce or inhibit cytochrome P450 metabolism [Citation38]. An average of 93% of defibrotide is bound to human plasma protein; the drug’s volume of distribution ranges from 8.1 to 9.1 L [Citation39]. Primarily during the first 4 h following administration of defibrotide 6.25–15 mg/kg doses as 2-h infusions, approximately 5–15% of the total dose is excreted in urine unmetabolized [Citation39]. There is no apparent accumulation of defibrotide after multiple doses in animals, healthy volunteers, and patients with renal impairment [Citation54,Citation55]. Defibrotide is not cleared by intermittent hemodialysis [Citation55].
3. Clinical efficacy of defibrotide in VOD/SOS
3.1. Treatment
3.1.1. Early-phase clinical studies for the treatment of VOD/SOS
summarizes the results from the early-phase clinical studies of defibrotide for the treatment of patients with post-HSCT hepatic VOD/SOS. A retrospective analysis of data from 19 patients enrolled in a US multicenter compassionate-use program (CUP) reported initial clinical safety, tolerability, and efficacy data of defibrotide in patients with hepatic VOD/SOS and MOD following HSCT [Citation56]. Eligible patients received defibrotide starting at a dose of 10 mg/kg/day given in four divided doses each as a 2-h IV infusion, for a planned 14-day minimum. Forty-two percent of patients had an observed complete response (CR), defined as evidence of improvement in VOD/SOS-related symptoms with a decrease in bilirubin to <2 mg/dL; 32% of patients survived past Day +100.
Table 3. Early clinical studies of DF for the treatment of severe hepatic VOD/SOS after HSCT.
A retrospective analysis of data from 40 patients enrolled in a European defibrotide CUP, including 65% with MOD, reported a 55% CR rate and a 43% survival rate at Day +100 [Citation57]. Updating and expanding on the initial US CUP defibrotide experience, an analysis of data from 88 patients reported a 36% CR rate and a 35% Day +100 survival rate, with no worsening of clinical bleeding and no grade 3–4 treatment-related adverse events (TRAEs) [Citation58]. Similarly, a retrospective exploratory analysis of 2008–2011 data from the Center for International Blood and Marrow Transplantation Research (CIBMTR) reported 39% survival at Day +100 for 41 first-time HSCT recipients with VOD/SOS treated with defibrotide compared with a 31% survival rate in patients with VOD/SOS not receiving defibrotide [Citation60]. CIBMTR further reported a higher rate of VOD/SOS resolution in defibrotide treated patients (51.2%) compared with patients not receiving defibrotide (22.1%).
A retrospective analysis of data from 45 children and adolescents treated with defibrotide for hepatic VOD/SOS after HSCT reported a 76% CR rate and a 64% Day +100 survival rate [Citation36]. Moreover, because the average time from diagnosis to defibrotide was 1 day in patients achieving CR but 5.5 in patients who did not achieve CR, this report showed for the first time that earlier initiation of defibrotide after VOD/SOS onset is associated with improved outcomes.
An open-label, prospective, multicenter, randomized dose-finding trial (25 vs. 40 mg/kg/day) identified defibrotide 25 mg/kg/day as the optimal dose in patients with post-HSCT VOD/SOS with MOD [Citation59]. Based on similar efficacy results between arms (CR rates of 49% and 43% and Day +100 survival rates of 44% and 39% in the 25 and 40 mg/kg day arm, respectively) and somewhat better tolerability in the 25 mg/kg/day arm, 25 mg/kg/day was chosen as the dose to test in future studies. In this study, the recommended minimum duration of treatment was 14 days; however, the median dosing was 19.5 days [Citation59]. Subsequent studies therefore adopted a minimum recommended treatment duration of 21 days [Citation59,Citation61].
3.1.2. Phase III and other large clinical studies for the treatment of VOD/SOS
summarizes the design and efficacy results of a phase III trial and two other large clinical studies of defibrotide for hepatic VOD/SOS. A historically controlled open-label phase III trial prospectively evaluated the efficacy and safety of defibrotide 25 mg/kg/day for ≥21 days in patients with post-HSCT VOD/SOS with MOD [Citation61]. Investigators used a historical control group because of logistical and ethical considerations of withholding a promising treatment from a control group of seriously ill patients for a disease with a typically dismal outcome and without proven treatment options. The trial found a statistically significant 23% estimated improvement in Day +100 survival among patients treated with defibrotide (median duration of treatment, 21.5 days) versus the historical control group treated with best supportive care only (P = .0109, propensity adjusted), a statistically significant 19% between group difference in CR rate (P = .0160, propensity adjusted), with tolerability that was generally similar between arms ()
Table 4. Phase III and other large clinical studies of DF for treatment or prophylaxisa of hepatic VOD/SOS after HSCT.
Figure 1. Kaplan-Meier estimates of overall survival (panel A) and time to CR (panel B) for defibrotide-treated patients and historical control group in the phase 3 treatment trial [Citation61].
CI: confidence interval; CR: complete response. Republished with permission of American Society of Hematology, from Phase 3 Trial of Defibrotide for the Treatment of Severe Veno-Occlusive Disease and Multi-Organ Failure, Richardson PG, et al, 127, 13 and 2016; permission conveyed through Copyright Clearance Center, Inc.
![Figure 1. Kaplan-Meier estimates of overall survival (panel A) and time to CR (panel B) for defibrotide-treated patients and historical control group in the phase 3 treatment trial [Citation61].CI: confidence interval; CR: complete response. Republished with permission of American Society of Hematology, from Phase 3 Trial of Defibrotide for the Treatment of Severe Veno-Occlusive Disease and Multi-Organ Failure, Richardson PG, et al, 127, 13 and 2016; permission conveyed through Copyright Clearance Center, Inc.](/cms/asset/ffe8c3ab-bb05-4f4b-b274-798e7fced642/ierh_a_1370372_f0001_b.gif)
A final analysis from a large international CUP program reported on data from 710 patients with hepatic VOD/SOS following HSCT (89%) or nontransplant-related chemotherapy/radiotherapy treatments (11%) [Citation62]. When the CUP started, the recommended defibrotide starting dose was 10 mg/kg/day (IV 4 divided doses each over 2 h) to be titrated up to a maximum of 60 mg/kg/day; in 2004 the recommended defibrotide dose was changed to 25 mg/kg/day based on a presentation of data from a phase II study later published in 2010 [Citation59]. The Day +100 survival rate was 54% for 701 patients with available data and 58% in the 25 mg/kg/day group (n = 272); in subgroup analyses of Day +100 survival, pediatric patients (65%) fared better than adults (46%; ), and patients without MOD (52%) fared better than patients with MOD (33%). In the subgroup of patients with chemo/radiotherapy, Day +100 survival was 67.5% in those with MOD and 74.2% for those without MOD.
Figure 2. Survival at Day +100 in pediatric patients (n = 303) and adults (n = 407) from a large compassionate use program [Citation62].
Reprinted from Biology of Blood and Marrow Transplantation, 22/10, Corbacioglu S, et al, Defibrotide for the Treatment of Hepatic Veno-Occlusive Disease: Final Results From the International Compassionate-Use Program, 1874–1882, Copyright (2016), with permission from American Society for Blood and Marrow Transplantation. doi:10.1016/j.bbmt.2016.07.001. URL for Creative Commons user license: https://creativecommons.org/licenses/by-nc-nd/4.0/.
![Figure 2. Survival at Day +100 in pediatric patients (n = 303) and adults (n = 407) from a large compassionate use program [Citation62].Reprinted from Biology of Blood and Marrow Transplantation, 22/10, Corbacioglu S, et al, Defibrotide for the Treatment of Hepatic Veno-Occlusive Disease: Final Results From the International Compassionate-Use Program, 1874–1882, Copyright (2016), with permission from American Society for Blood and Marrow Transplantation. doi:10.1016/j.bbmt.2016.07.001. URL for Creative Commons user license: https://creativecommons.org/licenses/by-nc-nd/4.0/.](/cms/asset/fb8cddd8-c7a2-48ae-a532-0f9e4f418ebc/ierh_a_1370372_f0002_b.gif)
As part of an expanded access treatment (T-IND) protocol, patients with VOD/SOS (with or without MOD) received IV defibrotide 6.25 mg/kg every 6 h (25 mg/kg/day) with a recommended treatment duration of ≥21 days [Citation63]. In an interim analysis of 681 patients enrolled in the study, 642 met VOD/SOS criteria and received defibrotide; 573 (89.3%) had undergone HSCT, and the other 69 (10.7%) had developed VOD/SOS following chemotherapy alone. The large HSCT population, with a mean age of 20.6 years, included 319 children (age ≤16 years) and 254 adults (age >16 years). Overall, 61.3% of patients had VOD/SOS with MOD and 38.7% did not have MOD. Kaplan–Meier estimated survival at Day +100 was 53.8% for the overall HSCT population, 54.5% for the pediatric subgroup, and 44.9% for the adult subgroup. A subgroup of 76 patients with late-onset VOD/SOS (VOD/SOS diagnosed >30 days post-HSCT (median age, 42 years) had a 34.2% Day +100 survival rate [Citation63]. A post hoc analysis of the smaller post-chemotherapy group (82 patients treated by day 30 after start of chemotherapy) showed a Day +70 survival rate of 74.1% [Citation64]. To explore the impact of time from VOD/SOS diagnosis to start of defibrotide treatment, a post hoc analysis of the HSCT population looked at Day +100 survival for all patients treated before/after days as well as the trend across days for patients treated on each particular day [Citation65]. Approximately one-third of patients were treated on the day of diagnosis and >90% by the end of day 7. The analysis of patients before/after specific days showed that shorter delay was associated with improved survival, which was confirmed by a statistically significant trend across specific days (P < .001). No specific day post-diagnosis proved to be a viable cutoff for this benefit [Citation65].
3.2. Prevention
There are no medications with regulatory approval for the prevention/prophylaxis of VOD/SOS. Several studies suggest that defibrotide has potential in this area. Corbacioglu et al. reported on 20 patients with infantile osteopetrosis, consecutively transplanted between 1996 and 2005 [Citation11]. Eleven patients were transplanted between 1996 and 2001 and experienced an overall incidence of VOD/SOS of 63.6% (7/11). VOD/SOS with MOD occurred in three patients and one patient died due to VOD/SOS-related MOD. Among 9 patients consecutively transplanted between 2001 and 2005 with defibrotide prophylaxis, only 1 patient (11.1%) was diagnosed retrospectively with moderate VOD/SOS.
Another study compared 52 consecutively transplanted adult patients treated with defibrotide prophylaxis versus 52 historical controls [Citation66]. Although none of the defibrotide-treated patients developed VOD/SOS (by Baltimore criteria), 19% of patients in the control group developed VOD/SOS and 3 died due to VOD/SOS. In an updated report of an expanded cohort of 258 treated patients and 258 controls, none of the patients receiving defibrotide developed VOD/SOS compared with 4.8% of controls (P < .001) [Citation67]. Another finding of interest in this HSCT population was that the rate of acute graft versus host disease (GVHD) was significantly lower in the defibrotide group at 1 year (31% vs. 42%; P = .026), although the Day +100 rates were similar (27% and 29%, respectively) [Citation67].
An uncontrolled retrospective analysis of data from 58 patients who received defibrotide prophylaxis during allogeneic HSCT found that none of the patients had VOD/SOS (Baltimore criteria) and none died of suspected VOD/SOS within 100 days of transplantation [Citation68]. Another retrospective analysis of 47 consecutive patients given defibrotide prophylaxis during HSCT found 4 cases of clinical VOD/SOS (modified Seattle criteria) that resolved within 14 days after the dose of defibrotide was increased [Citation69].
A 2012 open-label, phase III RCT compared defibrotide prophylaxis versus no prophylaxis in pediatric patients (age <18 years) with ≥1 risk factors for developing VOD/SOS post-HSCT () [Citation26]. Considered risk factors included preexisting liver disease, second myeloablative HSCT, allogeneic HSCT for leukemia beyond second relapse, conditioning with busulfan and melphalan, previous treatment with gemtuzumab ozogamicin, and diagnosis of inherited hemophagocytic lymphohistiocytosis, adrenoleukodystrophy, or osteopetrosis. The study demonstrated a reduction in VOD/SOS onset by Day +30 post-HSCT for the defibrotide prophylaxis versus control (12% vs. 20%; P = .0488 Z test; P = .0507 log-rank test). Adverse events (AEs) were similar between groups (). The rate of acute GVHD was reduced in the defibrotide arm at 30 days (P = .0057) and 100 days (P = .0034).
Table 5. Defibrotide safety/tolerability in phase III and other large clinical studies.
The results of these and other studies were analyzed in a meta-analysis of 1230 patients, which found an overall mean incidence of VOD/SOS of 4.7% among patients receiving prophylactic defibrotide compared with an incidence of 13.7% in 135 studies not using VOD/SOS prophylaxis (P < .005) [Citation70]. Further, the incidence of severe VOD/SOS was 0.8% in defibrotide patients with a relative risk of 0.31 compared with no prophylaxis [Citation70].
The ongoing, manufacturer-supported, NCT02851407 phase III clinical trial is comparing the efficacy and safety of defibrotide with best supportive care versus best supportive care alone in the prevention of hepatic VOD/SOS in pediatric (age ≥1 month and ≤16 years) and adult patients undergoing HSCT. With a planned enrollment of 400 patients, this study is currently enrolling.
4. Safety profile and tolerability
summarizes safety data from the larger, more recent clinical studies. In the studies that compared defibrotide treatment with a control group (best supportive care), the incidence of AEs was comparable between groups [Citation26,Citation61], suggesting that observed AEs might have been associated with preexisting disease, comorbidities, and/or complications of the HSCT.
For example, the phase III historically controlled trial in patients with VOD/SOS and MOD reported that all but 1 of the 102 defibrotide-treated patients and all 32 historical control patients experienced ≥1 AE [Citation61]. Hypotension was the most frequent AE (39% for defibrotide, 50% for controls), and common hemorrhagic AEs, which included pulmonary alveolar and gastrointestinal hemorrhage, occurred in 64% of defibrotide-treated patients and 75% of controls. Related AEs in the defibrotide arm included hemorrhagic events and hypotension. In the interim report of the expanded-access T-IND study, which included patients with and without MOD as well as patients who had received chemotherapy without HSCT, AEs that occurred in >10% of patients were hypotension (13.8%) and new or worsening MOD (12.7%), and AEs that were attributed by investigators to treatment occurred in 21.6% of patients [Citation63]. In the pediatric prevention trial, the incidence of AEs was much the same between groups: 154/177 (87%) in the defibrotide prophylaxis group and 155/176 (88%) in the controls [Citation26]. For hemorrhage, the most common treatment-related AE, cumulative incidence was also similar, with 39/177 (22%) in the defibrotide prophylaxis group and 37/176 (21%) in the controls [Citation26].
5. Regulatory affairs
Defibrotide was first approved in Italy in 1986 for deep vein thrombosis prophylaxis and thrombophlebitis treatment, and then in 1993 for the treatment of peripheral obstructive (mild-to-moderate) arteriopathy [Citation54]. The manufacturer withdrew the marketing authorization in 2009 primarily to support the evaluation of defibrotide in clinical trials in the USA and Europe. In October 2013, the European Commission granted orphan marketing authorization with an ‘orphan designation’ for severe hepatic VOD/SOS in HSCT in adults and children older than 1 month [Citation38,Citation71]. In March 2016, the US FDA approved defibrotide for the treatment of adult and pediatric patients with hepatic VOD/SOS, with renal or pulmonary dysfunction following HSCT [Citation39].
6. Conclusions
Clinical studies of defibrotide support its use in the regulatory-approved indication of treatment for hepatic VOD/SOS in children and adults following HSCT, with concomitant renal or pulmonary dysfunction in the USA and in patients with severe VOD/SOS in the European Union [Citation38,Citation39,Citation59,Citation61–Citation63]. Response rates appear favorable in comparison with historical controls, and the safety profile is similar to that of best supportive care [Citation61]. There are promising data on the use of defibrotide for VOD/SOS prophylaxis in high-risk children undergoing HSCT [Citation26], and an ongoing RCT in children and adults will provide data to better assess the clinical value of defibrotide as a preventive medication.
7. Expert commentary
Defibrotide is the drug with the largest body of evidence for therapeutic efficacy in the treatment of patients with VOD/SOS, with and without MOD. There is mounting evidence that early intervention and even preemptive/prophylactic use of defibrotide in certain high-risk patients may be associated with a superior outcome [Citation11,Citation26,Citation65,Citation66,Citation68,Citation69]. Evidence from a subgroup analysis of Day +100 survival from large studies suggests that defibrotide may be particularly effective in children [Citation62,Citation63] and suggested potential in a phase III pediatric prophylaxis study [Citation26].
Established diagnostic criteria, modified Seattle and Baltimore, may have substantial drawbacks for sensitivity and, considering the lack of hyperbilirubinemia in children, also specificity. Therefore, the new EBMT diagnostic criteria for VOD/SOS in children may well raise the awareness for early symptoms and consecutive preemptive intervention with defibrotide. This might be reflected in a reduced incidence of morbidity and mortality, properly assessable with the new criteria for severity.
In adults, the recognition of late-onset VOD/SOS has the potential to improve recognition and treatment of VOD/SOD after hospital discharge. As with children, new diagnostic and severity criteria also account for recent developments in treatment (e.g. RIC, alternative donors), and future refinements (e.g. imaging modalities as well as identification of signs including refractory thrombocytopenia and biomarkers), are expected to provide increased sensitivity and specificity. When available, anticipated data on prophylactic use of defibrotide in high-risk adult and pediatric patients will provide important new information regarding optimal management of VOD/SOS.
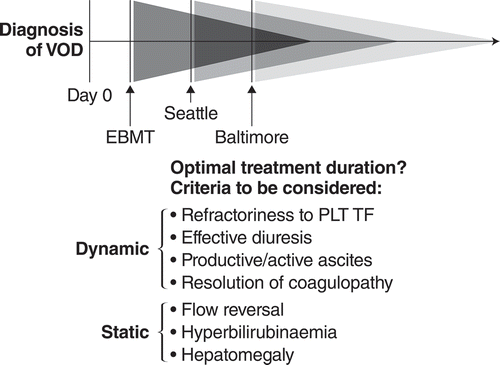
The duration of treatment may be placed in direct correlation to the start of treatment based on diagnostic criteria used (). On one end, the rigid Baltimore criteria with the obligatory hyperbilirubinemia might not trigger the diagnosis of VOD/SOS in a third of pediatric patients at all, or may be a late indicator, potentially delaying treatment initiation. On the other end are the pediatric EBMT criteria with the sensitive triggers of refractory thrombocytopenia and the dynamic of weight gain and hyperbilirubinemia. These differences between the Baltimore and new pediatric EBMT criteria might lead to prolonged treatment with futile outcome (Baltimore) versus early onset and a shorter treatment duration (EBMT).
The duration of treatment must be individualized to reflect the dynamics of the diseases. While the labeling for defibrotide recommends treatment for at least 21 days, factors that can affect treatment duration can be divided into 2 categories: dynamic and static. Dynamic criteria are persistent refractoriness to platelet transfusions under treatment; reduced third-spacing reflected in an effective diuresis; ceasing productive/active ascites reflected in a reduced drainage; and the resolution of coagulopathy, reflecting amelioration of hepatocytic damage. Static criteria are persistent flow reversal (if ever present), hyperbilirubinemia, and hepatomegaly. The decision to stop treatment should be based on the following consideration: dynamic criteria must be fulfilled because they represent disease activity on the endothelial level, and static criteria can be fulfilled, since hyperbilirubinemia and hepatomegaly in particular will take longer to resolve, are the consequences of post-endothelial damage, and do not reflect active disease as directly as the dynamic criteria.
There is also a subset of patients (especially pediatric patients) with a very high risk for VOD/SOS who will benefit from the prophylactic use of defibrotide. These include patients with osteopetrosis, hemophagocytic lymphohistiocytosis, and other macrophage activation syndromes, infants, and patients with high-risk neuroblastoma.
8. Five-year view
Areas of potential interest for future studies of defibrotide include the use of higher doses of defibrotide in infants, prophylaxis in specific subgroups (see above), and combination therapies with defibrotide and other endothelial-targeting drugs. Building on data in the treatment of patients receiving chemotherapy without HSCT [Citation62,Citation63], further areas of investigation might include efficacy of defibrotide to treat VOD/SOS due to alkaloid ingestion or solid-organ transplant. The diagnosis of VOD/SOS based on updated pediatric and adult criteria and severity scales is expected to improve timely identification of patients who would benefit from treatment. Future validated biomarkers will help identify patients at risk of VOD/SOS. Another area where defibrotide might find a role is in the prevention of GVHD (as suggested in the phase III pediatric prophylaxis study), transplant-associated microangiopathy, and other systemic endothelial complications.
Key issues
Clinical studies of defibrotide support its use in the regulatory-approved indication of treatment for hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) following hematopoietic stem cell transplantation (HSCT) in children and adults, in severe VOD/SOS (European Union) or with concomitant renal or pulmonary dysfunction (United States).
Day +100 survival and complete response rates significantly higher in comparison with historical controls.
The safety profile is manageable.
There are promising data on the use of defibrotide for VOD/SOS prophylaxis in high-risk children undergoing HSCT.
An ongoing RCT in children and adults will better assess the clinical value of defibrotide as a preventive medication.
Declaration of interest
Selim Corbacioglu served as a consultant for and received honoraria from Gentium/Jazz Pharmaceuticals. Paul G. Richardson has served on advisory committees and as a consultant, and received research funding from Jazz Pharmaceuticals.
The authors thank Monica Nicosia, PhD, and John Norwood of The Curry Rockefeller Group, LLC, Tarrytown, NY, USA, for providing medical writing support, and The Curry Rockefeller Group for editorial support in formatting, proofreading, copy editing and fact checking, which was funded by Jazz Pharmaceuticals in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Jazz Pharmaceuticals also reviewed and edited the manuscript for scientific accuracy. Although Jazz Pharmaceuticals was involved in the topic concept and fact checking of information, the content of this manuscript, the ultimate interpretation, and the decision to submit it for publication in Expert Review of Gastroenterology & Hepatology was made independently by the authors.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Funding
References
- Wallhult E, Kenyon M, Liptrott S, et al. Management of veno-occlusive disease: the multidisciplinary approach to care. Eur J Haematol. 2017;98(4):322–329.
- Mohty M, Malard F, Abecassis M, et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016;51(7):906–912.
- Corbacioglu S, Carreras E, Ansari M, et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: a new classification from the European Society For Blood and Marrow Transplantation. Bone Marrow Transplant. published online July 31 2017. doi: 10.1038/bmt.2017.161.
- Dalle JH, Giralt SA. Hepatic veno-occlusive disease after hematopoietic stem cell transplantation: risk factors and stratification, prophylaxis, and treatment. Biol Blood Marrow Transplant. 2016;22(3):400–409.
- Coppell JA, Richardson PG, Soiffer R, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16(2):157–168.
- Dvorak CC, Nejadnik B, Cao Z, et al. Hospital cost associated with veno-occlusive disease (VOD) in patients with hematopoietic stem cell transplant (HSCT) [ASH abstract 4452]. Biol Blood Marrow Transplant. 2016;22(3 Suppl):S275.
- Barker CC, Butzner JD, Anderson RA, et al. Incidence, survival and risk factors for the development of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2003;32(1):79–87.
- Corbacioglu S, Kernan N, Lehmann L, et al. Defibrotide for the treatment of hepatic veno-occlusive disease in children after hematopoietic stem cell transplantation. Expert Rev Hematol. 2012;5(3):291–302.
- Carreras E, Diaz-Beya M, Rosinol L, et al. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant. 2011;17(11):1713–1720.
- Tsirigotis PD, Resnick IB, Avni B, et al. Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. 2014;49(11):1389–1392.
- Corbacioglu S, Honig M, Lahr G, et al. Stem cell transplantation in children with infantile osteopetrosis is associated with a high incidence of VOD, which could be prevented with defibrotide. Bone Marrow Transplant. 2006;38(8):547–553.
- Schulz AS, Classen CF, Mihatsch WA, et al. HLA-haploidentical blood progenitor cell transplantation in osteopetrosis. Blood. 2002;99(9):3458–3460.
- Cesaro S, Spiller M, Sartori MT, et al. Veno-occlusive disease in pediatric patients affected by Wilms tumor. Pediatr Blood Cancer. 2011;57(2):258–261.
- Elli M, Pinarli FG, Dagdemir A, et al. Veno-occlusive disease of the liver in a child after chemotherapy for brain tumor. Pediatr Blood Cancer. 2006;46(4):521–523.
- Sulis ML, Bessmertny O, Granowetter L, et al. Veno-occlusive disease in pediatric patients receiving actinomycin D and vincristine only for the treatment of rhabdomyosarcoma. J Pediatr Hematol Oncol. 2004;26(12):843–846.
- Czauderna P, Katski K, Kowalczyk J, et al. Venoocclusive liver disease (VOD) as a complication of Wilms’ tumour management in the series of consecutive 206 patients. Eur J Pediatr Surg. 2000;10(5):300–303.
- Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44(6):778–783.
- McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267.
- Mohty M, Malard F, Abecassis M, et al. Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2015;50(6):781–789.
- Rio B, Andreu G, Nicod A, et al. Thrombocytopenia in venocclusive disease after bone marrow transplantation or chemotherapy. Blood. 1986;67(6):1773–1776.
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood. 1995;85(11):3005–3020.
- Dignan FL, Wynn RF, Hadzic N, et al. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol. 2013;163(4):444–457.
- Carreras E, Diaz-Ricart M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011;46(12):1495–1502.
- Johnson DB, Savani BN. How can we reduce hepatic veno-occlusive disease-related deaths after allogeneic stem cell transplantation? Exp Hematol. 2012;40(7):513–517.
- Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142(3):348–360.
- Corbacioglu S, Cesaro S, Faraci M, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379(9823):1301–1309.
- Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. Br J Haematol. 2015;168(4):481–491.
- Cheuk DK, Chiang AK, Ha SY, et al. Interventions for prophylaxis of hepatic veno-occlusive disease in people undergoing haematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2015;(5):CD009311.
- Imran H, Tleyjeh IM, Zirakzadeh A, et al. Use of prophylactic anticoagulation and the risk of hepatic veno-occlusive disease in patients undergoing hematopoietic stem cell transplantation: a systematic review and meta-analysis. Bone Marrow Transplant. 2006;37(7):677–686.
- Attal M, Huguet F, Rubie H, et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood. 1992;79(11):2834–2840.
- Or R, Nagler A, Shpilberg O, et al. Low molecular weight heparin for the prevention of veno-occlusive disease of the liver in bone marrow transplantation patients. Transplantation. 1996;61(7):1067–1071.
- Marsa-Vila L, Gorin NC, Laporte JP, et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Haematol. 1991;47(5):346–354.
- Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100(6):1977–1983.
- Tay J, Tinmouth A, Fergusson D, et al. Systematic review of controlled clinical trials on the use of ursodeoxycholic acid for the prevention of hepatic veno-occlusive disease in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2007;13(2):206–217.
- Tsochatzis EA, Feudjo M, Rigamonti C, et al. Ursodeoxycholic acid improves bilirubin but not albumin in primary biliary cirrhosis: further evidence for nonefficacy. Biomed Res Int. 2013;2013:139763.
- Corbacioglu S, Greil J, Peters C, et al. Defibrotide in the treatment of children with veno-occlusive disease (VOD): a retrospective multicentre study demonstrates therapeutic efficacy upon early intervention. Bone Marrow Transplant. 2004;33(2):189–195.
- Grupp SA, Smith AR, Triplett BM, et al. An exploratory post hoc analysis of survival by age and time to defibrotide initiation after diagnosis of hepatic veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) in patients receiving hematopoietic stem cell transplantation (HSCT) Poster session presented at: 5th Annual American Society of Pediatric Hematology/Oncology–Pediatric Blood and Marrow Transplant Consortium (ASPHO-PBMTC) Educational Meeting; 2017 Apr 26; Montreal, Quebec, Canada. Poster 1007.
- Defitelio® (defibrotide) [EU SPC]. Summary of product characteristics (Europe). Villa Guardia, 22079 Italy: Gentium Srl; 2016.
- Defitelio® (defibrotide sodium) [US PI]. Prescribing information (United States). Palo Alto, CA 94304: Jazz Pharmaceuticals, Inc; 2016.
- Bianchi G, Barone D, Lanzarotti E, et al. Defibrotide, a single-stranded polydeoxyribonucleotide acting as an adenosine receptor agonist. Eur J Pharmacol. 1993;238(2–3):327–334.
- Pescador R, Capuzzi L, Mantovani M, et al. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol. 2013;59(1–2):1–10.
- Bracht F, Schror K. Isolation and identification of aptamers from defibrotide that act as thrombin antagonists in vitro. Biochem Biophys Res Commun. 1994;200(2):933–937.
- Palomo M, Diaz-Ricart M, Rovira M, et al. Defibrotide prevents the activation of macrovascular and microvascular endothelia caused by soluble factors released to blood by autologous hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17(4):497–506.
- Benimetskaya L, Wu S, Voskresenskiy AM, et al. Angiogenesis alteration by defibrotide: implications for its mechanism of action in severe hepatic veno-occlusive disease. Blood. 2008;112(10):4343–4352.
- Echart CL, Graziadio B, Somaini S, et al. The fibrinolytic mechanism of defibrotide: effect of defibrotide on plasmin activity. Blood Coagul Fibrinolysis. 2009;20(8):627–634.
- Falanga A, Vignoli A, Marchetti M, et al. Defibrotide reduces procoagulant activity and increases fibrinolytic properties of endothelial cells. Leukemia. 2003;17(8):1636–1642.
- Palomo M, Mir E, Rovira M, et al. What is going on between defibrotide and endothelial cells? Snapshots reveal the hot spots of their romance. Blood. 2016;127(13):1719–1727.
- Schroder H. Defibrotide protects endothelial cells, but not L929 tumour cells, from tumour necrosis factor-alpha-mediated cytotoxicity. J Pharm Pharmacol. 1995;47(3):250–252.
- Scalia R, Kochilas L, Campbell B, et al. Effects of defibrotide on leukocyte-endothelial cell interaction in the rat mesenteric vascular bed: role of P-selectin. Methods Find Exp Clin Pharmacol. 1996;18(10):669–676.
- Eissner G, Multhoff G, Gerbitz A, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood. 2002;100(1):334–340.
- Nunziata A, Mariani MF, Cascone M, et al. Increased plasma t-PA activity and urinary PGE2 after repeated oral administration of defibrotide to the rat and the mouse. Thromb Res. 1991;64(2):279–284.
- Zhou Q, Chu X, Ruan C. Defibrotide stimulates expression of thrombomodulin in human endothelial cells. Thromb Haemost. 1994;71(4):507–510.
- Coccheri S, Biagi G, Legnani C, et al. Acute effects of defibrotide, an experimental antithrombotic agent, on fibrinolysis and blood prostanoids in man. Eur J Clin Pharmacol. 1988;35(2):151–156.
- Palmer KJ, Goa KL. Defibrotide. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in vascular disorders. Drugs. 1993;45(2):259–294.
- Tocchetti P, Tudone E, Marier J-F, et al. Pharmacokinetic profile of defibrotide in patients with renal impairment. Drug Des Devel Ther. 2016;10:2631–2641.
- Richardson PG, Elias AD, Krishnan A, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood. 1998;92(3):737–744.
- Chopra R, Eaton JD, Grassi A, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: results of the European compassionate-use study. Br J Haematol. 2000;111(4):1122–1129.
- Richardson PG, Murakami C, Jin Z, et al. Multi-institutional use of defibrotide in 88 patients after stem cell transplantation with severe veno-occlusive disease and multisystem organ failure: response without significant toxicity in a high-risk population and factors predictive of outcome. Blood. 2002;100(13):4337–4343.
- Richardson PG, Soiffer RJ, Antin JH, et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant. 2010;16(7):1005–1017.
- Strouse C, Richardson P, Prentice G, et al. Defibrotide for treatment of severe veno-occlusive disease in pediatrics and adults: an exploratory analysis using data from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2016;22(7):1306–1312.
- Richardson PG, Riches ML, Kernan NA, et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. 2016;127(13):1656–1665.
- Corbacioglu S, Carreras E, Mohty M, et al. Defibrotide for the treatment of hepatic veno-occlusive disease: final results from the international compassionate-use program. Biol Blood Marrow Transplant. 2016;22(10):1874–1882.
- Richardson PG, Smith AR, Triplett BM, et al. Defibrotide for patients with hepatic veno-occlusive disease/sinusoidal obstruction syndrome: interim results from a treatment IND study. Biol Blood Marrow Transplant. 2017;23(6):997–1004.
- Kernan NA, Richardson PG, Triplett BM, et al. Efficacy and safety of defibrotide to treat hepatic veno-occlusive disease/sinusoidal obstruction syndrome after primary chemotherapy: a post hoc analysis of final data. J Clin Oncol. 2017;35(suppl):abstr 10513.
- Richardson PG, Smith AR, Triplett BM, et al. Earlier defibrotide initiation post-diagnosis of veno-occlusive disease/sinusoidal obstruction syndrome improves Day +100 survival following haematopoietic stem cell transplantation. Br J Haematol. 2017. cited 2017 Jun 9. DOI:10.1111/bjh.14727.
- Chalandon Y, Roosnek E, Mermillod B, et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2004;10(5):347–354.
- Chalandon Y, Simonetta F, Dantin C, et al. Efficient prophylaxis with defibrotide for sinusoidal obstruction syndrome (SOS) after allogeneic hematopoietic stem cell transplantation (HSCT). Blood. 2016;128:2204.
- Dignan F, Gujral D, Ethell M, et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from veno-occlusive disease. Bone Marrow Transplant. 2007;40(1):79–82.
- Qureshi A, Marshall L, Lancaster D. Defibrotide in the prevention and treatment of veno-occlusive disease in autologous and allogeneic stem cell transplantation in children. Pediatr Blood Cancer. 2008;50(4):831–832.
- Zhang L, Wang Y, Huang H. Defibrotide for the prevention of hepatic veno-occlusive disease after hematopoietic stem cell transplantation: a systematic review. Clin Transplant. 2012;26(4):511–519.
- Richardson PG, Corbacioglu S, Ho VT, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf. 2013;12(1):123–136.