1. Introduction
Discriminating intestinal tuberculosis (ITB) and Crohn disease (CD) remains an eternal dilemma for clinicians. These two granulomatous diseases are remarkably similar in their clinical presentation, endoscopic appearances, radiological aspects and histopathological findings. While a number of different parameters have been noted to be suggestive of one diagnosis over the other, only very few parameters are deemed characteristic for a particular diagnosis [Citation1,Citation2]. In a large study from South Koreas, around 18% of CD patients were initially misdiagnosed as ITB, while 11% of ITB patients were misdiagnosed as CD [Citation3]. In fact, a large number of patients with CD are treated initially as ITB in TB endemic areas [Citation2]. There are apparent risks with such an approach: risk of hepatotoxicity with antitubercular therapy (ATT), delay in the diagnosis of CD, and possible risk of progression and stricture formation [Citation4]. Various features, as reported in a large meta-analysis, to suggest the diagnosis of CD include male gender, bleeding per rectum, perianal or extraintestinal involvement, linear ulcers, cobble-stoning, mucosal bridge, and left sided involvement, histology suggesting focally enhanced colitis; imaging suggesting asymmetrical intestinal thickening and wall stratification, presence of comb sign, and proliferation of mesenteric fat. On the contrary, certain findings which may favour ITB include pyrexia, pulmonary or peritoneal involvement, transverse ulcers, ileo-cecal involvement; and imaging findings of necrotic lymph nodes and short segment involvement and a positive interferon-γ release assay [Citation5]. However, even combining these multiple parameters into a Bayesian model may not discriminate all the cases [Citation5]. Therefore, newer and better strategies are urgently required to differentiate these two entities and the present editorial will enumerate some newer approaches which have been evaluated to differentiate the two conditions.
1.1. Endoscopic approaches: old wine in a new bottle
Certain colonoscopic findings like circumferential or transverse ulcer, ileo-cecal involvement are deemed to be more suggestive for ITB, whereas certain others like linear ulcers, aphthous ulcers, left sided involvement, and skip lesions are more frequent in CD [Citation1,Citation2,Citation5]. Unfortunately, none of these is very specific for either of the diseases. Attempts have been made to improve the diagnostic yield using small bowel endoscopy by capsule endoscopy. Two studies have compared capsule endoscopic findings in ITB and CD, while ileocecal involvement was more frequent in ITB, aphthous ulcers, or more than one segment involvement and proximal bowel involvement were more frequent in CD [Citation6,Citation7]. Unfortunately, none of these findings is discriminative of one disease from another. Although image enhanced endoscopy has been used in many diseases, no comparative studies comparing these in ITB and CD have been published.
1.2. Radiological findings: hope or hype?
A recent review in the journal summarized the imaging findings for discriminating ITB and CD.1 Radiological discrimination has been evaluated in a number of studies and apart from certain findings like necrotic lymph nodes (specific for ITB but not sensitive), most findings do not have sufficient discriminative value [Citation1]. Changes in visceral fat have been well recognized to occur in CD and mesenteric fat changes are often considered the driver of inflammation. This has led to the belief that visceral fat expansion could help in discriminating ITB and CD. In a study which evaluated the use of visceral fat and compared the ratio of visceral fat to total fat and of visceral fat to subcutaneous fat for discrimination, the value of VF/TF >0.46 had good specificity but low sensitivity for the diagnosis of CD [Citation8].
1.3. Histology and tissue-based approaches: flattering to deceive
Intestinal tissue is used both for histological and microbiological studies to discriminate ITB and CD. Although the presence of microbiological positivity (AFB, culture, or PCR) or certain histological findings (caseating granuloma confluent granulomas, or ulcer base lined by histiocytes) are considered specific for ITB, these suffer on account of very low sensitivity (Figure 1) [Citation9]. The reported sensitivities of AFB stain, culture, IS6110 PCR test, caseating granuloma, confluent granuloma, and ulcer base lined by histiocytes is <5%, 6–54%, 47%, 21%, 38% and 41% respectively [Citation2,Citation9]. Recent reports have evaluated to the use of Xpert-Mtb/Rif in the diagnosis of ITB, but the reported sensitivity reported is low (8–32%) [Citation2,Citation10]. Therefore, other approaches based to discriminate these two entities using testing of intestinal tissue have been evaluated but have met only limited success.
A study reported the use of Liquid chromatography–mass spectrometry-based approach for assessment of mucosal proteomics and found 11 proteins to be differentially expressed in multiple experiments. However, when six of these proteins were used for immunohistochemistry in another cohort of patients, none was reported to be differentially expressed in the ITB and CD patients [Citation11]. Further studies to look at tissue proteomics between these two diseases must match the site and depth of biopsies to ensure the comparability of the findings. Another approach which had initially been reported useful was the detection of mesenchymal stem cells in the tubercular granulomas in the belief that the accumulation of these cells helped somehow in the persistence of infection. This study suggested that the tubercular granulomas had CD73 expression along with the markers of MSC (CD29, CD90 and CD105) which distinguished it from the granulomas of CD [Citation12]. However, a study from a South African cohort tempered these exciting findings and reported that CD73 positivity could occur in both ITB and CD in 52% and 30% of the patients. It is unlikely that CD73 staining would be useful in discriminating these two entities because of the low sensitivity, possibility of use limited to only patients with granulomas, and a substantial positivity in CD [Citation13].
1.4. Early response to therapy: crème de la crème
Response to therapy, first postulated by Logan in 1969, has been used frequently to differentiate ITB and CD. In countries where TB is endemic, the cases where diagnosis is unclear, ATT is initiated and response assessed to ascertain if the underlying diagnosis was CD or ITB. Interestingly, even patients with CD could have symptomatic response and some reduction in inflammatory markers with ATT, while some patients with ITB (especially those with strictures) could remain symptomatic. Therefore, mere symptom assessment is inadequate as a response to therapy. It is now clear that healing of ulcers (mucosal response) is the appropriate criterion to determine a response to therapy [Citation1]. While this was initially done using colonoscopy at the end of therapy, it has now become clear that mucosal response can be assessed much earlier. Since prolonged ATT in patients with CD delays initiation of CD directed treatment and may predispose to stenosing complications, we had evaluated the feasibility of early mucosal response (ulcer healing at 2 months) and found it to be discriminative for ITB and CD (Figure 1) [Citation2,Citation14]. In this study, four patients lacked mucosal response and three of them had ongoing symptoms, whereas of the 30 patients with complete mucosal response, nine had persistent symptoms. Other workers have also reported the utility of a short course of chemotherapy to discriminate these two entities using endoscopic changes [Citation15]. Seeking an early mucosal response should become the norm for assessing response to therapy at present. Other components of mucosal response could be an improvement in strictures, reduction in hyperemia, but pseudo-polyps, strictures, and nodularity may persist.
Since repeating a colonoscopy is often not possible because of issues of availability, patient acceptability, costs and invasiveness, there is an urgent need to look at non-invasive markers for mucosal response. In one of our studies where we looked at a decline in CRP levels with ATT, we found that normalization of CRP at 2 months was predictive of mucosal response, whereas lack of decline was suggestive of underlying CD or drug resistant tuberculosis [Citation16]. However, some patients have a normal underlying CRP even at the baseline emphasizing the need to look at additional markers. Also, some reports suggest that CRP could decline with ATT even in patients with CD [Citation17]. In a recent work, we combined the use of CRP and fecal calprotectin fecal calprotectin appeared to be better at predicting mucosal response. A decline in both CRP and fecal calprotectin (by 35%) could obviate the need for colonoscopy in a subset of these patients [Citation18]. Imaging modalities which have been evaluated to assess response to therapy include gastrointestinal ultrasound, computed tomography and MRI based methods. However, the data is limited and the assessment was done at the end of treatment rather than after 2 months of therapy. Therefore, the use of imaging cannot at present be recommended to optimize the duration of the therapeutic trial of ATT [Citation1].
1.5. Serum biomarkers: work in progress
1.5.1. T regulatory cells
Inflammatory bowel disease including CD is considered as a manifestation of the dysregulated immune response. Important regulators of immune function are the natural (produced by Thymus) or induced (peripherally produced) regulatory T cells (T regs or CD4+ CD25+ FOXP3+). These T regs have the ability to home or accumulate in the gut. Defects in the number, function and the ability of T regs to home to the gut have been implicated in the genesis of Crohn disease. The comparison of T regulatory cells in ITB and CD has demonstrated that the T reg cells are increased in patients with ITB vis-à-vis CD and a cutoff of 32.5% cells had a good sensitivity (75%) and specificity (90.6%) for diagnosis of ITB. Further, the elevated T regs decline with antimycobacterial treatment. The tissue expression of FOXP3 mRNA in peripheral blood and colonic tissue CD4+ FOXP3+ cells on immunohistochemistry were increased in patients with ITB. The discriminative ability of the T regs is preserved even in patients where the diagnosis was deemed indeterminate [Citation19].
1.5.2. Proteomics
The other approach has been a proteomic based approach using high throughput technologies which help in characterization of various proteins, their structure, amount of expression, posttranslational modifications and changes with time. In a study using matrix-assisted laser desorption ionization- time of flight (MALDI-TOF) based mass spectrometry on serum, ten most differentially expressed proteins were used to develop discriminative models and eventually a model utilizing three proteins had the best discriminative ability with a sensitivity of 76.2% and specificity of 80%. Two of these biomarker proteins (Appetite peptide and LOXL-2) were also purified and identified [Citation20]. In another proteomic based approach using tandem mass tag labeled technology, certain proteins like TNF ligand superfamily 13, perioxiredoxin, fibulin-5, CutA and T-complex protein subunit – gamma etc. were differentially expressed in CD [Citation21]. While the proteomic based approach seems promising, validation studies to confirm these findings are required before these findings can be translated to the bedside.
To summarize, there are many unexplored areas like assessment of microbiome, tissue antigen expression and molecular imaging which should be evaluated to discriminate these two entities. Such modalities are required both to improve the diagnosis at the baseline and to improve the assessment of response to therapy. Till then, assessment of early mucosal response to therapy using colonoscopy at 2 months should remain the method of choice to distinguish ITB and CD in indeterminate cases in endemic regions () until better tools for discrimination are available to the clinicians.
Figure 1. Janus shaped pictorial representation of the parameters used for diagnosis of intestinal tuberculosis in the past, currently or likely to be of use in future. These apply to cases where the diagnosis is un clear after routine microbiological and histological tests.
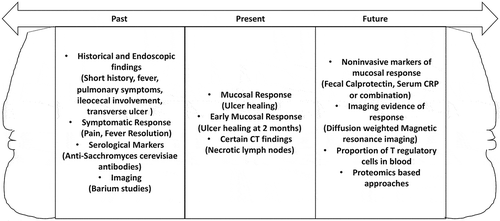
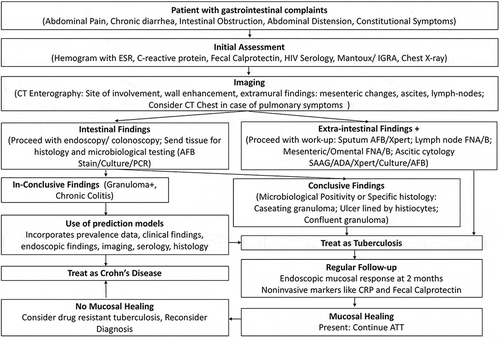
Figure 2. Flow chart to discriminate ITB and CD in regions where TB is endemic (Reproduced from Goyal P, Shah J, Gupta S, Gupta P, Sharma V. Imaging in discriminating intestinal tuberculosis and Crohn’s disease: past, present and the future. Expert Rev Gastroenterol Hepatol. 2019;13(10):995‐1007. with permission.).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Goyal P, Shah J, Gupta S, et al. Imaging in discriminating intestinal tuberculosis and Crohn’s disease: past, present and the future. Expert Rev Gastroenterol Hepatol. 2019;13(10):995–1007.
- Sharma V, Debi U, Mandavdhare H, et al. Tuberculosis and other mycobacterial infections of the abdomen. In: Kuipers EJ, editor. Encyclopedia of gastreonterology. 2nd ed. Academic Press, Elsevier; 2020. p. 646–659.
- Seo H, Lee S, So H, et al. Temporal trends in the misdiagnosis rates between Crohn’s disease and intestinal tuberculosis. World J Gastroenterol. 2017;23(34):6306‐6314.
- Banerjee R, Pal P, Girish BG, et al. Risk factors for diagnostic delay in Crohn’s disease and their impact on long-term complications: how do they differ in a tuberculosis endemic region? Aliment Pharmacol Ther. 2018 May;47(10):1367–1374. 2019 Oct;13(10):995–1007.
- Limsrivilai J, Shreiner AB, Pongpaibul A, et al. Meta-analytic bayesian model for differentiating intestinal tuberculosis from Crohn’s disease. Am J Gastroenterol. 2017;112(3):415‐427.
- Rana SS, Sharma V, Sharma R, et al. Capsule endoscopy in small bowel Crohn’s disease and tuberculosis. Trop Doct. 2017 Apr;47(2):113–118.
- Kim YG, Kim KJ, Min YK. Comparison of small bowel findings using capsule endoscopy between Crohn’s disease and intestinal tuberculosis in Korea. Yeungnam Univ J Med. 2020 Apr;37(2):98–105.
- Ko JK, Lee HL, Kim JO, et al. Visceral fat as a useful parameter in the differential diagnosis of Crohn’s disease and intestinal tuberculosis. Intest Res. 2014;12(1):42–47.
- Du J, Ma YY, Xiang H, et al. Confluent granulomas and ulcers lined by epithelioid histiocytes: new ideal method for differentiation of ITB and CD? A meta analysis. PLoS One. 2014 Oct 9;9(10):e103303.
- Bellam BL, Mandavdhare HS, Sharma K, et al. Utility of tissue Xpert-Mtb/Rif for the diagnosis of intestinal tuberculosis in patients with ileocolonic ulcers. Ther Adv Infect Dis. 2019;6:2049936119863939.
- Rukmangadachar LA, Makharia GK, Mishra A, et al. Proteome analysis of the macroscopically affected colonic mucosa of Crohn’s disease and intestinal tuberculosis. Sci Rep. 2016;6:23162.
- Banerjee R, Balaji M, Sasikala M, et al. Granulomas of intestinal tuberculosis and Crohn’s disease can be differentiated by CD73 cell surface marker expression: a pilot study. Dig Dis Sci. 2013 Aug;58(8):2301–2307.
- Watermeyer GA, Locketz M. CD73 expression in tissue granulomas in distinguishing intestinal tuberculosis from Crohn’s disease in a South African cohort. Scand J Gastroenterol. 2018 Oct–Nov;53(10–11):1217–1221.
- Sharma V, Mandavdhare HS, Dutta U. Letter: mucosal response in discriminating intestinal tuberculosis from Crohn’s disease-when to look for it? Aliment Pharmacol Ther. 2018 Mar;47(6):859–860.
- Park YS, Jun DW, Kim SH, et al. Colonoscopy evaluation after short-term anti-tuberculosis treatment in nonspecific ulcers on the ileocecal area. World J Gastroenterol. 2008 Aug 28;14(32):5051–5058.
- Sharma V, Mandavdhare HS, Lamoria S, et al. Serial C-reactive protein measurements in patients treated for suspected abdominal tuberculosis. Dig Liver Dis. 2018 Jun;50(6):559–562.
- Agrawal G, Clancy A, Sharma R, et al. Targeted combination antibiotic therapy induces remission in treatment-naïve Crohn’s disease: a case series. Microorganisms. 2020 Mar 6;8(3):pii: E371.
- Sharma V, Verma S, Kumar-M P, et al. Serial measurements of fecal calprotectin help discriminate intestinal tuberculosis and Crohn’s disease in patients started on antitubercular therapy trial. J Gastroenterol Hepatol. 2019;34(Suppl. 3):534.
- Tiwari V, Kedia S, Garg SK, et al. CD4+ CD25+ FOXP3+ T cell frequency in the peripheral blood is a biomarker that distinguishes intestinal tuberculosis from Crohn’s disease. PLoS One. 2018 Feb 28;13(2):e0193433.
- Zhang F, Xu C, Ning L, et al. Exploration of serum proteomic profiling and diagnostic model that differentiate Crohn’s disease and intestinal tuberculosis. PLoS One. 2016;11(12):e0167109.
- Ning L, Shan G, Sun Z, et al. Serum proteome profiles to differentiate Crohn disease from intestinal tuberculosis and primary intestinal lymphoma: A pilot study. Medicine (Baltimore). 2019;98(50):e18304.
