Clinically, the term ‘pulmonary gas exchange’ is used to describe a wide range of global and regional physiological measurements. Global measurements aspire to characterize the general gas exchange status of the patient by using single output parameters (e.g. arterial blood gas tensions of O2 (PaO2) and CO2 (PaCO2), total O2 uptake or CO2 elimination, or ventilation/perfusion (V/Q) distribution parameters), arguably making them easy to apply diagnostically. Their shortcoming is that they provide no spatial information on how well different anatomical units within the lung are contributing to gas exchange. In contrast, the data provided by imaging regional ventilation, perfusion, and V/Q provide a more detailed picture of the physiological factors affecting the patient’s gas exchange state but tend to be more difficult to interpret. It is perhaps for that reason that V/Q imaging has generally been restricted to a small number of clinical applications (diagnosing pulmonary embolisms and presurgical screening in chronic obstructive pulmonary disease (COPD) and lung cancer being the primary ones).
In pulmonary physiology, the term regional gas exchange is very often used as a synonym of regional V/Q ratio. While it is true that the local value of V/Q is an important determinant of local end-capillary and alveolar O2 and CO2 levels, it cannot be used as a surrogate for the local rates of gas exchange, and it is not possible to infer the functional contribution of different lung regions to global gas exchange exclusively based on local values of V/Q.Footnote1
We recently described an algorithm to generate 3D maps of regional gas exchange rates of O2 and CO2 [Citation1]. The first method for deriving regional gas exchange rates was originally described more than 60 years ago using low-resolution imaging data (nine isogravitational slices) [Citation2]. However, the clinical use of regional gas transfer rates never caught on, in great part because the low spatial resolution of the then-available imaging methods was not sufficient to reflect gas transfer abnormalities in lung disease. In the intervening years, the spatial resolution and accuracy of ventilation and perfusion imaging have increased by orders of magnitude, and resolution elements smaller than one cubic centimeter are now feasible, making it possible to calculate local rates of pulmonary gas exchange at scales approaching those of the functional lung unit.
In our article [Citation1], we presented an iterative method to derive local values of O2 and CO2 transfer rates within the lungs using imaging data of local ventilation and perfusion as inputs. We illustrated the method by generating 3D gas transfer maps for a healthy subject and for a patient with asthma after a methacholine provocation, showing regional impairment of gas exchange. By combining our method with CT lung image segmentation algorithms, it is now possible to quantify the magnitude of O2 and CO2 exchange rates at the lobar, segmental, or subsegmental level and express them as absolute values, percent of predicted, or as fractions of total gas exchange of the lung. One can also estimate regional gas transfer rates normalized by lung tissue volume – a descriptor of gas exchange efficiency.
Quantifying gas exchange at lobar or segmental levels may be especially useful for presurgical evaluation of candidates to pulmonary lobectomy or segmentectomy therapies for lung cancer, nonmalignant masses, suppurative lesions, tuberculosis, and fungal infections, as well as in emphysema (i.e. lung volume reduction surgery [LVRS] or bullectomy).
Currently, American Thoracic Society guidelines for evaluating eligibility of lung cancer patients for lobectomy [Citation3] are based, among other indicators, on an algorithm to predict postoperative diffusion capacity of the lung for carbon monoxide (DLCO) (and/or forced expiratory volume in 1 second (FEV1)) as:
where ‘DLCOPPO’ (or FEV1PPO) is a predicted postoperative value, ‘DLCOpre’ (or FEV1pre) is a preoperative measured value, ‘nres’ is the number of lung segments to be resected, and ‘ntotal’ is the total number of functional segments preoperation.
Based on the values of DLCOPPO and FEV1PPO, patients can be either cleared for surgery (DLCOPPO and FEV1PPO > 60% of predicted) or need to undergo further cardiopulmonary testing to ascertain their operability. Even so, the decision to operate or not involves high degrees of uncertainty, and more accurate methods for predicting operability are needed.
By mapping CO2 and O2 transfer rates, it is possible to quantify the contribution to overall gas exchange of lung segments to be resected. Given that each segment in the lung may contribute differently to gas exchange, the 3D maps of gas exchange should allow for a more accurate prediction of the surgical impact on overall lung function and gas exchange status compared with the simpler calculation based on number of resected segments.
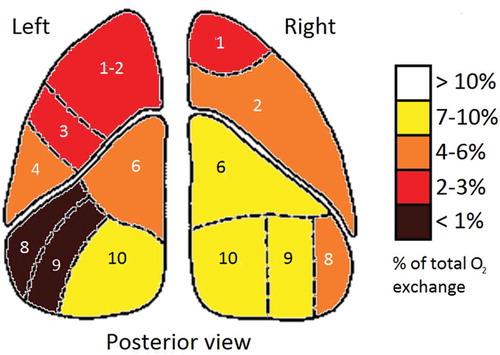
In the fictive example of a regional O2 exchange image illustrated in , it is obvious that the left lung’s anterior and lateral basal segments (8 and 9, left lung) could be removed with little impact on O2 exchange, whereas removing the right lung’s posterior lateral and anterior basal segments (9 and 10, right lung) would greatly impact pulmonary O2 exchange.
Figure 1. Fictive example of pulmonary oxygen uptake by lung segment. Posterior view of the coronal plane.
As an alternative to the algorithm based on number of segments, it is possible to calculate a DLCOPPO value based on the fraction of total gas exchange in the segments to be resected (which would also obviate the need to define the cutoff for what constitutes a ‘functional’ segment).
As a first approximation, DLCO can be assumed to be proportional to the ratio of the exchange of O2 () to the driving pressure (
PO2) across the capillary membranes. Since the fraction of DLCO/
for a given
PO2 should be constant,
where ΣVO2,s is the sum of oxygen uptake in the segments to be spared by the surgery, and is the total oxygen uptake in all segments. It follows that
where is the fraction of oxygen uptake in the segments to be resected. Note that Equation (2) is similar to Equation (1) but using segmental O2 transfer rates derived from the gas exchange images instead of the number of segments.
It is important to point out that the total postoperative gas exchange rate depends on the metabolic rate of the body and not on the lungs. For the patient to survive the operation, the segments spared by the surgery must increase their local gas exchange in order to compensate for the loss of gas exchange in the resected segments, in turn preserving total VO2. These increases are produced by increased ventilation, perfusion, and/or changes in baseline blood gas tensions. The complexity of such changes may reduce the accuracy of Equation 2, but it should still constitute a marked improvement for estimating the potential impact of surgery on lung function.
As a further line of inquiry, the close relationship between regional ventilation and CO2 transfer rate observed in our study supports the concept that the contribution to CO2 transfer of a lung section is correlated to its contribution to the FEV1. If that is correct, a similar equation could then be derived to estimate the postoperative predicted value of FEV1 (FEV1PPO), using the regional information obtained from the CO2 transfer maps:
(ΣVCO2,res = sum of CO2 elimination in the segments to be resected; VCO2,total = total CO2 elimination in all segments).
In LVRS for COPD with upper lobe-predominant emphysema, the choice of segments to resect is often clear, whereas in less localized emphysema, LVRS is often deemed too risky because results tend to be highly variable [Citation4]. It is conceivable that gas transfer mapping, used in conjunction with lung mechanical considerations, could enable surgeons to better evaluate the operability of patients with less localized disease distribution, allowing more patients to receive the benefits of LVRS. Similarly, gas transfer mapping could be helpful in planning radiotherapy, to minimize the impact on gas exchange induced by radiation damage on tissues.
In pulmonary critical care patients (acute respiratory distress syndrome, COPD exacerbations, etc.), arterial blood gases are in general the main guiding lights for interventions to improve gas exchange, and the balance between acid–base status and oxygenation is often especially pertinent. By utilizing both O2 and CO2 transfer maps in conjunction with knowledge of local shunt, the predictions – or even computational simulations – of the effect of interventions on arterial pH and oxygenation may be strengthened.
Another potential use for the present method is as a tool for cross-validation of different imaging techniques aiming directly or indirectly at measuring regional pulmonary O2 uptake rate [Citation5]. Last but not least, gas transfer maps could see use in basic research on pulmonary physiology and as a teaching tool for visualizing and clarifying the physiology of pulmonary gas transfer.
Overall, we believe that the gas transfer mapping method can yield valuable contributions to the understanding of pulmonary gas exchange in both health and disease; by visualizing the actual rates of gas transfer, in contrast to regional ventilation, perfusion, or V/Q ratio, the functional impact of a particular ventilation and perfusion derangement may be displayed and understood in practical and intuitive terms. Furthermore, the method could be used to enhance our understanding of the dissimilarities between the anatomical distribution of O2 and CO2 transfer respectively and how these differences impact PaO2, PaCO2, and arterial pH and oxygen saturation.
We thus envision for the future a number of new avenues of diagnosis and research that could be opened by quantifying and imaging the most basic of the lung’s functions: the distributed transfer of O2 and CO2 between air and blood.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Additional information
Funding
Notes
1. This can be illustrated by a simple example of two lung segments A and B: segment A having ventilation and perfusion of 0.5 L/min, and segment B having ventilation and perfusion of 0.01 L/min. Both segments have the ‘ideal’ unity value for V/Q, and therefore, – all other factors being equal – identical values of local end-capillary and alveolar PO2 and PCO2. Even so, the magnitudes of V and Q obviously imply that the gas transfer rate in segment B will be almost negligible.
References
- Johansen T, Winkler T, Kelly VJ, et al. A method for mapping regional oxygen and CO2 transfer in the lung. Respir Physiol Neurobiol. 2016;222:29–47.
- West JB. Regional differences in gas exchange in the lung of erect man. J Appl Physiol. 1962;17:893–898.
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 SUPPL):e166S–e190S.
- Marchetti N, Criner GJ. Surgical approaches to treating emphysema: lung volume reduction surgery, bullectomy, and lung transplantation. Semin Respir Crit Care Med. 2015;36(4):592–608.
- Kadlecek S, Mongkolwisetwara P, Xin Y, et al. Regional determination of oxygen uptake in rodent lungs using hyperpolarized gas and an analytical treatment of intrapulmonary gas redistribution. NMR Biomed. 2011;24(10):1253–1263.
