ABSTRACT
Introduction: The number of patients with pulmonary disease caused by non-tuberculous mycobacteria (NTM) is increasing globally. Poor resistance against infections, for example, due to pre-existing lung diseases, immune deficiency and immune-modulating treatment, predisposes the population to developing pulmonary NTM disease. The incidence of pre-existing lung diseases such as chronic obstructive pulmonary disease and bronchiectasis has also increased. NTM disease diagnosis is often delayed due to non-specific symptoms. The therapeutic arsenal is limited and adherence to treatment guidelines is often low since the treatment regimens are complex, lengthy and side effects are common. Thus, current disease management is far from satisfactory and needs to be improved.
Areas covered: This review provides an overview of the current knowledge of NTM infections and includes pathogenesis, disease patterns, epidemiology, disease management, unmet needs and future perspectives.
Expert commentary: NTM disease is becoming more prevalent, in part with our increased awareness and improved diagnostic methods. However, our understanding of the disease pathogenesis is limited and treatment decisions are challenging, with difficult to employ drug regimens. Optimal management requires collaboration between healthcare providers, patients and expert centers.
1. Introduction
Nontuberculous mycobacteria (NTM) – also called environmental mycobacteria or mycobacteria other than tuberculosis (MOTT) – encompass all mycobacterial species other than those of the Mycobacterium tuberculosis complex (MTB) and Mycobacterium leprae [Citation1]. Previously, NTM were called ‘anonymous’ or ‘atypical mycobacteria,’ but these terms are no longer in use. NTM are ubiquitous in nature and inhabit a wide variety of environmental reservoirs, including natural and municipal water, soil, animals, and humans. Many NTM are opportunistic pathogens of humans, domestic and wild animals [Citation1]. They vary widely in their pathogenicity and clinical relevance, dependent on concomitant diseases, immunologic status, as well as, age and gender of the host [Citation2,Citation3].
In humans, NTM disease can occur in all organs, but the most common manifestation is NTM pulmonary disease (NTMPD), and this article will focus on such infections. It is widely accepted that transmission of NTM infection is via the environment [Citation4]. However, some evidence is emerging showing patient-to-patient transmission within the cystic fibrosis (CF) community [Citation5].
A handful of NTM species account for the vast majority of patient cases with NTMPD [Citation2]. The NTM most commonly associated with pulmonary disease are those of the Mycobacterium (M.) avium complex (MAC), which encompasses several species including M. avium, M. intracellulare, and M. chimaera [Citation2]. The latter has been identified as the causative agent of some prolonged outbreaks after open-chest surgery [Citation6]. Other NTM frequently associated with NTMPD are M. kansasii and the M. abscessus complex, which encompasses the subspecies abscessus, bolletii, and massiliense [Citation7]. Based on their growth rate in culture, NTM are divided into slow-growing and rapid-growing mycobacteria [Citation1]. The species of the MAC and M. kansasii belong to the slow-growing mycobacteria whereas M. abscessus complex species are rapid growers [Citation2].
The accurate identification of the NTM species (etiologic agent) is critical for diagnosis and management of patients with NTMPD [Citation8].
In the following, we will provide an overview of disease pathogenesis, epidemiology, and characteristics of NTMPD, its current management, unmet clinical needs and future perspectives.
2. Pathogenesis and risk factors of mycobacterial disease
The cellular and physiologic conditions causing NTM disease are poorly understood. Macrophages are crucial for infection control and have a key role in disease pathogenesis. If inhaled, NTM encounter alveolar macrophages, which they can enter via multiple receptor-mediated pathways, and thereafter be taken up in primary phagosomes [Citation9]. The macrophages process mycobacterial antigens for surface presentation to T lymphocytes leading to recruitment and expansion of antigen-specific T lymphocyte clones. The lymphocytes ultimately interact with the infected macrophages to induce intracellular destruction of the mycobacteria or to destroy the infected macrophage itself. These cellular events lead to the formation of granulomas in which infected macrophages become surrounded by mononuclear inflammatory cells and epithelioid histiocytes. Cytokines such as IL-12, TNF-α, and IFN-γ are especially important in the antimycobacterial immune response and regulation. Antibodies seem to play a minor role in human mycobacterial infection, although some studies indicate that they contribute to protective immunity to infection [Citation10].
The formation of granulomas is a defense mechanism and encapsulates mycobacteria, but the granuloma may erupt and viable mycobacteria disseminate. Thus, the resolution of infection may be incomplete or even absent depending on the host factors and the NTM species involved. Consequently, viable NTM persisting in the host can thrive and cause disease in certain circumstances [Citation9].
Patients with NTMPD may have a known predisposing condition () [Citation27,Citation28]. In patients without a clear predisposition, body morphotype may be important with slender women with low body fat content overrepresented in NTM cases [Citation24]. Post-menopausal women may also be predisposed, and the reduced level of dehydroepiandrosterone has been discussed as a possible risk factor [Citation26]. Gastroesophageal reflux disease – also without typical symptoms – may contribute to the development of NTMPD, possibly due to recurrent aspiration into the tracheobronchial tree [Citation25].
Table 1. Host risk factors for pulmonary disease caused by nontuberculous mycobacteria.
Defects in the pathways in which cytokines such as IL-12, TNF-α, and IFN-γ are key factors, genetically or acquired, can lead to increased disease susceptibility [Citation9]. Iatrogenic factors also contribute to NTMPD risk, such as, immunosuppressive treatments including anti-TNF-α therapy and disease-modifying antirheumatic drugs [Citation13,Citation14], and organ transplantation [Citation15,Citation16].
A major and worldwide common cause of immunosuppression is infection with human immunodeficiency virus (HIV). Disseminated NTM is one of the opportunistic infections that are part of the acquired immune deficiency syndrome, especially in patients not receiving highly active antiretroviral therapy [Citation18]. The affected patients suffer from sweats, fever, anorexia, and diarrhea. HIV-infected patients with disseminated NTM may also have pulmonary manifestations and noncharacteristic radiologic presentations. The NTM diagnosis can be confirmed despite nonspecific symptoms by culture from a normally sterile site and/or a respiratory sample. HIV-infected patients may also develop an NTM immune reconstitution syndrome after initiation of highly active antiretroviral therapy.
NTMPD often occurs in people without recognized severe immune dysfunction, but is also strongly associated with preexisting lung diseases [Citation27,Citation28]. The latter include underlying lung pathologies due to chronic obstructive pulmonary disease (COPD), bronchiectasis, prior tuberculosis infection and pneumoconiosis [Citation11]. It has been shown that 9–50% of patients with bronchiectasis may have active NTM lung infections [Citation29–Citation31]. However, cause and effect are hard to differentiate: it is unclear whether NTM invade patients with preexisting bronchiectasis or lead to the initiation of bronchiectasis. The current evidence suggests that both can occur [Citation11,Citation19,Citation32]. COPD is one of the most frequent NTMPD-associated conditions, and the incidence of both diseases is increasing [Citation33,Citation34]. Many patients with NTMPD have at least one additional lung disease, either bronchiectasis or COPD, or both [Citation35]. NTMPD may interact with these and other comorbid conditions and may facilitate or be facilitated by them, affecting clinical observations [Citation36].
A further major risk factor is CF, an autosomal recessive systemic disease that involves the respiratory tract among other organs [Citation12]. Due to the highly viscous airway secretions, CF patients have impaired mucociliary clearance, predisposing them to bacterial infections. The prevalence of NTM infection in patients with CF ranges between 1.3% and 32.7% [Citation37]. NTMPD, and in particular M. abscessus, may impact on suitability for transplantation [Citation37,Citation38].
Individuals with α-1-antitrypsin anomalies [Citation19] and CF transmembrane conductance regulator (CFTR) mutations [Citation20] are predisposed to NTMPD. Some familial clustering of NTMPD has been identified with most of the cases found in siblings with high prevalence of scoliosis and CFTR mutations [Citation11,Citation39]. Patients with NTMPD have a higher burden of immune, CFTR, cilia, and connective tissues gene variants compared with controls, and they often have variants in multiple genes [Citation21].
There is a trend for a shift in the types of comorbidities associated with NTMPD over time. For example, in Germany from 2005 to 2011, a significant decline of immune deficiencies and tuberculosis among NTMPD-associated hospitalizations has been noted, whereas other comorbid conditions such as CF and COPD have been increasing from 2005 to 2011 [Citation33].
In addition to the host, the environment seems to play a role in NTM prevalence and individual susceptibility.
3. Epidemiology
Accurate epidemiologic estimates of NTMPD are challenging. Unlike tuberculosis, NTM disease is not reportable to most public health authorities; hence data are not readily available [Citation40]. Furthermore, NTM infection is acquired from the environment rather than transmitted from person to person. Exposure to NTM is common given their nearly ubiquitous environmental presence, and laboratory isolation is not necessarily equivalent to disease [Citation27].
Environmental conditions play a role in NTM prevalence and individual susceptibility. In the USA, for example, there are regions of higher risk as already demonstrated during the 1950s [Citation41]. According to more recent studies, these correspond to greater population density, higher education and income of inhabitants, large areas of surface water with high evaporation and soil composition [Citation42]. Furthermore, the prevalence of NTM species varies substantially by geographic region, perhaps due to growth conditions associated with different settings. Such geographical variations have important implications, given the inherent differences between NTM species. Nevertheless, MAC predominate in the USA and most western and European Union countries. However, MAC is more frequent in Northern Europe than in Southern Europe [Citation43,Citation44].
The prevalence and incidence of NTMPD has been reported to be increasing throughout the world. For example, an analysis of a 5% sample of US Medicare beneficiaries aged ≥65 years determined a prevalence of 20 NTMPD cases/100,000 in 1997 and 47 cases/100,000 in 2007, an annual increase of 8.2% per year [Citation45]. An analysis of positive culture results of a tertiary cardiothoracic center in London, UK, showed an increase in the annual prevalence of NTM infection from 0.7% in 2011 to 1% of patients in 2014 [Citation46]. Similar trends have also been described in many other countries. Consequently, hospitalization for NTMPD is increasing. For example, in Germany, the annual number of all NTMPD-associated hospitalizations increased from 665 in 2005 to 1039 in 2011, an average annual increase of 4.9%. Overall, there were almost 6000 NTMPD infection-associated hospitalizations during this period [Citation33]. The reasons behind the rise in NTMPD are unclear but several possibilities have been proposed. These include an overall increase in detection due to increased awareness, improved microbiologic detection and identification techniques [Citation47], and changing demographics, with aging populations, increased comorbidities, and immunosuppression [Citation48,Citation49]. Increased pollution, particularly in the large cities, as well as, individual exposure to smoke and organic and chemical products may also have an impact.
The burden of NTM infection and disease in developing countries is not well known, and only few epidemiological studies exist [Citation50,Citation51]. A major problem is that the patients are generally diagnosed based on clinical findings and smear examination, which precludes a proper discrimination between tuberculosis and NTM infection [Citation51,Citation52]. This leads to a high number of patients falsely diagnosed and to large underreporting of NTM infections in these countries [Citation51].
Study results on 5-year all-cause mortality are heterogeneous with rates ranging between 10% and 45% [Citation53–Citation59]. In the randomized-controlled trials by the British Thoracic Society, the 5-year all-cause mortality rates were approximately 40% [Citation54,Citation60]. In all these studies, the NTM-specific mortality rates are much lower, which may represent the accuracy and difficulty in recording cause of death and highlights the comorbidities that many of these patients have. Increasing age, male sex, low income, underlying conditions, as well as, fibrocavitary disease and pulmonary hypertension are significant risk factors for death [Citation59,Citation61].
The considerable costs for care and treatment of patients with NTMPD have an impact on the healthcare system. Furthermore, NTMPD is associated with remarkable indirect costs, e.g. due to sick leave and loss of productivity [Citation62–Citation64].
4. Clinical manifestations of NTMPD
The clinical manifestations of NTMPD are related to a significant negative impact on patient quality of life [Citation65]. A Canadian study showed that the quality of life impairment due to NTMPD is at least as severe as that of several common chronic diseases, including ischemic heart disease, arthritis, diabetes, COPD, and congestive heart failure [Citation65].
The clinical manifestations, the diagnostic findings, as well as the signs and symptoms of NTMPD vary between patients. The clinical disease presentation is strongly dependent on the patients’ characteristics and comorbidities. The main presentations of the disease are described in the following section.
4.1. Fibrocavitary disease
The tuberculosis-like fibrocavitary form of the disease is the traditionally recognized presentation of NTMPD [Citation27]. Apical fibrocavitary lesions are typical. Symptoms include coughing, often with purulent sputum, dyspnea, hemoptysis, and constitutional symptoms [Citation36]. Radiographic findings are characterized by areas of increased opacity and cavitations, usually localized in the upper lobes, with or without calcification (). Apical pleural thickening and fibrosis with volume loss and traction bronchiectasis are frequent [Citation32].
Figure 1. Radiographic presentation of the tuberculosis-like fibrocavitary form of NTMPD. Fibrocavitary disease is characterized by areas of increased opacity and cavitation (arrow), usually in the upper lobes. HRCT image kindly provided by Dr L-O. Larsson.
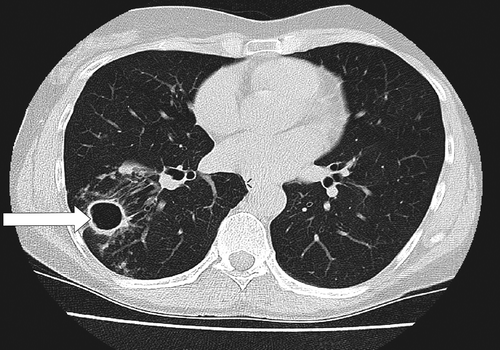
Fibrocavitary disease is generally associated with MAC, M. kansasii and M. abscessus complex. Other reported pathogens include M. malmoense, and M. xenopi [Citation36,Citation66,Citation67].
The fibrocavitary form of NTMPD is the most progressive [Citation36]. Untreated cases can result in extensive cavitary lung destruction and respiratory failure within 1–2 years [Citation27]. This form is associated with greater mortality compared to other forms of NTMPD [Citation55].
Fibrocavitary disease is often observed in white male smokers over 50 years old. Preexisting underlying structural lung diseases such as tuberculosis, COPD, bronchiectasis, and pneumoconiosis are common. In addition, alcohol abuse in these patients may lead to malnutrition, resulting in a weakened immune defense [Citation2,Citation27,Citation66].
4.2. Nodular-bronchiectatic disease
The nodular-bronchiectatic form of NTMPD appears to represent the most common form of NTM disease in many areas today [Citation36]. It often manifests without underlying lung disease [Citation68].
The symptoms are nonspecific with chronic indolent cough, with or without sputum [Citation36]. Fatigue, dyspnea, fever, and weight loss are common; hemoptysis and chest pain occur less commonly [Citation47]. Symptoms often become more prevalent as disease progresses [Citation68]. Nodular bronchiectasis may be visible by chest radiograph but is best observed via high-resolution computed tomography (HRCT) [Citation2]. An example is shown in . The abnormalities are primarily found in the mid and lower lung field. Up to 90% of patients have associated multifocal bronchiectasis, and many patients have clusters of small (5 mm) predominantly peripheral nodules in associated areas of the lung, typically in a bronchovascular distribution. These clusters have been termed ‘tree-in-bud.’ These findings correspond histopathologically to bronchiectasis, bronchiolar and peribronchiolar inflammation and granuloma formation [Citation27]. Small cavitary lesions may also occur in advanced stages of disease [Citation27,Citation36,Citation47].
Figure 2. Radiographic presentation of the nodular-bronchiectatic form of NTMPD. Peripheral nodules and bronchiectasis (arrow) in the lower lung areas. HRCT image kindly provided by Dr L. Codecasa.
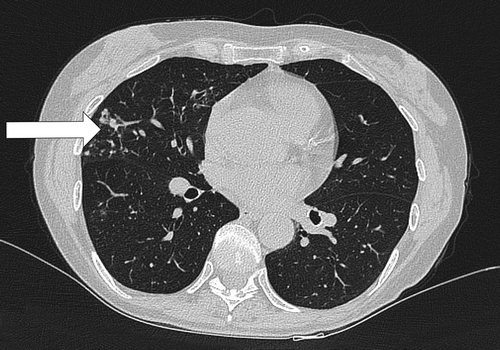
Nodular-bronchiectatic disease is most frequently associated with MAC [Citation36,Citation66,Citation67]. The nodular-bronchiectatic form tends to progress more slowly than cavitary disease, occurring over months, years or not at all [Citation36]. As a result, long-term follow up (months to years) may be necessary to demonstrate clinical or radiographic changes [Citation27].
4.3. Hypersensitivity pneumonitis
Hypersensitivity pneumonitis (HSP), also known as ‘hot-tub lung’ or ‘hypersensitivity-like disease,’ is often not a proper infection, but rather an inflammatory reaction to a large amount of inhaled NTM [Citation27,Citation69,Citation70]. The disease results from exposure to aerosols containing NTM, typically in settings involving hot tubs or metalworking fluids. In such settings, the NTM are able to survive and thrive in harsh environments, even with disinfection [Citation27,Citation69]. Inadequate ventilation, wet storage of filters, and aerosol-producing procedures are the main risk factors in the hot-tub setting [Citation70], in which the causative species are generally MAC [Citation71].
HSP appears to involve components of both infection and inflammation; inflammation is predominant and infection of less importance [Citation27,Citation69,Citation71]. These conditions result in unique features that differ distinctly from other NTM lung diseases [Citation27].
Symptom onset is subacute, and dyspnea, cough, and fever are most frequently observed. In some cases, intensive care is required due to hypoxemic respiratory failure [Citation27]. Findings on chest radiographs and/or CT scans include diffuse pulmonary infiltrates with nodules throughout all lung fields (). In addition, ground glass opacities and a mosaic attenuation pattern are often present on HRCT scans. Pulmonary function tests demonstrate varying degrees of ventilation and diffusion capacity reduction [Citation27].
Figure 3. Radiographic presentation of the hypersensitivity pneumonitis form of NTMPD. Diffuse infiltrates with prominent nodularity are visible throughout all lung fields. HRCT image kindly provided by Dr H. Fjallbrant, Sahlgrenska University Hospital, Gothenburg, Sweden.
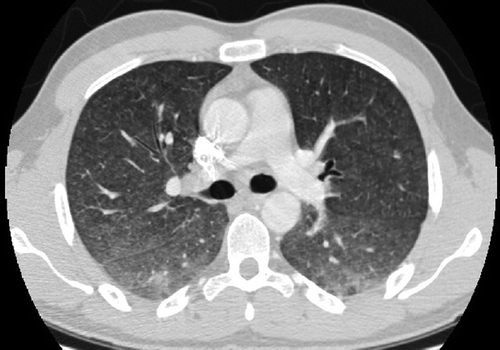
Some patients improve after corticosteroids or simple removal of exposure, while others only improve with antibiotic therapy [Citation71]. Prognosis of patients exposed to large amounts of NTM, rather than infected, is generally good [Citation27].
5. Management of NTMPD
NTMPD poses many challenges concerning diagnosis and treatment, both of which are difficult and thus protracted. These challenges are compounded by the paucity of evidence with which to guide decisions. In 2007, the American Thoracic Society (ATS) and Infectious Disease Society of America (IDSA) jointly published an updated consensus statement on NTMPD. The ATS/IDSA statement included criteria for the diagnosis and treatment of NTMPD, outlined below [Citation27].
5.1. Diagnosis
Diagnosis of NTMPD is often delayed due to nonspecific symptoms and overlapping signs with underlying lung diseases, such as, COPD [Citation27]. Diagnosis is established based on clinical symptoms, as well as, radiologic and microbiologic findings () [Citation27,Citation72]. The most conclusive criterion for NTMPD is a positive NTM culture. Mixed cultures may occasionally occur, e.g. with NTM and M. tuberculosis [Citation73] or with two NTM species, such as, MAC and M. abscessus complex, especially in immunocompromised hosts [Citation74]. Since NTM culture results are only available after 6–12 weeks, methods for direct molecular detection are highly warranted. Although such tests are commercially available (e.g. PCR restriction analysis based on the rpoB gene, quantitative real-time PCR), they are not clinically validated and therefore not yet recommended for routine clinical use [Citation72,Citation75,Citation76]. Radiologic findings may support NTMPD diagnosis, though they cannot be distinctly separated from the findings due to other differential diagnoses. Exclusion of other diagnoses is crucial [Citation27]. Cavitary lesions may not only be caused by mycobacterial disease (including tuberculosis), but also by malignancies or invasive pulmonary fungal and bacterial infections [Citation77].
Figure 4. Requirements for diagnosis of NTMPD (based on ATS/IDSA guidelines [Citation27] and BTS guidelines [Citation72]). HRCT, high-resolution computed tomography; NTMPD, non-tuberculous mycobacterial disease.
![Figure 4. Requirements for diagnosis of NTMPD (based on ATS/IDSA guidelines [Citation27] and BTS guidelines [Citation72]). HRCT, high-resolution computed tomography; NTMPD, non-tuberculous mycobacterial disease.](/cms/asset/3f40ef6b-eb69-4ea8-9d1e-e3c51c917e66/ierx_a_1386563_f0004_b.gif)
5.2. Treatment
5.2.1. The treatment decision
A microbiologic finding of NTM, even with species and drug susceptibility identification, does not always prompt the initiation of treatment. Treatment regimens are complicated, difficult, and costly undertakings, usually involving 1–2 years of combination antibiotic therapy, with attendant adverse effects, interactions, and compliance issues [Citation27,Citation78]. Thus, patients should be preferably treated in or with contact to an expert center.
The decision to treat, therefore, entails a risk-benefit analysis, taking into account symptoms, progression and other factors, including comorbidities, preexisting lung disease, and predisposing genetic or iatrogenic conditions, as well as, the patient’s preferences. The bacterial load and clinical significance of the infecting species are also important factors as basis for treatment decision [Citation27,Citation78]. Low-virulence NTM, such as, M. fortuitum, and environmental ones, such as, M. gordonae and M. terrae, are often associated with contamination and usually do not warrant treatment. Infection with the rapid-growing species M. kansasii closely resembles tuberculosis-like illness and is generally easy to treat, resulting in a low threshold for antibiotic therapy. In contrast, the M. abscessus complex is difficult to eradicate in the majority of cases [Citation27,Citation78].
The decision to treat also depends on the goals of therapy for that patient as eradication is not always possible and improvement or stability may be the major aim of treatment. For eradication, the primary microbiologic treatment end point is 12 months of negative sputum cultures during therapy [Citation27].
Not only microbiologic but also symptomatic improvement is another important goal for the patients. Progression of underlying diseases including bronchiectasis and COPD may, however, hamper symptom improvement. Likewise, radiographic improvement is desirable, although radiographic assessment may be complicated by concomitant lung disease and the limited potential for resolution of consolidated radiologic infiltrates [Citation27].
Treatment failure generally relates to the lack of microbiologic, clinical, or radiographic response following 6 months of appropriate therapy. Some authors have suggested failure as a lack of sputum conversion following either 6 [Citation79] or 12 months [Citation27] of therapy.
5.2.2. Antibiotic treatment
Few studies exist that guide the treatment of NTMPD. The ATS/IDSA guidelines are based largely on expert opinion. The current evidence base is composed of single-center observational studies and randomized-controlled trials from the British Thoracic Society [Citation54,Citation80]. Treatment regimens differ by species, with the most important distinction being between slow-growing and rapid-growing mycobacteria. For most slow-growing species, the recommended regimen consists of oral drugs including rifampin (or rifabutin), ethambutol, and a macrolide antibiotic, given for 12 months following culture conversion; amikacin or streptomycin can be added in the initial 3 months in severe cases () [Citation27]. As M. kansasii is clinically more closely related to M. tuberculosis than to other NTM species [Citation90], pulmonary disease caused by M. kansasii is usually treated with a combination of isoniazid, rifampin, and ethambutol [Citation27]. For the rapid-growing species, regimens are primarily based on in vitro drug susceptibility testing results. For the M. abscessus complex, the most notorious causative agent of disease among rapid growers, these regimens often include a macrolide antibiotic and amikacin in combination with other intravenous drugs often selected from cefoxitin, imipenem, or tigecycline [Citation91].
Table 2. Standard antibiotic drugs currently used for treatment of nontuberculous mycobacterial pulmonary diseasea.
5.2.2.1. Treatment of MAC lung disease
The recommended treatment for MAC lung disease always involves a multidrug regimen and differs depending on disease type, severity, and previous treatment (). The standard regimen contains a macrolide, a rifamycin, and ethambutol [Citation27]. Intermittent therapy, i.e. three times weekly administration, is an option in patients with nodular-bronchiectatic disease and moderate or mild symptoms [Citation27]. Addition of aminoglycosides should be considered in patients with advanced and/or fibrocavitary disease or previously treated disease, or those with macrolide resistance [Citation27]. The roles of other potential drugs, such as, clofazimine, moxifloxacin, oxazolidinones (linezolid, tedizolid), bedaquiline, and amikacin for inhalation require further evaluation [Citation27,Citation92–Citation100]. Recently, a regimen of clofazimine, ethambutol, and a macrolide showed similar response rates compared with the standard regimen containing rifampin [Citation101].
Table 3. ATS/IDSA-recommended treatment of MAC lung disease [Citation27].
It is essential to monitor patients for toxicity, given the number and nature of the drugs, the length of treatment, and their typical advanced age. The potential for rifabutin toxicity in patients receiving clarithromycin and rifabutin requires particular caution. The parameters that should be monitored are listed in [Citation27].
Table 4. ATS/IDSA-recommended monitoring for drug toxicity of standard antimycobacterial drugs [Citation27].
If the patient has refractory MAC disease, there are few choices for effective treatment [Citation79]. Potential strategies include: switching to another drug in the same class (i.e. clarithromycin to azithromycin or rifampin to rifabutin), switching from intermittent to daily therapy, adjunctive surgery or parenteral aminoglycoside therapy [Citation27,Citation79]. However, the response rate to these options is low, which underscores the importance of prevention of macrolide-resistant disease [Citation95,Citation102].
5.2.2.2. Treatment of M. abscessus complex lung disease
Accurate identification of the disease-causing strain before treatment initiation is critical, since the subspecies differ in their antibiotic susceptibility and treatment outcomes. M. abscessus subsp. massiliense is often susceptible to macrolides, and treatment outcomes are better than for M. abscessus subsp. abscessus, which has a gene that confers inducible macrolide resistance [Citation103,Citation104]. Treatment is often guided by in vitro resistance, however, there is only small data for correlation between in vitro resistance and outcomes for macrolides and aminoglycosides, and clinicians need to be aware of the limitations of drug susceptibility testing for M. abscessus complex [Citation105]. In fact, mutations in the erm(41) gene of M. abscessus group organisms are associated with differences in inducible macrolide resistance. Resultantly, current recommendations are to hold rapid-growing isolates for up to 14 days when performing susceptibility testing to ensure that slowly developing resistance can be detected [Citation106].
The initial treatment consists of two or more parenteral antibiotics and an oral macrolide if the species is macrolide susceptible [Citation27,Citation107]. The most active parenteral agents are amikacin, cefoxitin, imipenem, and tigecycline [Citation27,Citation107]. After the initial treatment phase and cessation of parenteral therapy, combinations of oral antibiotics are recommended e.g. macrolide, fluoroquinolones, linezolid, cotrimoxazole, minocycline, or clofazimine [Citation107,Citation108].
Surgical resection of localized disease combined with optimized multidrug chemotherapy is another therapeutic strategy for M. abscessus complex lung disease [Citation27]. The prognosis is generally discouraging in cases where a medical and joint surgical intervention is not possible [Citation27,Citation109]. Suppressive therapy, including periodic parenteral antibiotic or oral macrolide therapy, may be all that can be realistically administered to control the disease symptoms in these patients [Citation27].
The therapy of M. abscessus complex lung disease is usually complicated, lengthy, and costly, and side effects are common [Citation27,Citation109]. Ideally, treatment decisions should be made by expert clinicians [Citation27]. New treatments for this challenging disease are currently being investigated [Citation97,Citation110].
5.2.2.3. Poor antibiotic treatment outcomes
NTMPD treatment may have a poor outcome. Sustained clinical improvement and culture conversion is not achievable for all patients. Cure is usually defined as sustained culture conversion >12 months after treatment [Citation27]. Cure rates differ by species, disease severity and line of treatment (first line, relapse/reinfection) and typically range from 30–50% in M. abscessus complex [Citation91,Citation111] to 50–70% in MAC [Citation91,Citation112].
Macrolides have become the mainstay of treatment regimens for MAC. Infection with a macrolide-resistant strain or acquisition of macrolide resistance during treatment hinders adequate therapy and is a negative prognostic factor [Citation27]. The major mechanism of acquired bacterial resistance to macrolides is posttranscriptional methylation of the 23S bacterial ribosomal RNA. Risk factors for acquisition of macrolide resistance include macrolide monotherapy or dual therapy with a macrolide and a quinolone only [Citation113]. The likelihood of developing macrolide resistance appears to be relatively low when the treatment regimen complies with the ATS/IDSA-recommended 3-drug therapy [Citation113,Citation114].
Therapeutic options are limited once resistance occurs. One such regimen for patients with macrolide-resistant MAC includes discontinuation of the macrolide and initiation of therapy with ethambutol, rifabutin, and injectable aminoglycosides. However, side effects with this regimen are common and the mortality rate is high, especially among those who do not achieve culture conversion upon treatment [Citation113]. Combined medical treatment, including injectable aminoglycosides, and surgery seems to offer the best treatment outcome in some patients [Citation113,Citation115].
New treatments for macrolide-resistant disease are warranted. Next-generation macrolides such as solithromycin are promising candidates for macrolide-resistant disease [Citation116]. These compounds are not susceptible to erythromycin-resistance methylases, which mediate macrolide resistance [Citation91]. Other drugs for evaluation in macrolide-resistant MAC include clofazimine, moxifloxacin, oxazolidinones (linezolid, tedizolid), and bedaquiline [Citation27,Citation92–Citation96,Citation100].
The options for MAC lung disease refractory to first-line therapy are also limited. There is a paucity of data on which alternative drugs to use. A moxifloxacin-containing regimen was shown to be effective in some cases of refractory MAC lung disease [Citation95], and the roles of other potential drugs have to be further evaluated [Citation27].
Even with treatment that is considered successful, there is a possibility of recurrence, either in the form of relapse or reinfection. Among patients who initially undergo sputum conversion but subsequently develop positive cultures for MAC after discontinuing therapy, many are reinfected by new MAC strains rather than having a relapse with the initial strain [Citation27]. Episodes of true relapse generally present earlier than episodes of reinfection [Citation117]. The rate of recurrence can be considerable, ranging between 8.3% and 48% for MAC lung disease [Citation118,Citation119]. A retrospective study including 158 patients with MAC lung disease showed that nodular-bronchiectatic disease was associated with an increased risk of recurrence [Citation118]. In fact, prolonged treatment may only prevent relapse but not a new infection.
5.2.3. Surgical treatment
In some cases, surgery may be appropriate, but there are no established criteria for patient selection. Patients whose NTMPD is predominantly localized to one lung and/or one lobe who can tolerate surgical resection may be candidates for surgery under some circumstances. These include poor response to antibiotic therapy, macrolide-resistant disease, or severe disease-related complications including hemoptysis. Since pulmonary resections for mycobacterial disease are potentially associated with serious complications, these should preferably be performed in centers with expertise in both medical and surgical management of mycobacterial diseases [Citation27]. To improve the outcome, surgery should only be performed when all other adjustable factors have been optimized, e.g. nutritional status. Several single-center, retrospective studies including small numbers of patients suggest that surgery can be associated with favorable treatment outcome [Citation120]. Surgical resection of a solitary pulmonary nodule due to NTM is considered by experts to be curative, though further data are needed to support this assertion [Citation27].
Overall, surgical resection of limited disease in suitable patients can be a valuable strategy in combination with multidrug therapy for treating MAC lung disease [Citation27].
5.2.4. Adjunctive therapies
Use of adjunctive therapies in addition to antibiotics should be considered. Modalities offering increased mucus clearance, especially in patients with bronchiectasis, include several methods such as inhalation of hypertonic saline or mannitol. Other interventions may be useful, depending on individual possibilities and accessible medical equipment, for example oscillating positive expiratory pressure devices and high-frequency chest compression devices [Citation27]. The patients should have a key role in daily disease management, and they should be made aware that smoking cessation is important for improved airway function. An individual physical training program in close cooperation with a physiotherapist is essential for the wellbeing of the patients and helps preserve their physical capacity [Citation121,Citation122]. Physical function can be monitored with an exercise test, such as, the 6-minute walk test [Citation123]. Involuntary weight loss is a negative prognostic factor, and patient nutritional status should be evaluated in cooperation with a dietitian [Citation124]. Severe vitamin D deficiency may predispose for development or progression of NTM disease [Citation47].
6. Unmet needs
Although the literature and understanding of NTMPD has improved over recent years, there remain significant unmet needs. Epidemiologic data are scant worldwide and influenced by clinical awareness and frequency and quality of testing. Although certain groups appear to be predisposed to NTMPD, we do not understand why some patients are affected, and the different manifestations of the disease process. Determining the impact of the NTM on the underlying condition can be difficult, and the lack of biomarkers can make disease monitoring and treatment decisions complex. All of these factors are complicated further by the fact that present treatment regimens have limited success and may cause significant intolerance. Furthermore, even successful treatment may be followed by high rate of relapse or recurrence.
7. Conclusion
NTM infections represent an increasing problem worldwide. This review highlights the challenges of diagnosis and treatment. The treatment outcomes with the currently recommended multidrug regimens are far from satisfactory and, even worse; guideline recommendations are often not followed. Only a handful of antibiotics with antimycobacterial activity are available, with uncertain efficacy, particularly in cases with expanded resistance and in refractory or recurrent disease. NTMPD considerably impacts all aspects of the patients’ life and is associated with a significant mortality risk. Present treatment recommendations should be followed and consecutively evaluated. A close cooperation between expert centers, healthcare providers, and patients is crucial to improve outcomes.
8. Expert commentary
Despite a worldwide trend to prolonged life expectancy and increasing healthcare resources, some opportunistic infections are becoming more and more common. The frequent combination of advanced age and numerous comorbidities, particularly chronic respiratory diseases, increases the population at risk for opportunistic infections. Modern medical front-line measures such as transplantation and immunomodulation create the basis for opportunistic infections in fragile patients. In particular, this is the case for NTM pulmonary infections, which are currently recognized as an emerging clinical challenge, particularly in developed countries. Can this be considered as a manifestation of species fight for survival between humans (with their antibiotics) and microorganisms? According to Darwin’s theory of evolution, we could easily believe that. However, we should also consider that our current diagnostic ability has noticeably improved in the last decade, therefore leading us to potentially uncover an underestimated disease. However, there is still a lack of adequate identification and epidemiological data on NTMPD, despite a worldwide increase in the rate of these infections.
Unfortunately, we do not understand all aspects of the pathogenesis underlying NTMPD, and identification of patients at risk for NTM infections is still challenging. Although we know that dysfunction of the immune system (primary or secondary immune depression) and preexisting respiratory diseases (CF, bronchiectasis, COPD) are an optimal substrate for NTM, there seem to be several environmental factors (such as pollution and ‘acidity’ in soil and water) and individual susceptibility, which can consistently modulate the risk and severity of NTMPD.
Diagnosis of NTMPD is often delayed due to unspecific symptoms and inadequate use of ATS/IDSA diagnostic criteria. This delay potentially leads to disease progression, since treatment is often started late. Frequently, antibiotic monotherapy is administered before the correct diagnosis is established, thereby increasing the risk of multidrug resistance. In addition, individual characteristics of patients, including immune response, comorbidities and socioeconomic factors can significantly influence clinical presentation and time to diagnosis of NTMPD.
Treatment decisions are also quite challenging since, beyond ATS/IDSA guidelines, there is still debate as to which patients should be treated according to medical background and comorbid conditions, on the drugs to combine according to microbiologic findings and clinical response, and finally on duration of treatment according to clinical, microbiologic, and radiologic response. Moreover, the long duration of multidrug treatment necessitates composite evaluation of: potential side effects, variable response to treatment due to individual factors (drug metabolism, drug–drug interaction), differences between in vitro and in vivo susceptibility for some NTM species such as members of the M. abscessus complex, and finally the high risk of relapse or reinfection.
Such a long and difficult treatment has several implications in terms of clinical management: strict clinical follow up is required including the identification of the most adequate interaction model between healthcare providers and patients. In addition, a comprehensive supportive strategy has to take into account numerous factors such as nutrition, respiratory physiotherapy, management of comorbidities, treatment adherence control and, when possible, a self-management plan.
From a scientific point of view, further research is surely needed to define the ‘immune fingerprint’ that predisposes to NTMPD alongside environmental factors. In addition, new treatment modalities based on scientific evidence and guided upon more reliable microbiologic/or biological markers of disease activity and response to treatment are desirable.
9. Five-year view
Numerous advances are expected in the field of NTMPD in the future. The development of an NTM disease registry will enable a better understanding of the epidemiology worldwide. The potential role of innate and adaptive immune system responses to NTM infections in determining NTM susceptibility, disease severity, and response to treatment has to be further investigated to understand the pathogenesis of NTMPD. A greater knowledge of defense mechanisms could build the basis to design new preventive strategies, such as, immune therapy or vaccination for selected patients and better treatments once the infection is established.
The difficulties in achieving a rapid diagnosis of NTM infections reinforce the need to develop more reliable and faster diagnostic tests compared to the currently used microbiologic cultures. Today’s molecular tests available in clinical practice are still insufficient to guide treatment initiation due to the lack of antibiotic susceptibility (antibiogram). Furthermore, they are often not sensitive enough depending on the sample used. In the future, volatile organic compounds could be a novel noninvasive detection system to diagnose a new NTM infection or to monitor its progression. Also, the development of an interferon gamma release assay for the most relevant NTM species may, for example, assist in providing direction for future epidemiologic studies.
Finally, different therapeutic advances are expected in the future to increase efficacy of antibiotic therapy regimes. For currently available treatments, the efficacy and safety could be improved through the use of therapeutic drug monitoring. In the short term, the introduction of inhaled antibiotics to the multidrug treatment of NTMPD has the potential to increase local antibiotic concentrations, thereby reducing their systemic side effects. Several interesting antimicrobial agents are still awaiting proper evaluation within randomized clinical trials such as clofazimine. The synergistic activity between clofazimine and amikacin [Citation96] observed in vitro is an interesting example that warrants further study.
New methods for testing the complex pharmacodynamic and pharmacokinetic properties of antimicrobial agents in vitro, such as, the hollow fiber model will help to assess various treatment strategies in different NTM to provide guidance for the potential study of promising treatment regimens in patients. In the longer term, the development of disease-specific immunotherapy could have the potential to increase clinical cure rates by potentiating host defenses.
Key issues
The incidence of non-tuberculous mycobacterial pulmonary disease (NTMPD) increases throughout the world. Reasons behind the rise may include an overall increase in detection due to increased awareness, improved microbiologic detection and identification techniques, and changing demographics, with aging populations and increased comorbidities.
NTMPD often occurs in people without recognized severe immune dysfunction, but is also strongly associated with pre-existing lung diseases such as COPD, bronchiectasis, prior active tuberculosis and pneumoconiosis.
Macrophages are crucial for infection control and have a key role in disease pathogenesis. Cytokines such as IL-12, TNF-α, and IFN-γ are especially important in the anti-mycobacterial immune response and regulation. Defects in the cytokine pathways, genetically or acquired, can lead to increased disease susceptibility.
Diagnosis of NTMPD is often delayed due to non-specific symptoms and overlapping signs with underlying lung diseases such as COPD. Diagnosis is established based on clinical symptoms, as well as, radiologic and microbiologic findings.
The long duration of multidrug treatment necessitates composite evaluation of: potential side effects, variable response to treatment due to individual factors (drug metabolism, drug-drug interaction), differences between in-vitro and in-vivo susceptibility for some NTM species such as members of the M. abscessus complex, and the high risk of relapse or reinfection.
Cure rates differ by species, disease severity and line of treatment and typically range from 30–50% in M. abscessus complex to 50–70% in MAC. Rates for 5-year all-cause mortality are heterogeneous, ranging between 10% and 45%.
Although the understanding of NTMPD has improved over recent years, significant unmet needs remain, especially with respect to knowledge of disease pathology, lack of biomarkers and the limited success and intolerance of present treatments.
NTMPD considerably impacts all aspects of the patients’ life. A close cooperation between expert centers, healthcare providers, and ‘empowered’ patients is crucial to improve outcomes. Open access/on demand clinical care for these patients should be ensured.
Declaration of interest
LO Larsson received payments for consultancy from Insmed. E Polverino received payments for consultancy from Bayer and Insmed. W Hoefsloot received payments for consultancy and an unrestricted study grant from Insmed. LR Codecasa received payments for consultancy from Janssen and Insmed. R Diel received payments for lectures and/or consultancy from Bayer, Insmed, and Riemser. SG Jenkins received payment for a consultancy with Insmed. MR Loebinger received payments for consultancy and lectures from Insmed. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
The authors thank Malin Ridell for valuable discussions on the content and linguistic questions.
Additional information
Funding
References
- Primm TP, Lucero CA, Falkinham JO 3rd. Health impacts of environmental mycobacteria. Clin Microbiol Rev. 2004;17:98–106.
- Johnson MM, Odell JA Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210–220.
- Han XY, Tarrand JJ, Infante R, et al. Clinical significance and epidemiologic analyses of Mycobacterium avium and Mycobacterium intracellulare among patients without AIDS. J Clin Microbiol. 2005;43:4407–4412.
- Falkinham JO 3rd. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107:356–367.
- Bryant JM, Grogono DM, Rodriguez-Rincon D, et al. Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 2016;354:751–757.
- Sax H, Bloemberg G, Hasse B, et al. Prolonged outbreak of mycobacterium chimaera infection after open-chest heart surgery. Clin Infect Dis. 2015;61:67–75.
- Sassi M, Drancourt M Genome analysis reveals three genomospecies in Mycobacterium abscessus. BMC Genomics. 2014;15:359.
- Tortoli E Microbiological features and clinical relevance of new species of the genus Mycobacterium. Clin Microbiol Rev. 2014;27:727–752.
- Griffith DE Pathogenesis of nontuberculous mycobacterial infections. UpToDate. (http://www.uptodate.com/contents/pathogenesis-of-nontuberculous-mycobacterial-infections; accessed 15. Dec 2015).
- Glatman-Freedman A, Casadevall A Role of antibody-mediated immunity in host defense against mycobacterium tuberculosis. In: Cole S, Eisenach K, McMurray D, et al., editors. Tuberculosis and the Tubercle Bacillus. Washington, DC: ASM Press; 2005.
- Sexton P, Harrison AC Susceptibility to nontuberculous mycobacterial lung disease. Eur Respir J. 2008;31:1322–1333.
- Skolnik K, Kirkpatrick G, Quon BS Nontuberculous mycobacteria in cystic fibrosis. Curr Treat Options Infect Dis. 2016;8:259–274.
- Brode SK, Jamieson FB, Ng R, et al. Increased risk of mycobacterial infections associated with anti-rheumatic medications. Thorax. 2015;70:677–682.
- Winthrop KL, Chang E, Yamashita S, et al. Nontuberculous mycobacteria infections and anti-tumor necrosis factor-alpha therapy. Emerg Infect Dis. 2009;15:1556–1561.
- Daley CL Nontuberculous mycobacterial disease in transplant recipients: early diagnosis and treatment. Curr Opin Organ Transplant. 2009;14:619–624.
- Knoll BM, Kappagoda S, Gill RR, et al. Non-tuberculous mycobacterial infection among lung transplant recipients: a 15-year cohort study. Transpl Infect Dis. 2012;14:452–460.
- Brode SK, Campitelli MA, Kwong JC, et al. The risk of pulmonary nontuberculous mycobacterial disease associated with inhaled corticosteroid use Am J Respir Crit Care Med. 2016;193:A7503.
- Jones D, Havlir DV Nontuberculous mycobacteria in the HIV infected patient. Clin Chest Med. 2002;23:665–674.
- Chan ED, Iseman MD Underlying host risk factors for nontuberculous mycobacterial lung disease. Semin Respir Crit Care Med. 2013;34:110–123.
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074.
- Szymanski EP, Leung JM, Fowler CJ, et al. Pulmonary nontuberculous mycobacterial infection. A multisystem, multigenic disease. Am J Respir Crit Care Med. 2015;192:618–628.
- Fowler CJ, Olivier KN, Leung JM, et al. Abnormal nasal nitric oxide production, ciliary beat frequency, and Toll-like receptor response in pulmonary nontuberculous mycobacterial disease epithelium. Am J Respir Crit Care Med. 2013;187:1374–1381.
- Kartalija M, Ovrutsky AR, Bryan CL, et al. Patients with nontuberculous mycobacterial lung disease exhibit unique body and immune phenotypes. Am J Respir Crit Care Med. 2013;187:197–205.
- Honda JR, Knight V, Chan ED Pathogenesis and risk factors for nontuberculous mycobacterial lung disease. Clin Chest Med. 2015;36:1–11.
- Koh WJ, Lee JH, Kwon YS, et al. Prevalence of gastroesophageal reflux disease in patients with nontuberculous mycobacterial lung disease. Chest. 2007;131:1825–1830.
- Danley J, Kwait R, Peterson DD, et al. Normal estrogen, but low dehydroepiandrosterone levels, in women with pulmonary Mycobacterium avium complex. A preliminary study. Ann Am Thorac Soc. 2014;11:908–914.
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416.
- Wu UI, Holland SM. Host susceptibility to non-tuberculous mycobacterial infections. Lancet Infect Dis. 2015;15:968–980.
- Mirsaeidi M, Hadid W, Ericsoussi B, et al. Non-tuberculous mycobacterial disease is common in patients with non-cystic fibrosis bronchiectasis. Int J Infect Dis. 2013;17:e1000–4.
- Tanaka E, Amitani R, Niimi A, et al. Yield of computed tomography and bronchoscopy for the diagnosis of Mycobacterium avium complex pulmonary disease. Am J Respir Crit Care Med. 1997;155:2041–2046.
- Faverio P, Stainer A, Bonaiti G, et al. Characterizing non-tuberculous mycobacteria infection in bronchiectasis. Int J Mol Sci. 2016;17:1913.
- Bonaiti G, Pesci A, Marruchella A, et al. Nontuberculous mycobacteria in noncystic fibrosis bronchiectasis. Biomed Res Int. 2015;2015:197950.
- Ringshausen FC, Apel RM, Bange FC, et al. Burden and trends of hospitalisations associated with pulmonary non-tuberculous mycobacterial infections in Germany, 2005-2011. BMC Infect Dis. 2013;13:231.
- Andrejak C, Nielsen R, Thomsen VO, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68:256–262.
- Griffith DE, Aksamit TR Bronchiectasis and nontuberculous mycobacterial disease. Clin Chest Med. 2012;33:283–295.
- Weiss CH, Glassroth J Pulmonary disease caused by nontuberculous mycobacteria. Expert Rev Respir Med. 2012;6:597–612; quiz 3.
- Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71 Suppl 1:i1–22.
- Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34:1–15.
- Colombo RE, Hill SC, Claypool RJ, et al. Familial clustering of pulmonary nontuberculous mycobacterial disease. Chest. 2010;137:629–634.
- Kendall BA, Winthrop KL Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94.
- Edwards LB, Palmer CE Epidemiologic studies of tuberculin sensitivity. I. Preliminary results with purified protein derivatives prepared from atypical acid-fast organisms. Am J Hyg. 1958;68:213–231.
- Adjemian J, Olivier KN, Seitz AE, et al. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. 2012;186:553–558.
- Faria S, Joao I, Jordao L General overview on nontuberculous mycobacteria, biofilms, and human infection. J Pathog. 2015;2015:809014.
- Stout JE, Koh WJ, Yew WW Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–134.
- Adjemian J, Olivier KN, Seitz AE, et al. Prevalence of nontuberculous mycobacterial lung disease in U.S. Medicare beneficiaries. Am J Respir Crit Care Med. 2012;185:881–886.
- Cowman S, Burns K, Benson S, et al. The antimicrobial susceptibility of non-tuberculous mycobacteria. J Infect. 2016;72:324–331.
- McShane PJ, Glassroth J. Pulmonary disease due to nontuberculous mycobacteria: current state and new insights. Chest. 2015;148:1517–1527.
- Al-Houqani M, Jamieson F, Mehta M, et al. Aging, COPD, and other risk factors do not explain the increased prevalence of pulmonary Mycobacterium avium complex in Ontario. Chest. 2012;141:190–197.
- Van Ingen J, Hoefsloot W, Dekhuijzen PN, et al. The changing pattern of clinical Mycobacterium avium isolation in the Netherlands. Int J Tuberc Lung Dis. 2010;14:1176–1180.
- Botha L, Gey van Pittius NC, van Helden PD. Mycobacteria and disease in southern Africa. Transbound Emerg Dis. 2013;60 Suppl 1:147–156.
- Gopinath K, Singh S Non-tuberculous mycobacteria in TB-endemic countries: are we neglecting the danger? PLoS Negl Trop Dis. 2010;4:e615.
- WHO 2002. An expanded DOTS framework for effective tuberculosis control (http://apps.who.int/iris/bitstream/10665/67232/1/WHO_CDS_TB_2002.297.pdf; 2017 Sep 06).
- Andrejak C, Thomsen VO, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. 2010;181:514–521.
- Jenkins PA, Campbell IA, Banks J, et al. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax. 2008;63:627–634.
- Hayashi M, Takayanagi N, Kanauchi T, et al. Prognostic factors of 634 HIV-negative patients with Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012;185:575–583.
- Ito Y, Hirai T, Maekawa K, et al. Predictors of 5-year mortality in pulmonary Mycobacterium avium-intracellulare complex disease. Int J Tuberc Lung Dis. 2012;16:408–414.
- Gommans EP, Even P, Linssen CF, et al. Risk factors for mortality in patients with pulmonary infections with non-tuberculous mycobacteria: a retrospective cohort study. Respir Med. 2015;109:137–145.
- Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. 2014;11:1–8.
- Marras TK, Campitelli MA, Lu H, et al. Pulmonary nontuberculous mycobacteria-associated deaths, Ontario, Canada, 2001-2013. Emerg Infect Dis. 2017;23:468–476.
- Research Committee of the British Thoracic Society. Pulmonary disease caused by Mycobacterium avium-intracellulare in HIV-negative patients: five-year follow-up of patients receiving standardised treatment. Int J Tuberc Lung Dis. 2002;6:628–634.
- Fleshner M, Olivier KN, Shaw PA, et al. Mortality among patients with pulmonary non-tuberculous mycobacteria disease. Int J Tuberc Lung Dis. 2016;20:582–587.
- Leber A, Marras TK The cost of medical management of pulmonary nontuberculous mycobacterial disease in Ontario, Canada. Eur Respir J. 2011;37:1158–1165.
- Collier SA, Stockman LJ, Hicks LA, et al. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol Infect. 2012;140:2003–2013.
- Diel R, Jacob J, Lampenius N, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J. 2017;49:1602109.
- Mehta M, Marras TK Impaired health-related quality of life in pulmonary nontuberculous mycobacterial disease. Respir Med. 2011;105:1718–1725.
- Stout JE Evaluation and management of patients with pulmonary nontuberculous mycobacterial infections. Expert Rev Anti Infect Ther. 2006;4:981–993.
- Wagner D, Young LS Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32:257–270.
- Jarzembowski JA, Young MB Nontuberculous mycobacterial infections. Arch Pathol Lab Med. 2008;132:1333–1341.
- Falkinham JO, 3rd. Nontuberculous mycobacteria in the environment. Clin Chest Med. 2002;23:529–551.
- Fjallbrant H, Akerstrom M, Svensson E, et al. Hot tub lung: an occupational hazard. Eur Respir Rev. 2013;22:88–90.
- Marras TK, Wallace RJ Jr., Koth LL, et al. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest. 2005;127:664–671.
- British Thoracic Society NTM Guideline Development Group. British Thoracic Society Guidelines for the diagnosis and management of non-tuberculous mycobacterial pulmonary disease (NTM-PD). 2017;under consultation cited 2017 March 03 Available: (https://www.brit-thoracic.org.uk/document-library/clinical-information/non-tuberculous-mycobacteria/ntm-guideline/bts-guidelines-for-the-diagnosis-and-management-of-ntm-pd/,
- Jun HJ, Jeon K, Um SW, et al. Nontuberculous mycobacteria isolated during the treatment of pulmonary tuberculosis. Respir Med. 2009;103:1936–1940.
- Levy-Frebault V, Pangon B, Bure A, et al. Mycobacterium simiae and Mycobacterium avium-M. intracellulare mixed infection in acquired immune deficiency syndrome. J Clin Microbiol. 1987;25:154–157.
- Kim S, Park EM, Kwon OJ, et al. Direct application of the PCR restriction analysis method for identifying NTM species in AFB smear-positive respiratory specimens. Int J Tuberc Lung Dis. 2008;12:1344–1346.
- Perry MD, White PL, Ruddy M Potential for use of the Seegene Anyplex MTB/NTM real-time detection assay in a regional reference laboratory. J Clin Microbiol. 2014;52:1708–1710.
- Gadkowski LB, Stout JE Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21:305–333, table of contents.
- Thomson RM, Yew WW When and how to treat pulmonary nontuberculous mycobacterial diseases. Respirology. 2009;14:12–26.
- Griffith DE, Aksamit TR Therapy of refractory nontuberculous mycobacterial lung disease. Curr Opin Infect Dis. 2012;25:218–227.
- Research Committee of the British Thoracic Society. First randomised trial of treatments for pulmonary disease caused by M avium intracellulare, M malmoense, and M xenopi in HIV negative patients: rifampicin, ethambutol and isoniazid versus rifampicin and ethambutol. Thorax. 2001;56:167–172.
- Barrow W Treatment of mycobacterial infections. Rev Sci Tech. 2001;20:55–70.
- Schönfeld N, Haas W, Richter E, et al. [Recommendations for diagnosis and treatment of nontuberculous mycobacterioses of the German Central Committee against tuberculosis and the German Respiratory Society]. Pneumologie. 2013;67:605–633.
- Rifadin prescribing information. cited 2016 Jan 05. Available from: (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.DrugDetails;.
- Mycobutin prescribing information. cited 2016 Jan 05. Available from: (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo;.
- Biaxin prescribing information. cited 2016 Jan 05 Available from: (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo;.
- Myambutol prescribing information. cited 2016 Jan 05 Available from: (http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo;.
- Streptomycin prescribing information. cited 2016 Jan 25 Available from: (http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/064210s009lbl.pdf;.
- Amikacin summary of product characteristics. cited 2017 Sep 01 Available from: (http://www.medicines.org.uk/emc/medicine/22773/SPC/AMIKIN+INJECTION+100MG+2ML;.
- Smith CRMR, Edwards CQ, Rogers JF, et al. Nephrotoxicity induced by gentamicin and amikacin. Johns Hopkins Med J. 1978;142:85–90.
- Evans SA, Colville A, Evans AJ, et al. Pulmonary Mycobacterium kansasii infection: comparison of the clinical features, treatment and outcome with pulmonary tuberculosis. Thorax. 1996;51:1248–1252.
- Van Ingen J, Boeree MJ, Van Soolingen D, et al. Resistance mechanisms and drug susceptibility testing of nontuberculous mycobacteria. Drug Resist Updat. 2012;15:149–161.
- Pang Y, Zheng H, Tan Y, et al. In vitro activity of bedaquiline against nontuberculous mycobacteria in China. Antimicrob Agents Chemother. 2017;61(5):e02627–16.
- Brown-Elliott BA, Wallace RJ Jr. In vitro susceptibility testing of tedizolid against nontuberculous mycobacteria. J Clin Microbiol. 2017;55:1747–1754.
- Cavusoglu C, Soyler I, Akinci P Activities of Linezolid against nontuberculous mycobacteria. New Microbiol. 2007;30:411–414.
- Koh WJ, Hong G, Kim SY, et al. Treatment of refractory Mycobacterium avium complex lung disease with a moxifloxacin-containing regimen. Antimicrob Agents Chemother. 2013;57:2281–2285.
- Van Ingen J, Totten SE, Helstrom NK, et al. In vitro synergy between clofazimine and amikacin in treatment of nontuberculous mycobacterial disease. Antimicrob Agents Chemother. 2012;56:6324–6327.
- Olivier KN, Griffith DE, Eagle G, et al. Randomized trial of liposomal amikacin for inhalation in nontuberculous mycobacterial lung disease. Am J Respir Crit Care Med. 2017;195:814–823.
- Winthrop KL, Ku JH, Marras TK, et al. The tolerability of linezolid in the treatment of nontuberculous mycobacterial disease. Eur Respir J. 2015;45:1177–1179.
- Martiniano SL, Wagner BD, Levin A, et al. Safety and effectiveness of clofazimine for primary and refractory nontuberculous mycobacterial infection. Chest. 2017;in press. DOI: http://dx.doi.org/10.1016/j.chest.2017.04175.
- Philley JV, Wallace RJ Jr., Benwill JL, et al. Preliminary results of bedaquiline as salvage therapy for patients with nontuberculous mycobacterial lung disease. Chest. 2015;148:499–506.
- Jarand J, Davis JP, Cowie RL, et al. Long-term follow-up of mycobacterium avium complex lung disease in patients treated with regimens including clofazimine and/or Rifampin. Chest. 2016;149:1285–1293.
- Jo KW, Kim S, Lee JY, et al. Treatment outcomes of refractory MAC pulmonary disease treated with drugs with unclear efficacy. J Infect Chemother. 2014;20:602–606.
- Nash KA, Brown-Elliott BA, Wallace RJ Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–1376.
- Koh WJ, Jeon K, Lee NY, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011;183:405–410.
- Maurer FP, Bruderer VL, Ritter C, et al. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother. 2014;58:3828–3836.
- Christianson S, Grierson W, Kein D, et al. Time-to-detection of inducible macrolide resistance in mycobacterium abscessus subspecies and its association with the Erm(41) Sequevar. PLoS One. 2016;11:e0158723.
- Kasperbauer SH, De Groote MA The treatment of rapidly growing mycobacterial infections. Clin Chest Med. 2015;36:67–78.
- Yang B, Jhun BW, Moon SM, et al. Clofazimine-containing regimen for the treatment of mycobacterium abscessus lung disease. Antimicrob Agents Chemother. 2017;61:e02052–16.
- Griffith DE, Girard WM, Wallace RJ Jr. Clinical features of pulmonary disease caused by rapidly growing mycobacteria. An analysis of 154 patients. Am Rev Respir Dis. 1993;147:1271–1278.
- Lerat I, Cambau E, Roth Dit Bettoni R, et al. In vivo evaluation of antibiotic activity against Mycobacterium abscessus. J Infect Dis. 2014;209:905–912.
- Diel R, Ringshausen F, Richter E, et al. Microbiological and clinical outcomes of treating non-mycobacterium avium complex nontuberculous mycobacterial pulmonary disease: a systematic review and meta-analysis. Chest. 2017;152:120–142.
- Kwak N, Park J, Kim E, et al. Treatment outcomes of mycobacterium avium complex lung disease: a systematic review and meta-analysis. Clin Infect Dis. 2017;65:1077–1084.
- Griffith DE, Brown-Elliott BA, Langsjoen B, et al. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006;174:928–934.
- Adjemian J, Prevots DR, Gallagher J, et al. Lack of adherence to evidence-based treatment guidelines for nontuberculous mycobacterial lung disease. Ann Am Thorac Soc. 2014;11:9–16.
- Morimoto K, Namkoong H, Hasegawa N, et al. Macrolide-resistant mycobacterium avium complex lung disease: analysis of 102 consecutive cases. Ann Am Thorac Soc. 2016;13:1904–1911.
- Buege MJ, Brown JE, Aitken SL Solithromycin: a novel ketolide antibiotic. Am J Health Syst Pharm. 2017;74:875–887.
- Boyle DP, Zembower TR, Qi C Relapse versus reinfection of mycobacterium avium complex pulmonary disease. Patient characteristics and macrolide susceptibility. Ann Am Thorac Soc. 2016;13:1956–1961.
- Lee BY, Kim S, Hong Y, et al. Risk factors for recurrence after successful treatment of Mycobacterium avium complex lung disease. Antimicrob Agents Chemother. 2015;59:2972–2977.
- Wallace RJ Jr., Brown-Elliott BA, McNulty S, et al. Macrolide/Azalide therapy for nodular/bronchiectatic mycobacterium avium complex lung disease. Chest. 2014;146:276–282.
- Van Ingen J, Verhagen AF, Dekhuijzen PN, et al. Surgical treatment of non-tuberculous mycobacterial lung disease: strike in time. Int J Tuberc Lung Dis. 2010;14:99–105.
- Bouchard C, Blair S, Haskell W Physical activity and health, 2nd edition: Human Kinetics; Champaign, IL, USA. 2012.
- Chapter 29: pulmonary rehabilitation. In: Gibson G, Loddenkemper R, Sibille Y, et al., editors. European Lung Whitebook (http://wwwerswhitebookorg/): European Respiratory Society. 2017.
- Singh SJ, Puhan MA, Andrianopoulos V, et al. An official systematic review of the European Respiratory Society/American Thoracic Society: measurement properties of field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1447–1478.
- Berthon BS, Wood LG Nutrition and respiratory health–feature review. Nutrients. 2015;7:1618–1643.
