ABSTRACT
Introduction: Asthmatic patients show a large heterogeneity in response to asthma medication. Rapidly evolving genotyping technologies have led to the identification of various genetic variants associated with treatment outcomes.
Areas covered: This review focuses on the current knowledge of genetic variants influencing treatment response to the most commonly used asthma medicines: short- and long-acting beta-2 agonists (SABA/LABA), inhaled corticosteroids (ICS) and leukotriene modifiers. This review shows that various genetic variants have been identified, but none are currently used to guide asthma treatment. One of the most promising genetic variants is the Arg16 variant in the ADRB2 gene to guide LABA treatment in asthmatic children.
Expert commentary: Poor replication of initially promising results and the low fraction of variability accounted for by single genetic variants inhibit pharmacogenetic findings to reach the asthma clinic. Nevertheless, the identification of genetic variation influencing treatment response does provide more insights in the complex processes underlying response and might identify novel targets for treatment. There is a need to report measures of clinical validity, to perform precision-medicine guided trials, as well as to understand how genetic variation interacts with environmental factors. In addition, systems biology approaches might be able to show a more complete picture of these complex interactions.
1. Introduction
Asthma is a heterogeneous chronic inflammatory disease affecting the airways. Worldwide over 300 million children and adults suffer from asthma [Citation1], and it is considered to be the most common chronic disease among children. Poor asthma control and asthma exacerbations have a substantial impact on the quality of life of patients, as well as a large impact on society in terms of direct and indirect costs. The total annual costs of asthma management and productivity loss due to asthma in the countries of the European Union have been estimated at 33.9 billion euros [Citation2], with hospitalizations and healthcare visits due to uncontrolled asthma substantially contributing to the direct medical costs.
Asthma management guidelines apply a stepwise approach for pharmacological treatment to gain and maintain asthma control [Citation1,Citation2]. The first step of asthma management consists of treatment with short-acting beta-2 agonists (SABA) as needed to relieve bronchoconstriction. When symptoms persist despite SABA use, the second step is adding a low dosage of inhaled corticosteroids (ICS) to suppress the airway inflammation. Subsequently, as a third step the dosage of ICS may be increased or long-acting beta-2 agonists (LABA) can be added. Alternatively, a leukotriene modifier can be prescribed instead of a LABA. However, the preferred options differ between asthma guidelines [Citation1,Citation3]. In step 4, the dosage of ICS is further increased, and a muscarinic antagonist (tiotropium) may be added to the regime in adults, but this is currently not indicated for children <12 years of age [Citation1]. The fifth step of asthma management consists of referral of the patients to specialized care, where anti-IgE treatment might be considered for patients with severe allergic asthma. In addition, other expensive biologics targeting Th2 cytokines (such as anti-IL-5, anti-IL-13, and combined anti-IL-4/13) are entering clinical practice to treat severe asthma patients [Citation4].
There is a large variation in the magnitude of the response to asthma medication. Response to SABA is often defined by the percentage change in lung function upon SABA administration, the so-called bronchodilator response (BDR). Clinical trials have shown a large range in BDR distribution upon SABA use. Although a large number of patients show a BDR >12%, approximately half of the patients have BDR values <12% and even a small subset of patients show a decrease in lung function upon SABA (negative BDR) [Citation5]. Most patients show a response of 5–10%. A large heterogeneity in treatment response is also observed for asthma maintenance treatment. A 16-week cross-over trial showed that children with mild-to-moderate asthma show a large distribution in improvement in asthma control days per week () [Citation5] or lung function improvement [Citation6] upon treatment with ICS or LTRA. Change in asthma control days per week varied from an increase of seven asthma-controlled days per week to a decrease of four asthma-controlled days per week. Change in lung function upon treatment showed an equal wide distribution ranging between a decrease of 30% in FEV1 to an increase of >30%. In addition, it is estimated that approximately 5–10% of all patients remains symptomatic, and/or at risk of severe asthma exacerbations despite high dosages of asthma treatment [Citation4]. This could be due to various factors, such as suboptimal adherence to treatment, poor inhalation technique, comorbidities that have not been managed well or continued exposure to allergens.
Figure 1. Heterogeneity in improvement in asthma-control upon treatment with inhaled corticosteroids (ICS) or leukotriene receptor agonists (LTRA). Change in asthma-control days per week from baseline was measured in children with mild-to-moderate asthma participating in a cross-over NHLBI-CARE Network trial. Based on [Citation5].
![Figure 1. Heterogeneity in improvement in asthma-control upon treatment with inhaled corticosteroids (ICS) or leukotriene receptor agonists (LTRA). Change in asthma-control days per week from baseline was measured in children with mild-to-moderate asthma participating in a cross-over NHLBI-CARE Network trial. Based on [Citation5].](/cms/asset/bf6b2654-8e58-456e-9af9-d5d1b955268e/ierx_a_1403318_f0001_b.gif)
However, even in clinical trials in which adherence to treatment is closely monitored and patients groups are carefully selected, subsets of patients remain symptomatic despite maintenance treatment [Citation5,Citation6], suggesting that genetic variance plays a role as well. Heritability studies reported heritability estimates between 10% and 29% for SABA response [Citation7,Citation8]. The effect of genetic variants on treatment outcome might occur through differences in disease subtypes or through influences on drug level or drug target.
Rapidly evolving genotyping technologies have led to the identification of various genetic variants associated with poor asthma treatment outcomes. Initially these pharmacogenomics asthma studies used candidate-gene approaches to study genetic variation associated with the observed treatment heterogeneity, later also Genome-Wide Association Studies (GWAS) became available. Both approaches have their own advantages and disadvantages (). In a candidate-gene approach, genetic variants are selected based on biological knowledge. This type of study is not designed to identify novel genomic areas associated with treatment response, but rather to confirm a hypothesized association. In contrast, GWAS can be used to identify novel genomic variants. In this data-driven approach, genes are not selected based on a priori knowledge but large numbers of genetic variants across the entire genome are assessed simultaneously using genotyping arrays. Although prices are rapidly decreasing, this approach is more expensive than a candidate-gene approach. In addition, GWAS require much larger sample sizes to achieve an adequate statistical power.
Figure 2. Candidate-gene approach versus Genome Wide Association Study (GWAS) approach to study genetic variants associated with treatment response in asthma.
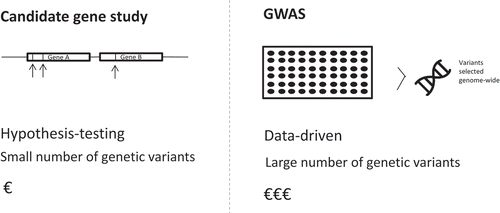
In this review, we will discuss the current knowledge of genetic variants influencing treatment response to the most commonly used asthma medicines: SABA, LABA, ICS, and leukotriene modifiers.
2. Beta-2 agonist response
2.1. Short-acting beta-2 agonists
SABA, e.g. salbutamol, albuterol, and terbutaline, are the most commonly prescribed asthma medication. They are often used as relief medication on ‘as needed’ basis to reduce bronchoconstriction. The primary action of these drugs is dilation of the bronchi. It is mediated through binding to the beta-2 adrenoceptors within the cell membrane of smooth muscle cells in the lower respiratory tract.
Candidate-gene studies have mostly focused on the gene encoding the beta-2 adrenoceptor; ADRB2. Two genetic variants have been associated with BDR to SABA. One variant leads to a substitution of glycine for arginine at position 16 of the receptor protein (Gly16Arg, allele frequency ~40%), and one leads to change of glutamic acid into glutamine at position 27 (Gln27Glu, allele frequency ~45%). In vitro studies showed that these variants influence the downregulation of the receptor [Citation9].
Retrospective genotyping of the participants in clinical studies, showed that Arg16Arg was associated with a poorer response to regularly used SABA [Citation10–Citation12]. This was confirmed by a prospective genotype-stratified cross-over trial with 78 asthmatic patients [Citation12]. The Beta-Adrenergic Response by Genotype (BARGE) trial showed that patients with an ADRB2 Arg16 variant who used albuterol had a small decline in lung function over the duration of the trial (measured as morning peak expiratory flow, mPEFR) compared to patients with a Gly16Gly genotype [Citation13]. The baseline mPEFR was not significantly different between both genotypes (450 L/min for Arg16Arg vs. 479 L/min for Gly16Gly). There was an effect of the genotype on mPEFR over time; however, it was minor and not clinically relevant (genotype-attributable treatment difference: −24 L/min).
In addition, six GWAS on SABA response have been performed assessing BDR as outcome (), including asthmatics as well as healthy individuals. Often these studies first perform a GWAS in a so-called discovery cohort and then replicate the most significant findings using a candidate-gene approach in one or more independent populations. Himes et al. performed a GWAS on SABA in 1644 non-Hispanic white asthmatic patients from 6 clinical trials assessing BDR as outcome [Citation14]. Replication of the most significant loci was attempted in an independent population of 501 white patients. The most significant associated locus (but not passing the conventional genome-wide significance threshold) was near the SPATSL2 gene (p-value: 9.7E-07). Patients with a TT genotype had a median BDR of 16.0 compared to 10.9 of patients with the CC or TC genotype. In vitro cell analyses showed that knockdown of SPATS2L resulted in significantly increased β2AR protein levels [Citation14].
Table 1. GWAS studies assessing genetic variants associated with asthma medication response.
Israel et al. studied 724 asthmatics from two pediatric trial populations and one adult population. The most significant results were subsequently replicated in an independent population of 439 adult asthmatics [Citation16]. Three SNPs in one region in chromosome 2 were associated with BDR. The nearest gene is a gene coding for ASB3 and SOCS. Patients homozygous for the risk allele had approximately a 20% mean decrease in BDR.
Another GWAS was performed by Drake et al. in 1782 Latino children with asthma [Citation17]. They subsequently performed a replication in 531 Latinos (children and adults). Using conventional GWAS analysis, as well as admixture mapping, they identified several rare variants to be associated with BDR in this population. In addition, they found two previously identified candidate genes to be associated with BDR (ADCY9 and CRHR2), but different variants in these genes than previously reported.
Duan et al. performed a GWAS in 403 child-parent trios from the Childhood Asthma Management Program [Citation15]. The most significant findings were subsequently studied during two replication phases. In total, 1397 SNPs were successfully genotyped in three non-Hispanic white adult asthma trials (n = 764), 13 SNPs were tested in two other asthma trials (n = 456). Six SNPs were nominally associated in both the primary replication phase and in one or more of the secondary replication populations, including SNPs in the COL22A1 and CLOCK genes.
BDR upon SABA use was also assessed as outcome in a GWAS by Padhukasahasram et al. in 328 healthy individuals from African-American descent. Three replication populations were included in the study, consisting of African-American asthmatics (n = 1968), healthy African-Americans (n = 149) and European asthmatics (n = 685) [Citation18]. Using a gene-based association (combination of p-values of all SNPs in a gene) variation in SPATA13 and its associated antisense RNA were found to be associated with BDR. The genetic variation was also associated with the number of SABA refills; however, effect estimates were not provided.
Brehm et al. performed a GWAS in 447 Puerto Rican children with asthma [Citation19]. Top hits were replicated in 568 children participating in the Childhood Asthma Management Program, 556 children from the Genetics of Asthma in Costa Rica study and 1858 children from the Genes-environment and Admixture in Latino Americans study. One SNP in FGF14 was replicated in the same direction of effect in all cohorts (combined p-value: 0.005, effect size: −58.06 ml on post-bronchodilator FEV1).
Although various GWAS have been performed assessing SABA response, all reported different genetic variants to be associated with response to SABA. This could be due to selection of the study population, sample size numbers or differences in genotyping platforms and/or imputation strategies.
2.2. Long-acting beta-2 agonists
LABA, e.g. salmeterol and formoterol, act on the same beta-2 adrenoceptor as SABA, but act longer (8–12 h vs. 3–5 h for SABA). LABA are not prescribed ‘as needed,’ but as maintenance treatment in combination with ICS to avoid the increased risks of adverse effects associated with LABA monotherapy. A complication in assessing treatment response to LABA, is the concurrent use of other medication, such as ICS and/or LTRA which may confound the findings. Clinical studies have shown that the combination of LABA and ICS might lead to additive or synergistic effects. It remains unclear whether this is the result of complementary mechanisms or due to a poorly understood anti-inflammatory mechanism of LABA [Citation20]. In line with the studies assessing genetic variants associated with SABA response, most pharmacogenetics studies on LABA response have focused on the ADRB2 gene. In adults, most retrospective pharmacogenetics studies showed no effect of the ADRB2 genotype on lung function improvement [Citation21,Citation22]. In addition, the prospective genotype stratified trial by Wechsler et al. confirmed these results [Citation23]. The Long-Acting Beta Agonist Response by Genotype (LARGE) trial [Citation23] assessed the effect of the Arg16 variant in adults with moderate asthma and did not find any associations with risk of exacerbations or poorer lung function improvement. Patients (n = 42 Arg16Arg and n = 45 Gly16Gly) were randomized to ICS + LABA or ICS + placebo in a double-blind cross-over design for 18 weeks periods. However, in an exploratory post hoc analysis, a genotypic effect was found in a subgroup of asthma patients with African-American descent.
In contrast, in children there seems to be a rather consistent effect of Arg16 on LABA response; when assessing exacerbations and persistent symptoms as response outcomes. A prospective randomized clinical trial (RCT) has been performed with 62 asthmatic children homozygous for the Arg16 variant [Citation24]. Participants were randomized for LTRA or LABA treatment as an add-on to ICS. The trial showed that asthmatic children treated with LTRA had fewer school absences, used less rescue medication, had less symptoms and a better quality of life compared to the group treated with LABA. However, the genotype did not have an impact on the lung function improvement. These results suggest that this variant might have an impact on LABA outcome, but mainly in the pediatric asthma population. This hypothesis is supported by a recent large meta-analysis of observational studies gathered in Pharmacogenomics in Childhood Asthma Consortium (PiCA). Turner et al. performed a meta-analysis of 4226 children and young adults and showed that patients treated with LABA had a 52% increased risk of exacerbations per copy of the Arg16 risk allele () [Citation25]. Remarkably, patients solely treated with ICS did not have an increased risk. Since asthma severity and treatment response are difficult to entangle, confounding by severity cannot be excluded. A Dutch RCT assessing whether ADRB2 Arg16-guided treatment will lead to better treatment outcomes compared to non-genotype guided treatment in asthmatic children is currently ongoing [Citation26]. This trial will provide more insights in the clinical value of ADRB2-guided asthma treatment, as well as the cost-effectiveness of such an approach.
Figure 3. Children with an ADRB2 Arg16 genotype have an increased risk of exacerbations when using LABA. Results of a meta-analysis of 5 studies participating in the Pharmacogenomics in Childhood Asthma (PiCA) consortium. OR and corresponding 95%CI for exacerbation for each copy of the Arg16 variant in children using ICS and LABA. The increased risk was not observed in children using ICS solely or ICS + LTRA. Based on [Citation25].
LABA: long-acting beta-2 agonists, ICS: inhaled corticosteroids, LTRA: leukotriene receptor antagonists, OR: odds ratio, CI: confidence intervals
![Figure 3. Children with an ADRB2 Arg16 genotype have an increased risk of exacerbations when using LABA. Results of a meta-analysis of 5 studies participating in the Pharmacogenomics in Childhood Asthma (PiCA) consortium. OR and corresponding 95%CI for exacerbation for each copy of the Arg16 variant in children using ICS and LABA. The increased risk was not observed in children using ICS solely or ICS + LTRA. Based on [Citation25].LABA: long-acting beta-2 agonists, ICS: inhaled corticosteroids, LTRA: leukotriene receptor antagonists, OR: odds ratio, CI: confidence intervals](/cms/asset/bc366448-9117-4fe5-8b5a-81a03b835242/ierx_a_1403318_f0003_b.gif)
In addition, rare variants in ADRB2 have been associated with an increased risk of asthma-related hospitalizations [Citation27]. These variants might be more frequent in certain ethnic groups (e.g. African Americans), which advocates for studying pharmacogenomics in diverse ethnic populations.
Few studies have assessed and replicated other genetic variants in the beta-2 agonist drug pathway [Citation28,Citation29], and no GWAS on LABA has currently been published.
3. ICS response
ICS, e.g. beclomethasone, budesonide, and fluticasone, are the cornerstone of maintenance therapy for patients with asthma. The primary mode of action of corticosteroids is to inhibit the expression of inflammatory genes. The anti-inflammatory effect of ICS is mediated through their binding to the corticosteroid receptor, which can subsequently influence the transcription of a wide variety of genes. Response to ICS is generally assessed as lung function improvement upon ICS use, exacerbations despite ICS use or persisting asthma symptoms despite ICS use.
Several GWAS on ICS response in asthmatic patients have been performed, mainly focusing on Caucasian asthma patients (). Six GWAS studies reported genetic variants to be associated with treatment response [Citation30–Citation35], while a large recent GWAS did not find any significantly associated genetic variants [Citation36]. The GWAS that did identify genetic variants to be associated with ICS response, all reported different genes to be most significantly associated with response to ICS. The first GWAS on ICS response was published in 2011 by Tantisira and coworkers. They studied 118 child-parent trios included in the Childhood Asthma Management Program (CAMP), a multicenter RCT trial (discovery phase) and subsequently aimed to replicate identified variants in 935 asthmatic adults and children from four different replication populations [Citation31]. Variation in the GLCCI1 gene, encoding the glucocorticoid-induced transcript 1 protein, was found to be associated with a poorer improvement in FEV1 upon treatment with ICS.
Another GWAS by Tantisira et al. [Citation32] in asthma patients included in the Single-Nucleotide Polymorphism Health Association-Asthma Resource Project (SHARP) identified three genetic variations in the T gene to be associated with an increase in lung function. SHARP consists of a large number of NHLBI-sponsored asthma clinical trials with genetic data and drug-treatment response data gathered, including the CAMP study. The GWAS was performed in 418 patients (pediatric and adults) and the most significant associations were subsequently genotyped in another population of 407 adult patients. Homozygotes carriers of the variant T gene showed a 2–3-fold higher response in FEV1 than homozygote wild-type carriers, with the largest effect of 9.9 ± 2.1% versus 2.8 ± 1.3% improvement in lung function in the CAMP population; however, not in the replication population.
A GWAS by Park et al. in 189 Korean adults with asthma reported variation in ALLC to be associated with lack of improvement in lung function in response to ICS; however, the association did not reach genome-wide significance and the study was only performed in a single population of patients [Citation34].
A different approach was used by Wang et al. [Citation35]; they applied a pharmacodynamic model to clinical trial GWAS data in order to identify loci affecting corticosteroid response curves. Five loci were significantly associated with pulmonary response to ICS in a trial with 120 mild-to-moderate asthmatics. Findings were replicated in three independent trial populations. Most significant effects were found for chr6 rs6924808 and chr11 rs1353649.
Moreover, a large recent GWAS study by Mosteller et al. in 2627 patients (≥12 years of age) originating from 7 industry-sponsored clinical studies did not find any significant associations of genetic variants and lung function improvement upon ICS treatment [Citation36]. Variations in GLCCI1 were nominally associated with lung function improvement, but did not reach the genome-wide significance threshold. Patients in this study had diverse ethnic backgrounds and Mosteller et al. corrected for potential population stratification. This is important since unrecognized population stratification can lead to both false-positive and false-negative findings [Citation37].
GWAS arrays are often designed to cover common genetic variants. Alternatively, exome arrays have been developed to assess functional and rare variants. Leusink et al. used an exome array to study functional and rare genetic variants associated with ICS treatment response in 110 asthmatic children who participated in the Children Asthma Therapy Optimal (CATO) study. Treatment response was based on changes in lung function and airway hyperresponsiveness to metacholine (Mch PD20) over time. Variants in the 17q12-21 locus were found nominally associated with treatment response to ICS, but none of the variants was significantly associated with treatment response upon correction for multiple testing [Citation38]. Genes in the 17q12-21 locus have previously been associated as risk factors for childhood-onset asthma [Citation39,Citation40].
In addition to lung function as an outcome for ICS response, symptoms and exacerbations as markers of ICS response have been assessed using GWAS approaches. Park et al. studied asthma scores instead of lung function [Citation30]. They selected 124 Caucasian children from the CAMP study for the initial GWAS, and subsequently aimed to replicate the most significant SNPs in three independent populations, consisting of 77 children and 220 adults. Variation in FBXL7 was found to be associated with altered response to ICS in children based on self-reported symptoms, but not in adults. Dahlin et al. performed a GWAS analysis in two populations of Caucasian asthmatic patients (n = 806 in total) and subsequently meta-analyzed the results [Citation33]. None of variants surpassed the threshold for genome‐wide significance, but the top candidate gene, CMTR1, was found to be overexpressed in nasal lavage samples of patients experiencing asthma exacerbations. The gene encodes for methyltransferase and it is thought to be involved in regulating immune responses to viral infections. Nevertheless, the reported effect of variation in this gene on the risk of asthma exacerbations was limited; with the largest effect estimate to be reported of OR: 1.07 (95%CI: 1.03–1.11).
Candidate-gene approaches have been used to attempt to replicate findings of GWAS with similar or different outcomes of ICS response, as well as assess genes with a biologically plausibility to influence corticosteroid pharmacokinetics or pharmacodynamics. A recent systematic review by Farzan et al. [Citation41] showed that genetic variants of three genes (GLCCI1, CRHR1, and FCER2) have been successfully replicated in at least one independent study.
GLCCI1 has been initially identified in a GWAS as a genetic marker associated with lung function improvement upon ICS use in Caucasian children of the CAMP trial [Citation31]. Three studies have subsequently attempted to replicate the results, using similar [Citation42,Citation43] or different [Citation44] outcome measures for ICS response. A smaller study in 224 adult Japanese asthmatics found variation in GLCCI1 to be associated with annual decline in lung function [Citation43]. However, a large study by Hosking et al. using pooled trial data (n = 1916 patients) found no association of GLCCI1 with improvement in lung function [Citation42]. In addition, a meta-analysis of three observational cohorts including 1791 asthmatic children and young adults populations did not find an association of variation in GLCCI1 and risk of exacerbations despite ICS use (n = 1791 patients) [Citation44].
The release of endogenous glucocorticoids is regulated by corticotrophin releasing hormone regulated by the hypothalamic pituitary adrenal (HPA axis). CRHR1 encodes for corticotrophin-releasing hormone receptor 1. It has been hypothesized that variation in this gene influences the level of endogenous corticosteroid secretion and thereby alters the response to ICS. Several SNPs in this gene have been studied and two variants have been replicated at least once [Citation30,Citation46,Citation47] in independent populations of children and adults with asthma; however, results have been inconsistent [Citation48].
The most consistent results in candidate-gene studies have been found for FCER2, which has been studied in three different populations. This gene encodes for a low affinity IgE receptor and was initially to be found associated with response to ICS in the CAMP trial [Citation49], with Hazard Ratio (HR) for severe exacerbations despite ICS use of 3.95 (95% CI: 1.64–9.51) in Caucasian children; and HR: 3.08; 95% CI: 1.00–9.47 in African-American children. Rogers et al. reported FCER2 also be associated with lung function improvement upon ICS use in the CAMP trial [Citation47], OR: 2.1 (95%CI: 1.2–3.5). Moreover, a meta-analysis of two North-European asthma cohorts (PACMAN and BREATHE) and data of the CAMP study also identified FCER2 as a risk factor for severe exacerbations despite ICS use (summary OR: 2.38, 95%CI: 1.47–3.85), as well as uncontrolled asthma symptoms (OR: 2.46, 95%CI: 1.38–4.39) [Citation50].
4. Leukotriene modifiers response
Leukotriene modifiers interfere in the leukotriene pathway, which is thought to mediate bronchoconstriction in asthma patients. One of the key enzymes in this pathway is 5-lipoxygenase, which converts arachidonic acid into leukotriene A4 (LTA4). This compound is subsequently modified to leukotriene E4 and D4, which on their turn can bind to leukotriene receptors on leukocytes and lung smooth muscle cells and cause bronchoconstriction. There are two types of leukotriene modifiers (LTM): leukotriene receptor antagonists (LTRA), e.g. montelukast, which prevent the leukotrienes from binding to the cysteinyl leukotriene receptor 1 and leukotriene inhibitors (e.g. zileuton), which inhibit leukotriene production through blockage of 5-lipoxygenase. LTRA are more commonly prescribed for asthma than leukotriene inhibitors.
Clinical trials have shown that 35–78% of patients show a poor improvement in lung function when treated with a leukotriene modifier [Citation6,Citation51,Citation52]. Initially, candidate-gene studies on LTRA response focused on genes within pharmacological leukotriene pathway, such as leukotriene A4 hydrolase (LTC4s), 5-lipoxygenase (ALOX5), leukotriene A4 hydrolase (LTA4H), and cysteinyl leukotriene receptor 1 (CysLTR1) [Citation41]. In addition, genes influencing LTM pharmacokinetics such as CYP enzymes (CYP3A4, CYP2C9) and transporter genes (SLCO2B1, MRP1/ABCC1) have also been investigated [Citation53,Citation54]. A recent systematic review showed that despite a large amount of pharmacogenetics LTRA studies; only variants in two genes, ALOX5 and MRP1, have been replicated to show similar outcomes of LTRA response at least once [Citation41]. Two genetic variants of ALOX5 have been associated with altered LTRA response; tandem repeat of the Sp1-binding domain and a SNP at position rs2115819. Tandem repeats are repeated patterns of one or more nucleotides adjacent to each other. Most individuals carry five tandem repeats in the Sp1 binding domain of ALOX5. Drazen et al. and Telleria et al. reported that patients carrying 5 tandem repeats in the Sp1 binding domain of ALOX5 showed a better improvement to leukotriene modifiers compared to patients with variant alleles [Citation55,Citation56]. Drazen et al. studied 114 adults in a 12-week trial with leukotriene inhibitor ABT-761 [Citation55]. Patients homozygous for the variant showed almost no improvement in lung function during the trial (FEV1%: −1.2 ± 3%), while patients with the wild type did show a substantial improvement in lung function (FEV1%: 18.8 ± 4%). In addition, Telleria et al. showed that asthma patients with less than 5 tandem repeats more often suffered exacerbations and required albuterol to control symptoms when treated with montelukast [Citation56]. In contrast, a study by Lima et al. found that patients carrying 5 tandem repeats had an increased risk of exacerbations compared to patients carrying variant alleles [Citation57]. Studies by Fowler et al. and Klotsman et al. reported no increased risk of poor montelukast response in patients carrying this genetic variant [Citation54,Citation58]. The effect of the ALOX5 promotor SP1 tandem repeat variant on treatment response seems to be context specific and may depend on the specific (non-5 tandem repeat) variant [Citation59].
Another genetic variant in ALOX5, a SNP at position rs2115819, has been found to be associated with less improvement in lung function when treated with LTRA [Citation57] or leukotriene inhibitor [Citation60]. Nevertheless, smaller studies by Mougey et al. (n = 65 patients) [Citation46] and Kotani et al. (n=21 patients) [Citation61] did not find any pharmacogenetics effect of this variant in studies with montelukast.
More recently, two GWAS on LTM have been published assessing genetic variants associated with montelukast and zileuton response [Citation62,Citation63] (). Both GWAS reported novel genetic variants, but did not identify genetic variants previously identified in the candidate-gene studies. However, it should be noted that SP-1 tandem repeat variants were not assessed in the GWAS analyses. The first GWAS in 2015 identified MLLT3 as a marker for LTRA response [Citation62]. A GWAS was performed in 133 asthmatic patients who participated in two clinical trials assessing montelukast response, and the 200 most significant hits were replicated using the data of two other montelukast trials (n = 184 patients). In the combined analyses (including adults as well as children with asthma), the top hit reached genome-wide significance. Patients from all four studies who were homozygous for a genetic variant of MLLT3 had more improvement in lung function upon montelukast use; with the largest reported difference of 344 mL improvement in FEV1 in the patients homozygous for the variant versus 4.66 mL in the wild-type patients upon 8 weeks of treatment in one of the trials.
The second GWAS published by the same research group [Citation63] assessed data of two zileuton trials (n = 309 patients, ≥12 yrs) and two other cohorts of montelukast users (n = 133 patients, ≥6 yrs). The GWAS was performed in 160 patients who received zileuton, and subsequently the top 50 SNPs were studied in the three replication populations with zileuton and montelukast users. Although none of the SNPs achieved genome-wide significance in the discovery cohort, two genes were found to be associated with LTM response in the combined analysis of the discovery and replication cohorts. Genetic variation in GLT1D1 was associated with a poor response to montelukast and zileuton when assessing change in lung function over time in the trial (beta ranging from −0.44 to −0.15L), while genetic variation of MRPP3 was found to be solely associated with a poorer response to zileuton. Patients carrying both variant alleles of MRPP3 showed a worsening of lung function upon treatment with zileuton (mean ΔFEV1 of −0.12 L), while patients carrying only one or no variant allele showed an increase in lung function (mean ΔFEV1 of 0.23 L). For both genes, it is unknown how their functioning would impact LTM response.
5. Summary
Although various genetic variants have been reported to influence response to short- and long-acting beta-2 agonists, inhaled corticosteroids and leukotriene modifiers, results remain largely inconsistent or effect sizes are small. GWAS approaches have identified various novel genetic variants, but often these variants do not reach the strict threshold of genome-wide significance, or have only a limited effect on the treatment heterogeneity. Moreover, genetic variants previously identified through candidate-gene approaches are not found to be associated with treatment outcome through these hypothesis-free approaches. One of the most promising genetic variants which might potentially change clinical asthma practice soon – at least in pediatric asthmatics – is the Arg16 variant in the ADRB2 gene to guide LABA treatment. Various studies have shown that children carrying this variant have a poorer response to LABA and might benefit from alternative treatment. The variant is common in the asthma population and has a substantial effect on LABA outcome such as exacerbations despite treatment.
6. Expert commentary
One of the main unmet clinical needs for asthma patients is the lack of clinically available biomarkers to guide treatment. It has been suggested that up to 80% of the interindividual variance in lung function response upon treatment in asthmatic patients is caused by genetic variations [Citation64]. Despite numerous pharmacogenetics studies, identified genetic variants do only partly attribute to this observed treatment heterogeneity and no pharmacogenetic markers have reached clinical asthma practice. This is partly due to poor replication of initial promising results in independent study populations. In addition, effect sizes are often limited and large sample sizes are needed to have enough power to detect significant effects. Most pharmacogenetic studies are underpowered. Despite the value of single replication studies, a joint effort is needed to enable large-scale pharmacogenomics studies and large-scale meta-analyses. Novel initiatives, such as the Pharmacogenomics in Childhood Asthma (PiCA) consortium [Citation65] are emerging and might identify new pharmacogenomics markers in asthma by conducting GWAS meta-analyses. In addition, electronic medical records are becoming increasingly available for research purposes, and might be valuable tools for large genomics studies [Citation66]. An important next step is to link loci identified in GWAS to genes and biological pathways. Cell-based models and expression Quantitative Trait Loci (eQTL) mapping might help us understand in which tissues or cells identified genetic variants influence gene expression (directly or indirectly) in order to affect treatment response [Citation67].
Novel analysis strategies might lead to a better understanding of the influence of genetic variation on treatment heterogeneity. Combining genetic variants in risk scores might explain more of the observed treatment heterogeneity than single variants. Furthermore, studying rare variants in specific subgroups of patients might identify novel genes [Citation17].
Most genetic markers are initially identified in observational studies or post hoc analyses of clinical drug trials by genotype. However, due to a lack of replication in independent study populations and inconsistent results, these genetic markers do often not reach a higher level of diagnostic testing assessment. Reporting is often based on strength of the association, and measures of clinical validity or population impact are generally not reported [Citation68]. Reporting measures of discriminative accuracy and predictive value will help to identify promising pharmacogenetic markers and facilitate gathering evidence of clinical value. One of the last steps toward clinical implementation; comparing genotype-guided treatment to usual care, is hardly ever reached. Few of the currently identified pharmacogenetic markers seem to be able to reach clinical practice, however, that does not imply that these markers hold no scientific value. The identification of genetic variation influencing treatment response can provide more insights in the complex processes underlying response and distinct asthma phenotypes in diverse asthma populations, and might identify novel targets for treatment.
7. Five-year view
A genomic susceptibility alone does not seem to be enough to drive asthma treatment outcomes and asthma phenotypes. Interaction with the environment might play a major role in driving a predisposed genetic background toward an asthma phenotype with a poor response to treatment. Genome-wide interaction studies (GWIS) are emerging [Citation69], which assess the interaction between genome-wide genetic variance and environmental factors and might provide more insights in these complex interactions.
Several studies have already shown associations between other -omics markers (e.g. epigenomics, transcriptiomics, microbiomics, breathomics) and asthma-related outcomes [Citation70–Citation73]. With rapid advances in the bio-informatics field and well-phenotyped cohorts, systems medicine approaches that link different -omics layers, might be able to show a more complete picture of the pathways underlying treatment response in asthmatics than pharmacogenomics solely [Citation74]. Nevertheless, these voluminous complex data require a close collaboration between respiratory experts and bioinformaticians. This should lead to well documented, preferably open-source, asthma omics databases.
Finally, precision-medicine-guided trials are needed to assess the value of biomarker-guided asthma treatment over current clinical practice. This might be especially relevant for the guidance of novel expensive biological drugs, such as anti-IL5 treatment. These trials should also assess the cost-effectiveness of such interventions to pave the way for successful implementation.
Key issues
There is a large heterogeneity in the magnitude of asthma medication response. Approximately 5–10% of the patients remains symptomatic, and/or at risk of severe asthma exacerbations despite high dosages of asthma treatment
One of the most promising genetic variants which might potentially change clinical asthma practice soon is the Arg16 variant in the ADRB2 gene to guide LABA treatment in children with asthma. Various studies have shown that children carrying this variant have a poorer response to LABA and might benefit from alternative treatment. The effect of this genetic variant on treatment response in adults is less consistent.
Genome-wide association studies identified various genetic variants to be associated with SABA, ICS and LTM response, but all reported different genes to be most significantly associated with treatment outcome. The strict threshold of genome-wide significance is often not passed.
Candidate-gene studies assessing LTM response, have shown that tandem repeats in the Sp1 binding domain of ALOX5 are associated with a poorer response to LTM, but results are inconsistent.
Most genetic markers are initially identified in observational studies or post hoc analyses of clinical drug trials by genotype. However, due to a lack of replication in independent study populations or inconsistent results, these genetic markers do often not reach a higher level of diagnostic testing assessment.
A single biomarker approach is increasingly seen as an outdated concept. Future research should include a multitude of biomarkers (genetic/non-genetic) to assess which combination has the most additive value to the currently used clinical parameters.
Precision-medicine guided trials are needed to pave the way for clinical implementation of biomarker-guided asthma treatment
Declaration of interest
SJH Vijverberg and AH Maitland-van der Zee have received an unrestricted grant from GlaxoSmithKline for their research on asthma pharmacogenomics in the past. They have also received funding from ERANET (H2020) for the SysPharmPediA consortium, The Netherlands Lung Foundation for the PUFFIN trial and Stichting Astmabestrijding for the PiCA consortium. AH Maitland-van der Zee has received consultancy fees from AstraZeneca in the past. N Farzan works as a PhD student on the PiCA consortium. EMA Slob works as a PhD student on the PUFFIN trial. AH Neerincx works as a post-doc on the SysPharmPediA study. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Global INitiative for Asthma (GINA) Report. Global strategy for asthma management and prevention global strategy for asthma management and prevention. [ Updated 2017; cited 2017 Nov Sep 1]. Available from: http://ginasthma.org/gina-reports/
- Gibson GJ, Loddenkemper R, Sibille Y, et al. The European lung white book. Sheffield: European Respiratory Society; 2013.
- British Thoracic Society. British guideline on the management of asthma. [Edition 2016; cited 2017 Sep 14]. Available from: https://www.brit-thoracic.org.uk/standards-of-care/guidelines/btssign-british-guideline-on-the-management-of-asthma/
- Hilvering B, Pavord ID. What goes up must come down: biomarkers and novel biologicals in severe asthma. Clin Exp Allergy. 2015;45(7):1162–1169.
- Zeiger RS, Szefler SJ, Phillips BR, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117(1):45–52.
- Szefler SJ, Phillips BR, Martinez FD, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115(2):233–242.
- Hersh CP, Soto-Quirós ME, Avila L, et al. Genome-wide linkage analysis of pulmonary function in families of children with asthma in Costa Rica. Thorax. 2007;62(3):224–230.
- McGeachie MJ, Stahl EA, Himes BE, et al. Polygenic heritability estimates in pharmacogenetics: focus on asthma and related phenotypes. Pharmacogenet Genomics. 2013;23(6):324–328.
- Lima JJ. Do genetic polymorphisms alter patient response to inhaled bronchodilators? Expert Opin Drug Metab Toxicol. 2014;10(9):1231–1240.
- Taylor DR, Drazen JM, Herbison GP, et al. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55(9):762–767.
- Hancox RJ, Sears MR, Taylor DR. Polymorphism of the beta2-adrenoceptor and the response to long-term beta2-agonist therapy in asthma. Eur Respir J. 1998;11(3):589–593.
- Israel E, Drazen JM, Liggett SB, et al. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162(1):75–80.
- Israel E, Chinchilli VM, Ford JG, et al. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364(9444):1505–1512.
- Himes BE, Jiang X, Hu R, et al. Genome-wide association analysis in asthma subjects identifies SPATS2L as a novel bronchodilator response gene. PLoS Genet. 2012;8(7):e1002824.
- Duan QL, Lasky-Su J, Himes BE, et al. A genome-wide association study of bronchodilator response in asthmatics. Pharmacogenomics J. 2014;14(1):41–47.
- Israel E, Lasky-Su J, Markezich A, et al. Genome-wide association study of short-acting β2-agonists. A novel genome-wide significant locus on chromosome 2 near ASB3. Am J Respir Crit Care Med. 2015;191(5):530–537.
- Drake KA, Torgerson DG, Gignoux CR, et al. A genome-wide association study of bronchodilator response in Latinos implicates rare variants. J Allergy Clin Immunol. 2014;133(2):370–378.
- Padhukasahasram B, Yang JJ, Levin AM, et al. Gene-based association identifies SPATA13-AS1 as a pharmacogenomic predictor of inhaled short-acting beta-agonist response in multiple population groups. Pharmacogenomics J. 2014;14(4):365–371.
- Brehm JM, Man Tse S, Croteau-Chonka DC, et al. A genome-wide association study of post-bronchodilator lung function in children with asthma. Am J Respir Crit Care Med. 2015;192(5):634–637.
- Remington TL, Digiovine B. Long-acting beta-agonists: anti-inflammatory properties and synergy with corticosteroids in asthma. Curr Opin Pulm Med. 2005;11(1):74–78.
- Bleecker ER, Yancey SW, Baitinger LA, et al. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118(4):809–816.
- Bleecker ER, Postma DS, Lawrance RM, et al. Effect of ADRB2 polymorphisms on response to longacting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007;370(9605):2118–2125.
- Wechsler ME, Kunselman SJ, Chinchilli VM, et al. Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009;374(9703):1754–1764.
- Lipworth BJ, Basu K, Donald HP, et al. Tailored second-line therapy in asthmatic children with the Arg(16) genotype. Clinical Science. 2013;124(8):521–528.
- Turner S, Francis B, Vijverberg S, et al. Childhood asthma exacerbations and the Arg16 beta2-receptor polymorphism: a meta-analysis stratified by treatment. J Allergy Clin Immunol. 2016;138(1):107–113.e105.
- Vijverberg SJ, Pijnenburg MW, Hövels AM, et al. The need for precision medicine clinical trials in childhood asthma: rationale and design of the PUFFIN trial. Pharmacogenomics. 2017;18(4):393–401.
- Ortega VE, Hawkins GA, Moore WC, et al. Effect of rare variants in ADRB2 on risk of severe exacerbations and symptom control during longacting β agonist treatment in a multiethnic asthma population: a genetic study. Lancet Respir Med. 2014;2(3):204–213.
- Litonjua AA, Lasky-Su J, Schneiter K, et al. ARG1 is a novel bronchodilator response gene: screening and replication in four asthma cohorts. Am J Respir Crit Care Med. 2008;178(7):688–694.
- Duan QL, Gaume BR, Hawkins GA, et al. Regulatory haplotypes in ARG1 are associated with altered bronchodilator response. Am J Respir Crit Care Med. 2011;183(4):449–454.
- Park HW, Dahlin A, Tse S, et al. Genetic predictors associated with improvement of asthma symptoms in response to inhaled corticosteroids. J Allergy Clin Immunol. 2014;133(3):664–669.e665.
- Tantisira KG, Lasky-Su J, Harada M, et al. Genomewide association between GLCCI1 and response to glucocorticoid therapy in asthma. N Engl J Med. 2011;365(13):1173–1183.
- Tantisira KG, Damask A, Szefler SJ, et al. Genome-wide association identifies the T gene as a novel asthma pharmacogenetic locus. Am J Respir Crit Care Med. 2012;185(12):1286–1291.
- Dahlin A, Denny J, Roden DM, et al. CMTR1 is associated with increased asthma exacerbations in patients taking inhaled corticosteroids. Immun Inflamm Dis. 2015;3(4):350–359.
- Park TJ, Park JS, Cheong HS, et al. Genome-wide association study identifies ALLC polymorphisms correlated with FEV₁ change by corticosteroid. Clin Chim Acta. 2014;436:20–26.
- Wang Y, Tong C, Wang Z, et al. Pharmacodynamic genome-wide association study identifies new responsive loci for glucocorticoid intervention in asthma. Pharmacogenomics J. 2015;15(5):422–429.
- Mosteller M, Hosking L, Murphy K, et al. No evidence of large genetic effects on steroid response in asthma patients. J Allergy Clin Immunol. 2017;139(3):797–803.e7.
- Li M, Reilly MP, Rader DJ, et al. Correcting population stratification in genetic association studies using a phylogenetic approach. Bioinformatics. 2010;26(6):798–806.
- Leusink M, Vijverberg SJ, Koenderman L, et al. Genetic variation in uncontrolled childhood asthma despite ICS treatment. Pharmacogenomics J. 2016;16(2):158–163.
- Moffatt MF, Kabesch M, Liang L, et al. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 2007;448(7152):470–473.
- Moffatt MF, Gut IG, Demenais F, et al. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363(13):1211–1221.
- Farzan N, Vijverberg SJ, Arets HG, et al. Pharmacogenomics of inhaled corticosteroids and leukotriene modifiers: a systematic review. Clin Exp Allergy. 2017;47(2):271–293.
- Hosking L, Bleecker E, Ghosh S, et al. GLCCI1 rs37973 does not influence treatment response to inhaled corticosteroids in white subjects with asthma. J Allergy Clin Immunol. 2014;133(2):587–589.
- Izuhara Y, Matsumoto H, Kanemitsu Y, et al. GLCCI1 variant accelerates pulmonary function decline in patients with asthma receiving inhaled corticosteroids. Allergy. 2014;69(5):668–673.
- Vijverberg SJ, Tavendale R, Leusink M, et al. Pharmacogenetic analysis of GLCCI1 in three north European pediatric asthma populations with a reported use of inhaled corticosteroids. Pharmacogenomics. 2014;15(6):799–806.
- Tantisira KG, Lake S, Silverman ES, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13(13):1353–1359.
- Mougey EB, Chen C, Tantisira KG, et al. Pharmacogenetics of asthma controller treatment. Pharmacogenomics J. 2013;13(3):242–250.
- Rogers AJ, Tantisira KG, Fuhlbrigge AL, et al. Predictors of poor response during asthma therapy differ with definition of outcome. Pharmacogenomics. 2009;10(8):1231–1242.
- Dijkstra A, Koppelman GH, Vonk JM, et al. Pharmacogenomics and outcome of asthma: no clinical application for long-term steroid effects by CRHR1 polymorphisms. J Allergy Clin Immunol. 2008;121(6):1510–1513.
- Tantisira KG, Silverman ES, Mariani TJ, et al. FCER2: a pharmacogenetic basis for severe exacerbations in children with asthma. J Allergy Clin Immunol. 2007;120(6):1285–1291.
- Koster ES, Maitland-van der Zee A-H, Tavendale R, et al. FCER2 T2206C variant associated with chronic symptoms and exacerbations in steroid-treated asthmatic children. Allergy. 2011;66(12):1546–1552.
- Malmstrom K, Rodriguez-Gomez G, Guerra J, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma. A randomized, controlled trial. Montelukast/Beclomethasone Study Group. Ann Intern Med. 1999;130(6):487–495.
- Israel E, Chervinsky PS, Friedman B, et al. Effects of montelukast and beclomethasone on airway function and asthma control. J Allergy Clin Immunol. 2002;110(6):847–854.
- Mougey EB, Feng H, Castro M, et al. Absorption of montelukast is transporter mediated: a common variant of OATP2B1 is associated with reduced plasma concentrations and poor response. Pharmacogenet Genomics. 2009;19(2):129–138.
- Klotsman M, York TP, Pillai SG, et al. Pharmacogenetics of the 5-lipoxygenase biosynthetic pathway and variable clinical response to montelukast. Pharmacogenet Genomics. 2007;17(3):189–196.
- Drazen JM, Yandava CN, Dubé L, et al. Pharmacogenetic association between ALOX5 promoter genotype and the response to anti-asthma treatment. Nat Genet. 1999;22(2):168–170.
- Telleria JJ, Blanco-Quiros A, Varillas D, et al. ALOX5 promoter genotype and response to montelukast in moderate persistent asthma. Respir Med. 2008;102(6):857–861.
- Lima JJ, Zhang S, Grant A, et al. Influence of leukotriene pathway polymorphisms on response to montelukast in asthma. Am J Respir Crit Care Med. 2006;173(4):379–385.
- Fowler SJ, Hall IP, Wilson AM, et al. 5-Lipoxygenase polymorphism and in-vivo response to leukotriene receptor antagonists. Eur J Clin Pharmacol. 2002;58(3):187–190.
- Mougey E, Lang JE, Allayee H, et al. ALOX5 polymorphism associates with increased leukotriene production and reduced lung function and asthma control in children with poorly controlled asthma. Clin Exp Allergy. 2013;43(5):512–520.
- Tantisira KG, Lima J, Sylvia J, et al. 5-lipoxygenase pharmacogenetics in asthma: overlap with Cys-leukotriene receptor antagonist loci. Pharmacogenet Genomics. 2009;19(3):244–247.
- Kotani H, Kishi R, Mouri A, et al. Influence of leukotriene pathway polymorphisms on clinical responses to montelukast in Japanese patients with asthma. J Clin Pharm Ther. 2012;37(1):112–116.
- Dahlin A, Litonjua A, Lima JJ, et al. Genome-wide association study identifies novel pharmacogenomic loci for therapeutic response to montelukast in asthma. PLoS One. 2015;10(6):e0129385.
- Dahlin A, Litonjua A, Irvin CG, et al. Genome-wide association study of leukotriene modifier response in asthma. Pharmacogenomics J. 2016;16(2):151–157.
- Drazen JM, Silverman EK, Lee TH, Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56(4):1054–1070.
- Farzan N, Vijverberg SJ, Andiappan AK, et al. Rationale and design of the multiethnic Pharmacogenomics in Childhood Asthma consortium. Pharmacogenomics. 2017;18(10):931–943.
- Almoguera B, Vazquez L, Mentch F, et al. Identification of four novel loci in asthma in European American and African American populations. Am J Respir Crit Care Med. 2017;195(4):456–463.
- Ober C. Asthma genetics in the post-GWAS era. Ann Am Thorac Soc. 2016;13 Suppl 1:S85–90.
- Tonk ECM, Gurwitz D, Maitland-van der Zee A-H, et al. Assessment of pharmacogenetic tests: presenting measures of clinical validity and potential population impact in association studies. Pharmacogenomics J. 2017;17(4):386–392.
- Forno E, Sordillo J, Brehm J, et al. Genome-wide interaction study of dust mite allergen on lung function in children with asthma. J Allergy Clin Immunol. 2017;140(4):996–1003.e7.
- Yang IV, Pedersen BS, Liu AH, et al. The nasal methylome and childhood atopic asthma. J Allergy Clin Immunol. 2014;133(2):587–589.
- Wysocki K, Conley Y, Wenzel S. Epigenome variation in severe asthma. Biol Res Nurs. 2015;17(3):263–269.
- Brinkman P, Van de Pol MA, Gerritsen MG, et al. Exhaled breath profiles in the monitoring of loss of control and clinical recovery in asthma. Clin Exp Allergy. 2017;47(9):1159–1169.
- Van der Schee MP, Palmay R, Cowan JO, et al. Predicting steroid responsiveness in patients with asthma using exhaled breath profiling. Clin Exp Allergy. 2013;43(11):1217–1225.
- Gustafsson M, Nestor CE, Zhang H, et al. Modules, networks and systems medicine for understanding disease and aiding diagnosis. Genome Med. 2014;6(10):82.
