ABSTRACT
Background: The preferences and opinions of patients are important when choosing the optimal inhaler device for asthma management. We compared patient satisfaction of three dry powder inhalers in patients with moderate to severe asthma.
Methods: We selected a group of patients treated with EasyhalerTM (n = 164) and a second group of patients treated with TurbuhalerTM (n = 100) or DiskusTM (AccuhalerTM) (n = 64) from the register of an observational, multicenter study. Data of patients were paired according to age, gender, and asthma severity. Patient satisfaction with the inhaler type was assessed with the specific ‘Feeling of Satisfaction with Inhaler’ (FSI-10) questionnaire.
Results: Specific satisfaction with inhaler was statistically significantly higher with EasyhalerTM, as well as the percentage of patients with high satisfaction with inhaler. (FSI-10 score ≥43). Scores for EasyhalerTM were also statistically significantly better for individual FSI-10 items such as learning how to use, inhaler preparation, inhaler use, weight and size, and portability. There were no significant differences in asthma control (ACT, Mini-AQLQ) and adherence (TAI global score).
Conclusions: Specific satisfaction with inhaler was higher with EasyhalerTM in a homogeneous population of patients with moderate to severe asthma. However, the relationship between satisfaction with the inhaler and adherence and asthma control deserves more investigation.
1. Introduction
When choosing asthma inhaled therapy, taking into account the preferences and opinions of patients can improve adherence and asthma control [Citation1,Citation2]. Patient satisfaction with inhaler has been related to more favorable clinical outcomes [Citation3], just as difficulties using the inhaler have been shown to contribute to poor adherence [Citation2]. Thus, inhaler choice is considered key for asthma control [Citation4].
Dry powder inhalers (DPIs) are used in asthma therapy [Citation2]. However, they present different features that may have clinical implications [Citation5]. EasyhalerTM (EH) (Orion Corporation, Finland), is a three-step, light, compact, and user-friendly DPI. It has advantages over older DPIs [Citation6], including dose uniformity in different real-world conditions [Citation7]. With regard to features such as perception of inhalation, size, discreetness, mouthpiece comfort, and dose counter, EH was rated higher than other inhaler devices by asthma patients [Citation8]. In an observational, multicenter study, patient satisfaction with inhaler was higher in asthma patients treated with EH [Citation9]. Here we present a post-hoc analysis of a homogeneous subpopulation of this study. Our objective was to compare EH and other DPIs regarding patient satisfaction with inhaler and potential clinical differences.
2. Materials and methods
The method used in the ASCONA (Asthma Satisfaction, CONtrol and Adherence) study has been previously reported [Citation9]. Briefly, the ASCONA scientific committee selected and invited the participating physicians. This selection aimed to achieve an appropriate geographical distribution across Spain, as well as involving centers providing different levels of healthcare. Seventy-three investigators (50.7% allergists and 49.3% pulmonologists) participated in the study. They selected 800 consecutive patients according to the inclusion criteria: age ≥ 18 years, physician-confirmed asthma diagnosis, moderate to severe asthma according to GINA guidelines [Citation2], and in receipt of any kind of asthma medication with the same inhaler for at least 3 months prior to their inclusion in the study. This last criterion was intended to assure that patients were under steady treatment without recent changes. Prior and current medications were registered and analyzed. Patients with disabling comorbidities and/or cognitive dysfunction were excluded. All patients signed an informed consent form, no personal data were recorded, and neither physicians nor patients received any compensation for their participation.
Physicians and patients completed electronic data collection forms in a single visit. We recorded sociodemographic and clinical data, including age at asthma diagnosis and at therapy onset, current asthma therapy, and asthma severity and control according to GINA [Citation2]. We also included several questionnaires and tests. We assessed satisfaction with the specific Feeling of Satisfaction with Inhaler (FSI-10) questionnaire [Citation10] and the more general Treatment Satisfaction Questionnaire for Medication (TSQM) [Citation11]. For adherence, we used the Test of Adherence to Inhalers (TAI) to assess the specific adherence to inhalers [Citation12] and the 4-item Morisky-Green scale for the general adherence to treatment (where 0 is no adherence and 4 is good adherence) [Citation13]. We also used the Asthma Control Test (ACT) [Citation14] and the reduced version of the Asthma Quality of Life Questionnaire (Mini-AQLQ) [Citation15,Citation16]. All these instruments have been validated in Spanish [Citation10,Citation12,Citation17–Citation20]. Furthermore, FSI-10 and TAI have been developed in Spain.
The specific FSI-10 questionnaire assesses the portability and usability of inhalers regardless of administered drugs. Patients answer ten questions, each with five options, using a Likert scale from 5 (very) to 1 (hardly at all). The FSI-10 maximum score is 50 (maximum satisfaction) [Citation10] (see Supplementary file 1).
In turn, TAI consists of two complementary questionnaires. The 10-item TAI is completed by the patient and identifies the level of adherence: good (50 points), intermediate (46–49 points), and poor (≤ 45 points). In turn, the 12-item TAI suggests the pattern of non-compliance. It includes the 10-item TAI plus two items that are answered by the physician: knowledge of the dosing regimen by the patient (item #11) and performance of inhalation technique without critical errors (item #12) [Citation12] (see Supplementary file 2).
The study was conducted according to the Declaration of Helsinki. The Clinical Research Ethics Committee of the Hospital Clinic in Barcelona (Spain) approved its protocol.
The more common DPIs in the ASCONA register were EH, TurbuhalerTM (TH) (AstraZeneca, Sweden) or DiskusTM (AccuhalerTM) (DKAH) (GSK, United Kingdom). They also were the only ones with an adequate number of patients to perform statistical analyses. In a prior analysis of the ASCONA registry, study investigators included 739 patients with moderate or severe asthma treated with EH, TH or DKAH. The comparison between these three DPIs showed a significant trend favorable to EH when compared both versus TH and versus AH individually in specific satisfaction to inhaler, satisfaction with treatment, quality of life, and asthma control [Citation21] (see Supplementary file 3), without significant differences in other variables. However, because of non-systematic recruitment in the ASCONA registry, DPI groups were heterogeneous (in size and clinical profile) and therefore not directly comparable. For instance, percentage of patients with moderate asthma was higher in the EH group (p < 0.001). Therefore, we decided to perform a post-hoc analysis using two randomized paired samples of patients matched according to age, gender and asthma severity, and treated with EH or with TH/DKAH. To explore whether this EH differences were relevant or not, we created homogeneous groups using paired matched samples approach, which will provide enough statistical power by unifying comparator groups (TH and AH) only under the assumption that they differ in a similar degree on the EH results.
2.1. Statistical analysis
Statistical analysis was performed with SPSS version 22.0 (SPSS Inc., Chicago, IL, USA). All statistical tests, tables and charts considered only the numbers of valid cases.
Categorical variables were described with number of valid cases and percentage, whereas mean and standard deviation (SD) were used for continuous variables. Dichotomous variables were created for each variable of interest using the median of the scores from the questionnaires and scales (FSI-10, TSQM, TAI and Mini-AQLQ) or a pre-established cut-off point (ACT [Citation22] and Morisky–Green scale [Citation13]).
Statistical tests included one-way analysis of variance (ANOVA) for group comparisons, Mann-Whitney test or Kruskal–Wallis test for continuous variables, and χ2 test for categorical variables. Pearson and Spearman correlation tests were used to assess relationships between scale scores. Finally, multivariate analyses were performed to examine factors that influenced the study dependent variable (specific satisfaction with inhaler according to FSI-10).
3. Results
We included a group of patients treated with EH (n = 164) and a second group of patients treated with TH (n = 100) or DKAH (n = 64). Data were paired by gender, age, and asthma severity (). There were no statistically significant differences between groups in the forced expiratory volume in one second (FEV1) test (p = 0.443); daytime asthma symptoms more than twice/week (p = 0.664); night waking due to asthma (p = 0.861); need for rescue medication (p = 0.975); and activity limitation due to asthma (p = 0.823). No prior or current medication affected satisfaction with inhaler.
Table 1. Characteristics of study patients.
shows the differences between groups in terms of patient satisfaction and adherence. Specific satisfaction with the inhaler (global score of FSI-10) was statistically significantly higher with EH compared with TH/DKAH (p < 0.01). With regard to individual FSI-10 items, patients considered EH to be easier to learn to use (p = 0.025), easier to prepare for inhalation (p = 0.01), easier to use (p = 0.012), and easier to carry (p = 0.018). Patients found EH easier to continue with regular activities (p = 0.038), and its weight and size were more convenient (p < 0.001). In addition, = they felt they had used it correctly after inhalation (p = 0.02). Overall, patients noted greater satisfaction with the device (p = 0.001). No statistically significant difference was found for device cleaning and maintenance, or for mouthpiece comfort. Moreover, the percentage of patients with high satisfaction with inhaler was statistically significantly higher with EH (p = 0.01).
Table 2. Comparison of questionnaire results between groups.
Regarding general satisfaction with therapy (TSQM), there were no statistically significant differences in global scores between DPIs. However, patients in EH group reported that it was easier to take their medication in its current form (p = 0.018) and to plan when they will use the medication each time (p = 0.004). Patients using EH also found it more convenient to take the medication as instructed (p = 0.042). Furthermore, all items of adverse event subscale showed a trend to be lower for EH group, but without statistical significance; this result is based on questionnaire answers, not on patient-reported adverse events of administered drugs. Moreover, convenience was statistically significantly higher in EH group (p < 0.01). In addition, the percentage of patients with high satisfaction with treatment was statistically significantly higher in the EH group (p = 0.027).
In respect of specific adherence to inhaler (TAI), there was no statistically significant difference in TAI global scores between groups. Nevertheless, mean scores of erratic and unwitting non-adherence patterns were higher in the EH group (p < 0.05 for both patterns), reflecting that these non-adherence patterns were less frequent with EH. There was no statistically significant difference between groups for general adherence in global scores of the Morisky–Green scale.
With regard to asthma control, there was no statistically significant difference in ACT global score or in the percentage of patients with controlled asthma (ACT score ≥ 20). Finally, there was no statistically significant difference in health-related quality of life (Mini-AQLQ).
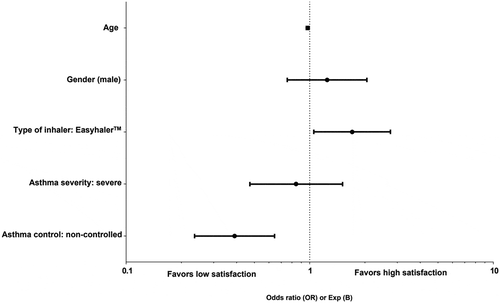
We performed a logistic regression analysis with specific satisfaction with inhaler (FSI-10) as a dependent variable (). The most important factor leading to high satisfaction was the use of EH (odds ratio [OR] 1.705; 95% CI 1.056–2.755). Moreover, male gender slightly inclined towards high satisfaction with inhaler (OR 1.245; 95% CI 0.756–2.051). Conversely, factors that led to low satisfaction with inhaler were severe asthma (OR 1.186; 95% CI 0.663–2.120) and non-controlled asthma (OR 0.390; 95% CI 0.236 −0.643).
4. Discussion
In our real-world study, patient satisfaction with inhaler was higher with EH than with TH/DKAH. Among other features, ease of use, convenient weight and size, and ease of portability contributed to higher satisfaction with EH. These results confirm the findings in the whole ASCONA population, where patient satisfaction was also higher with EH than with TH or DKAH [Citation9]. Moreover, although the aim of our study was not to prove the clinical relevance of differences in FSI-10 score, results of ASCONA main analysis [Citation9] showed that these differences are clinically relevant.
The preference for EH has been found in other studies in patients with asthma. Ahonen et al. [Citation23] performed a meta-analysis of nine clinical trials (n = 802) of EH and metered-dose inhalers (MDIs) with and without a spacer, TH, and DiskhalerTM in patients with asthma. Ease of use was statistically significantly better for EH vs. the pooled data (p < 0.001) and for almost all individual studies. Inhalation through the device was also easier with EH, as was learning how to use it. Patients preferred EH to the MDI and spacer (p < 0.001) and to TH (p < 0.01) [Citation23]. Preference of patients for EH has also been shown in other randomized clinical trials in patients with asthma. Jäger et al. [Citation24] compared EH and TH; almost 59% of patients preferred EH and scores of device acceptability were higher for EH (p = 0.001). Similarly, in a study by Tukiainen et al. [Citation25] again 59% of patients preferred EH vs. TH. Moreover, overall acceptability was higher for EH (p < 0.0001). In a study by Giner et al. [Citation8], nine aspects of the inhalers were rated from 0 to 10 for each device. The overall score was higher for EH vs. TH (p = 0.015) and vs. DKAH (p = 0.003). When patients were asked to rank the inhalers in order of preference, EH was placed first by 53% of patients, TH by 27% and DKAH by 20%. In two real-world multicenter studies by Gálffy et al. [Citation26], patients considered that EH was easy to learn and use, and 95% were satisfied or very satisfied with this inhaler.
Erratic and unwitting non-adherence patterns were less frequent in patients using EH. With respect to erratic non-adherence, we could not find a direct relationship between forgetting to take the dose and the different inhaler devices. Regarding the lower unwitting non-adherence with EH, we suggest that it was due to its ease of use, with only three steps and therefore fewer technical errors. There were no statistically significant differences between groups in terms of global adherence scores. Similarly, asthma control was comparable in both groups. A relationship between satisfaction and adherence has been found in other studies [Citation3,Citation27], but these did not compare different inhalers. In the main analysis of ASCONA, high patient satisfaction with inhaler was related to adherence and asthma control [Citation9]. However, because of the sample size in our subanalysis, the difference in satisfaction was insufficient to be reflected in adherence and asthma control.
The main strengths of our study are the population homogeneity and the use of validated and specific scales and questionnaires. Regarding potential limitations, adherence was self-reported except for items 11 and 12 of TAI. However, these two physician-reported items define the unwitting non-adherence pattern. Another potential limitation could be that different drug combinations may affect results.
5. Conclusions
Physicians should keep in mind patient satisfaction with asthma therapy, and especially specific satisfaction with the inhaler. In this setting, EH is well valued by patients. In our homogeneous population of patients with moderate to severe asthma, specific satisfaction with the inhaler was higher with EH.
However, the relationship between satisfaction with the inhaler and adherence and asthma control deserves more investigation.
Key issues
Physicians should keep in mind patient satisfaction with asthma therapy, and especially specific satisfaction with the inhaler.
Dry powder inhalers (DPIs) present different features that may have clinical implications.
EasyhalerTM has been shown to have advantages over older DPIs in prior studies.
In a homogeneous population of patients with moderate to severe asthma, specific satisfaction with inhaler (measured with the FSI-10 questionnaire) was statistically significantly higher with EasyhalerTM (43.8 ± 7.1) compared with TurbuhalerTM or DiskusTM (AccuhalerTM) (41.3 ± 7.6) (p < 0.01).
Moreover, high satisfaction with inhaler was statistically significantly higher with EasyhalerTM (62.4% of patients) than with TurbuhalerTM or DiskusTM (AccuhalerTM) (43% of patients) (p = 0.01).
This preference for EH has been found in other studies in patients with asthma.
Author contribution
All authors have contributed significantly to conception, design and execution of the study. All authors have participated in drafting, reviewing, and/or revising the manuscript, and have approved its submission.
Declaration of interest
A.V. has previously received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi and Novartis, and is a consultant for Astrazeneca and Sanofi.
L.M. has participated in advisorys and training activities for Zambon, Esteve and Orion Pharma.
M.C. has received speaker fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Menarini, and Novartis, and has received consulting fees from GlaxoSmithKline, Gebro Pharma and Novartis.
C.C. and P.R. are both employed by Orion Pharma.
V.P. previously received honoraria for speaking at sponsored meetings from AstraZeneca, Chiesi, GlaxoSmithKline and Novartis. They also received assistance in traveling to meetings from Chiesi and Novartis. They act as a consultant for ALK, AstraZeneca, Boehringer, MundiPharma and Sanofi. They have also received funding/grant support for research projects from a variety of government agencies and not-for-profit foundations, as well as AstraZeneca, Chiesi and Menarini.
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewers Disclosure
Peer reviewers on this manuscript have no relevant financial relationships or otherwise to disclose.
Supplemental Material
Download Zip (38 KB)Acknowledgments
Writing and editorial assistance was provided by Content Ed Net (Madrid, Spain).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Wilson SR, Strub P, Buist AS, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. Am J Respir Crit Care Med. 2010;181:566–577.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention [Internet]. 2018. [cited 2018 Jun 15]. Available from: http://ginasthma.org/2018-gina-report-global-strategy-for-asthma-management-and-prevention/
- Price D, Harrow B, Small M, et al. Establishing the relationship of inhaler satisfaction, treatment adherence, and patient outcomes: a prospective, real-world, cross-sectional survey of US adult asthma patients and physicians. World Allergy Organ J. 2015;8:1–11.
- Lavorini F, Levy ML, Dekhuijzen PR, et al. Inhaler choice and inhalation technique: key factors for asthma control. Prim Care Respir J. 2009;18:241–242.
- Chrystyn H, Price D. Not all asthma inhalers are the same: factors to consider when prescribing an inhaler. Prim Care Respir J. 2009;18:243–249.
- Chrystyn H. Closer to an “Ideal Inhaler” with the Easyhaler®. Clin Drug Investig. 2006;26:175–183.
- Haikarainen J, Rytilä P, Roos S, et al. Dose uniformity of budesonide Easyhaler® under simulated real-life conditions and with low inspiration flow rates. Chron Respir Dis. 2018 ;15:265-271.
- Giner J, Torrejón M, Ramos A, et al. Patient preference in the choice of dry powder inhalers. Arch Bronconeumol. 2004;40:106–109.
- Plaza V, Giner J, Calle M, et al. Impact of patient satisfaction with his or her inhaler on adherence and asthma control. Allergy Asthma Proc. 2018;39:437–444.
- Perpiñá Tordera M, Viejo JL, Sanchis J, et al. [Assessment of patient satisfaction and preferences with inhalers in asthma with the FSI-10 Questionnaire]. Arch Bronconeumol. 2008;44:346–352.
- Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12.
- Plaza V, Fernández-Rodríguez C, Melero C, et al. Validation of the “Test of the Adherence to Inhalers” (TAI) for asthma and COPD patients. J Aerosol Med Pulm Drug Deliv. 2016;29:142–152.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74.
- Nathan RA, Sorkness CA, Kosinski M, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65.
- Juniper EF, Buist AS, Cox FM, et al. Validation of a standardized version of the asthma quality of life questionnaire. Chest. 1999;115:1265–1270.
- Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the mini asthma quality of life questionnaire. Eur Respir J. 1999;14:1044–1048.
- Trujols J, Iraurgi I, Siñol N, et al. Satisfaction with methadone as a medication: psychometric properties of the Spanish version of the treatment satisfaction questionnaire for medication. J Clin Psychopharmacol. 2012;32:69–74.
- Val Jiménez A, Amorós Ballestero G, Martínez Visa P, et al. [Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the morisky and green test]. Aten Primaria. 1992;10:767–770.
- Vega JM, Badia X, Badiola C, et al. Validation of the Spanish version of the Asthma Control Test (ACT). J Asthma. 2007;44:867–872.
- Sanjuás C, Alonso J, Ferrer M, et al. Adaptation of the asthma quality of life questionnaire to a second language preserves its critical properties: the Spanish version. J Clin Epidemiol. 2001;54:182–189.
- Valero A, Ribo P, Giner J, et al. Ease of use of inhalers and its impact on treatment adherence and control of asthma. An observational study. Allergy. 2017;72(S103):419.
- Thomas M, Kay S, Pike J, et al. The Asthma Control TestTM (ACT) as a predictor of GINA guideline-defined asthma control: analysis of a multinational cross-sectional survey. Prim Care Respir J. 2009;18:41–49.
- Ahonen A, Leinonen M, Ranki-Pesonen M. Patient satisfaction with Easyhaler® compared with other inhalation systems in the treatment of asthma: a meta-analysis. Curr Ther Res. 2000;61:61–73.
- Jäger L, Laurikainen K, Leinonen M, et al. Beclomethasone dipropionate Easyhaler is as effective as budesonide Turbohaler in the control of asthma and is preferred by patients. German study group. Int J Clin Pract. 2000;54:368–372.
- Tukiainen H, Rytilä P, Hämäläinen K, et al. Safety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid-treated asthmatic patients. Respir Med. 2002;96:221–229.
- Gálffy G, Mezei G, Németh G, et al. Inhaler competence and patient satisfaction with Easyhaler®: results of two real-life multicentre studies in asthma and COPD. Drugs R D. 2013;13:215–222.
- Small M, Anderson P, Vickers A, et al. Importance of inhaler-device satisfaction in asthma treatment: real-world observations of physician-observed compliance and clinical/patient-reported outcomes. Adv Ther. 2011;28:202–212.