ABSTRACT
Introduction
Pulmonary embolism (PE) is a potentially life-threatening disease, making an accurate and prompt diagnosis thus very important. However, the normally used diagnostic algorithms may not be as efficient and safe in special patient populations. The diagnostic management of suspected PE in these patients is particularly challenging.
Areas covered
Current diagnostic strategies in patients with malignancy, elderly patients and patients with renal insufficiency are discussed in this review. A special focus is on reviewing the literature supporting the use of adjusted D-dimer cutoffs in these patient categories and the current guideline statements. Information is obtained through an extensive literature search of the following databases: PubMed, Embase, Web of Science, COCHRANE Library and Emcare (searched September 2019).
Expert opinion
A diagnostic strategy starting with clinical decision rules (CDRs) and D-dimer testing is clinically useful, also in these three patient categories, since it reduces the need for computed tomography pulmonary angiography (CTPA). The use of adjusted D-dimer cutoffs is preferred over a fixed cutoff as it safely improves the yield of the CDR/D-dimer combination.
1. Introduction
Pulmonary embolism (PE), together with deep vein thrombosis (DVT) referred to as venous thromboembolism (VTE), is a potentially life-threatening disease and an accurate and prompt diagnosis is thus very important. However, signs and symptoms of patients with PE are nonspecific and show a wide variety, ranging from shortness of breath to hemodynamic instability [Citation1]. Especially in patients with comorbidity, symptoms of other conditions may mimic the symptoms of PE [Citation2]. Consequently, objective imaging tests are required to confirm the diagnosis, of which computed tomography pulmonary angiography (CTPA) is the current standard [Citation3]. Still, imaging tests are expensive, time-consuming and may cause harm to the patient. CTPA is associated with radiation exposure and contrast material induced complications, but also overdiagnosis is an increasing challenge due to more sensitive scanning techniques, leading to diagnosing smaller (isolated) subsegmental emboli. Therefore, various diagnostic algorithms have been developed, with the aim of simplifying the diagnostic management of patients with suspected PE, and to reduce the number of required CTPA. The algorithms are based on CDRs, followed by D-dimer testing and, in case of high pretest probability and/or abnormal D-dimer, imaging [Citation4].
These algorithms have proven to be safe and efficient in the general population, where PE in approximately one third of patients can be ruled out based on CDR and D-dimer testing alone, with a low 3-month incidence of symptomatic venous thromboembolism (failure rate) [Citation1,Citation4,Citation5]. Unfortunately however, D-dimer testing has a low specificity and is often abnormal in the absence of thrombosis. Moreover, CDRs may not be as efficient and safe in some patients, patients in whom imaging tests are relatively contraindicated or yield more non-diagnostic test results. This is especially the case in patients with malignancy, elderly patients and patients with renal insufficiency, three patients categories that happen to be associated with a higher risk for VTE [Citation1,Citation4]. A clinical diagnosis of acute PE is thus frequently considered in these patients, while also being particularly challenging.
Several advances have been made to improve the performance of the diagnostic algorithms, including age-adjusted D-dimer cutoffs and D-dimer cutoffs adapted to clinical probability ( and ) [Citation6–Citation8]. However, results of posthoc analyses of studies on adjusted D-dimer cutoffs in different subgroups are often inconclusive or conflicting, and they have only been externally validated to a limited extent. The optimal diagnostic approach in these three patient groups is therefore unknown.
Figure 1. Validated diagnostic algorithms for suspected acute PE. (a) Conventional algorithm applying the age-adjusted D-dimer cutoff (see also .). (b) YEARS algorithm with a D-dimer cutoff adapted to clinical probability. PE, pulmonary embolism; CTPA, computed tomography pulmonary angiography; DVT, deep vein thrombosis. Figure printed with permission from Nature Reviews (Huisman et al, 2018).
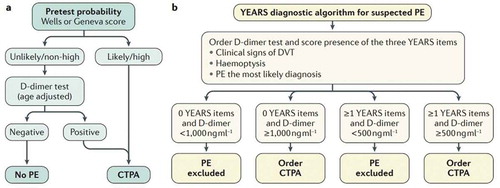
Table 1. The Wells rule and the Revised Geneva Score.
In this review, we will focus on the challenge of diagnosing PE in patients with cancer, elderly patients and patients with renal insufficiency. We searched the following databases: PubMed, Embase, Web of Science, COCHRANE Library and Emcare from inception to September 2019. The search strategy involved the general keywords Pulmonary Embolism and Diagnosis and the following specific keywords per special patient population: Neoplasms, Cancer, Malignancy, Renal Insufficiency, Renal failure, Kidney disease, Aged or Elderly. The searches were limited to studies in the English language and case reports were excluded. The aim of this review is to discuss the most important and recent studies on the diagnostic management of PE in these special patient categories and to provide clinicians with practical diagnostic management guidance using evidence from the literature. In addition, future perspectives will be discussed as well.
2. Suspected PE in cancer patients
Cancer patients frequently visit the emergency department with signs and symptoms suggestive of acute PE. It is known that cancer patients have a four- to seven-fold increased risk for VTE compared with patients without cancer. This is due to the pro-coagulant effects of cancer and cancer therapies as well as other risk factors such as immobility, frequent invasive procedures and the use of central venous catheters [Citation9,Citation10]. Current guidelines are very clear about the diagnostic approach in non-cancer patients with suspected PE, where validated clinical decision rules and D-dimer testing are recommended to assess clinical probability and to reduce the need for unnecessary and potentially harmful imaging tests. However unfortunately, no further guidance is given for the diagnostic approach in cancer patients with suspected PE [Citation11,Citation12].
Consequently, the cancer patient still represents a clinical challenge. The presence of cancer alone increases the pretest probability if using the commonly used Wells or Geneva CDRs [Citation13,Citation14]. In addition, D-dimer values are often abnormal in cancer patients [Citation15]. This was illustrated in a large management study in 12 hospitals in the Netherlands: 474 cancer patients (of the 3306 in total) were all managed with a diagnostic strategy of CDR, D-dimer testing and CTPA. A total of 51% of the cancer patients had an unlikely pretest probability but only 12% had a normal D-dimer test (using the fixed cutoff of <500 µg/L). This resulted in 49 patients (10%) in whom PE could be ruled out based on CDR and D-dimer testing alone. Despite this low efficiency, the approach was safe as only 1 patient developed VTE during 3 month follow-up, but confidence intervals were wide owing to the small number of patients (failure rate 2.0% 95% CI 0.05–10.9) [Citation16]. Similar findings were observed in a post-hoc analysis of 114 cancer patients, in which 9.4% of patients had a normal D-dimer test (using the fixed D-dimer cutoff) [Citation17]. These findings are in great contrast to the efficacy and safety in the non-cancer population, where approximately one third of patients can be ruled out based on CDR and D-dimer testing alone, at a low failure rate [Citation13,Citation18]. Hence, the clinical utility of the traditional diagnostic approach appears doubtful in cancer patients. Oncologists often perceive that D-dimer values are ‘always’ abnormal in their patients and thus CTPA scanning as a sole test is frequently the standard approach to establish or rule out PE in cancer patients.
More recently, studies tried to adjust the D-dimer cutoff, based on the patient’s age or in combination with clinical probability (YEARS and PEGeD). These methods, as is stated in the ESC guidelines, should be considered as an alternative to the fixed cutoff level, although the optimal method to adjust the cutoff still remains to be determined [Citation11]. In a recent post hoc analysis of the ADJUST-PE study [Citation6], the age-adjusted D-dimer cutoff in patients with and without cancer was assessed. The age-adjusted cutoff level of D-dimer has been validated in the general population and is calculated as age ˣ 10 µg/L for patients above 50 years of age. Of the 3324 patients in this study in total, 429 patients had cancer. 9.9% of the cancer patients had a D-dimer <500 µg/L as compared with 19.7% of the cancer patients when using the age-adjusted cutoff for D-dimer. Together with the pretest probability this doubled the proportion of cancer patients in whom PE could be excluded without further CTPA imaging from 6.3% to 12.6%. This approach appeared to be safe as no VTE events occurred during the three month follow-up (failure rate 0% 95% CI 0.0–6.9) [Citation19]. Furthermore, there is evidence from a post hoc analysis of the Christopher Study [Citation18] that raising the cutoff of D-dimer to 700 µg/L may help to increase the proportion of cancer patients in whom PE could be ruled out without imaging. The number of patients in whom PE could be ruled out increased from 8% to 13%, if using the Wells CDR and a D-dimer cutoff of 700 µg/L. Applying the age-adjusted cutoff in this group of patients yielded comparable results as 13% of patients could be ruled out based on CDR and D-dimer alone [Citation15].
Recently a new CDR (YEARS) has been published [Citation8]. All patients in this large (3465 patients) prospective study were managed by simultaneous assessment of the YEARS CDR (consisting of three items) and D-dimer testing, of which the D-dimer cutoff was dependent on whether YEARS items were present or not (explained in )). This study showed that CTPA could be safely avoided in an additional 13% of patients compared with the Wells CDR and fixed D-dimer cutoff. This reduction in CTPA was still substantial when compared with the age-adjusted D-dimer cutoff, namely 7.6%. This reduction could be achieved at an only 0.78% (95% CI 0.49–1.2) failure rate (worst case scenario) with regard to the 3-month incidence of recurrent VTE. However, this approach showed a higher failure rate (2.6%, 95% CI 1.3–5.2) in the relatively small subgroup of cancer patients. Yet, it should be noted that the absolute number of failures was low in the cancer subgroup (7 developed an event of the 279 patients with PE excluded at baseline) and most patients had either other strong risk factors for VTE (mostly surgery; after baseline and prior to developing VTE) or PE could not be excluded as cause of death (patients died at home and adjudication committee could not rule out PE) [Citation8]. The same failure rate was observed in a recent meta-analysis, focussing on the Wells rule with the fixed D-dimer cutoff of <500 µg/L (2.6%, 95% CI 0.57–11.0) [Citation4]. Another study that evaluated a diagnostic strategy of ruling out PE based on the Wells rule and a D-dimer, of which the cutoff was adapted to clinical probability, was published more recently. In patients with a low clinical probability and a D-dimer below 1000 ng/mL or in patients with a moderate clinical probability and a D-dimer below 500 ng/mL, PE was ruled out without imaging. This PEGeD study was consistent with previous results regarding efficiency and safety of D-dimer cutoffs adapted to clinical probability, since the number of required CTPA’s could be reduced with 17.6% (compared with the fixed D-dimer cutoff of 500 ng/mL), in the presence of a low failure rate (0% 95% CI 0.0–0.29). This study did not perform a subgroup analysis in cancer patients [Citation20].
A prospective diagnostic management study in cancer patients, called the Hydra study, is currently under way (Netherlands Trial Register NL7752). In this randomized study, the safety and efficiency of the YEARS algorithm will be compared with the safety and efficiency of CTPA alone. The results of this study are expected by the end of 2022 and will contribute to guidance on the optimal diagnostic approach in daily practice. Meanwhile, current guidelines do not provide clear recommendations on the diagnostic approach in cancer patients with suspected PE [Citation11,Citation12]. Nevertheless, studies in cancer patients have shown that a reduction in CTPA for up to 13% can be achieved when using CDRs and adjusted D-dimer cutoffs [Citation15,Citation19]. This approach has been demonstrated to be safe in cancer patients very recently in a retrospective study in 380 cancer patients. This study evaluated the performance of the ACP (American College of Physicians) guideline, which recommends the use of validated CDRs in conjunction with D-dimer testing or Pulmonary Embolism Rule-out Criteria (PERC), in cancer patients. 78 patients (21%) in this study underwent unnecessary CTPA not indicated by the combination of CDR and D-dimer, of whom only 1 was diagnosed with PE (failure rate 1.3% 95% CI 0.2–6.9). Important limitation of this study however was that only patients who underwent CTPA were included [Citation21].
In conclusion, we suggest to use these adjusted D-dimer cutoff methods (age-adjusted or adjusted to clinical probability) in cancer patients with suspected PE, as they increase the diagnostic utility of the CDR/D-dimer combination and reduce the need for CTPA. This will result in less contrast material induced complications, a reduction of potentially irrelevant sub-segmental emboli detection, as well as lower health care costs [Citation22].
3. Suspected PE in elderly patients
The incidence of VTE increases exponentially with age, particularly after age 60. Consequently, the majority of VTE events occur in older adults, as does the risk of potentially fatal major bleeding events [Citation23–Citation25]. In addition, short-term mortality of acute PE is also greater in the elderly than in younger patients [Citation26]. An accurate and prompt diagnosis in older patients is thus of importance, but at the same time challenging for multiple reasons as well. First of all, aging is associated with an increasing prevalence of cardiac and pulmonary comorbidities, of which the symptoms can mimic the nonspecific symptoms of PE [Citation2]. Moreover, the clinical presentation of older patients with confirmed PE differs considerably from the one of younger patients. For instance, syncope was shown to be present more often in small retrospective studies in the elderly whereas pleuritic chest pain, considered often as one of the hallmark symptoms of PE diagnosis, was less prevalent [Citation27,Citation28]. This may render the initial assessment of patients, including answering the common item in CDRs of ‘PE most likely diagnosis’ and establishing the pretest probability, complex. In addition, D-dimer levels increase physiologically with age and thus D-dimer testing is known to have a low specificity in the elderly [Citation29,Citation30]. In a study with 1029 patients on the performance of common diagnostic tests in different age groups, results showed a clear age dependent diminishing specificity of the D-dimer. The specificity was as low as 10% in patients above 80 years old, resulting in the exclusion of PE in only 5% of the patients above 80 years old (with the fixed D-dimer cutoff of 500 µg/L) [Citation29]. Hence, after D-dimer testing the majority of patients must be referred for imaging tests. Not only will the number of patients with actually confirmed PE on imaging be small, the number of suboptimal and nondiagnostic imaging tests also increases with age [Citation29]. Furthermore, transitions for hospital care are especially stressful in the very elderly [Citation31], which makes the physician reluctant to refer older patients for suspicion of PE.
Since assessing clinical probability in elderly patients is difficult, it is interesting to know which CDR performs best. A previous systematic review tried to evaluate different CDRs in elderly patients (and perform a diagnostic meta-analysis), but unfortunately pooling of data was not possible as a result of the limited number of studies and heterogeneity among studies [Citation32]. Small single-center (mostly retrospective) studies have compared the diagnostic performance of the Wells with the revised Geneva CDR in elderly patients. These studies were consistent in demonstrating superiority of Wells for assessing clinical probability in elderly patients. This finding could, at least partially, be related to the absence of an item 'immobilization for reasons other than surgery or fracture' in the Geneva rule (see also ) [Citation33–Citation35].
In an attempt to improve the performance of D-dimer in elderly patients, the age-adjusted D-dimer cutoff was introduced. The age-adjusted cutoff level of D-dimer is calculated as age ˣ 10 µg/L in patients 50 years or older. This strategy has been prospectively validated in a large (3346 patients) diagnostic management outcome study [Citation6]. Patients with unlikely clinical probability and D-dimer below age-adjusted cutoff did not undergo further testing and were left untreated: 766 patients in this study were 75 years or older. Applying the age-adjusted D-dimer cutoff in these patients instead of the fixed D-dimer cutoff increased the number of patients in whom PE could be excluded based on CDR and D-dimer alone from 6.4% (43/673) to 29.7% (200/673). The diagnostic failure rate of the age-adjusted D-dimer cutoff was 0/195 (0.0% 95% CI 0.0%-1.9%) in patients 75 years or older and 1/331 (0.3% 95% CI 0.1%-1.7%) in all patients managed based on the age-adjusted cutoff [Citation6]. A large meta-analysis including over 12.000 patients with an unlikely clinical probability supported the use of the age-adjusted D-dimer cutoff and showed increased specificity without significantly reduced sensitivity when compared with the fixed D-dimer cutoff [Citation36].
In the meantime another adjusted D-dimer cutoff method was derived and validated in a large (3465 patients) prospective diagnostic management outcome study. This study used the YEARS clinical decision rule and was already discussed in the first part of this review. Importantly, an adjusted D-dimer cutoff of 1000 µg/L was applied in patients in whom none of the YEARS items were present, whereas a D-dimer cutoff of 500 µg/L was used when one or more items were present. Patients below this D-dimer cutoff were left untreated. Overall results of this study showed an additional 7.6% of patients in whom CTPA could be safely avoided compared with the Wells CDR and age-adjusted D-dimer cutoff. The 3-month failure rate, according to the worst case scenario 0.78% (95% CI 0.49%-1.2%), of this approach was low. Since the D-dimer cutoff of this approach is very high (1000 µg/L in the case of no YEARS items) and would only be reached in a 100 year old patient when using the age-adjusted D-dimer cutoff approach, this is very relevant in the elderly population. Unfortunately no subgroup analysis was performed in the very elderly in this study (aged>75 or >80 years old) [Citation8]. A previous retrospective study did studied the age-adjusted D-dimer cutoff and the fixed higher D-dimer cutoff of 1000 µg/L in patients aged 80 years or older. Both methods increased the specificity of the D-dimer in the elderly, but owing to the small number of patients a good comparison could not been made [Citation37]. Nevertheless, it was shown by a recent post hoc analysis of the YEARS study that there was no added value of implementing ADJUST in the YEARS algorithm, likely because of the already high D-dimer cutoff in the YEARS algorithm [Citation38].
Meanwhile current guidelines advice physicians to consider using the adapted D-dimer cutoffs as an alternative to the fixed cutoff, in patients with an unlikely clinical probability [Citation11,Citation12]. The recent ASH guideline states that ‘the use of an age-adjusted D-dimer cutoff in outpatients older than 50 years is as safe as the standard cutoff and increases the diagnostic utility of the test’ [Citation12]. However, guidelines provide no further specific recommendations on the diagnostic management of elderly patients (>75 years), and the ADJUST approach has never been directly compared with the YEARS approach [Citation11,Citation12].
In summary, awareness of the differences in the clinical presentation of elderly patients with suspected PE and the limitations of diagnostic tests in that setting are important in clinical daily care. Nevertheless, is it worthwhile limiting the use of imaging tests, especially in elderly patients who are more prone to develop contrast material induced complications and for whom transfers to the hospital are very burdensome [Citation39]. An accurate diagnostic strategy that safely over excludes PE in a timely manner while reduces the need for CTPA is necessary. In line with current guidelines, we suggest the use of adjusted D-dimer cutoff methods such as that used in the ADJUST-PE study [Citation6] and the YEARS study [Citation8] in order to improve the yield of noninvasive diagnostic tests in elderly patients.
4. Suspected PE in patients with renal insufficiency
Thrombosis is common in patients with renal insufficiency and comprises not only DVT and PE, but also hemodialysis vascular-access-related thrombosis and arterial thrombosis. The exact mechanism for this increased risk, whether this is by activation of procoagulants, decreased anticoagulants or decreased fibrinolytic activity, likely differs depending on the cause of renal insufficiency [Citation40–Citation42]. Not only does the presence of renal disease limits the use of anticoagulants and/or makes dose reduction necessary, it also increases the risks associated with CTPA and the administration of intravenous contrast. This makes diagnosing these patients challenging.
Problems are encountered when relying on the common diagnostic sequential use of clinical assessment, D-dimer testing and CTPA in patients with renal disease and suspected PE. The specificity of the D-dimer in patients with renal insufficiency is low and decreases further with declining renal function. The yield of the D-dimer approach in these patients is thus limited [Citation43]. Consequently, patients with renal insufficiency are overexposed to the risks of CTPA.
Most studies that reported on the diagnostic performance of D-dimer testing in patients with impaired renal function were retrospective and used the fixed D-dimer cutoff of 500 µg/L. In a post hoc analysis on 351 patients assessing the proportion of normal D-dimer level, 30% of the patients had a normal renal function (eGFR>89 mL/min), 59% a mild impaired renal function (eGFR 60–89 mL/in) and 11% a moderate impaired renal function (eGFR 30–59 mL/min). Patients with an eGFR<30 mL/min were excluded from the primary study and were thus not examined. Normal D-dimer levels were found in 58% of the patients with a normal renal function, 54% of patients with a mild impaired renal function and in 28% of patients with a moderate impaired renal function [Citation44].
Another study included 1305 patients, of whom 82% had an eGFR exceeding 60 mL/min (mild impaired to normal renal function), 16% an eGFR of 30–60 mL/min (moderate impaired renal function) and 2% an eGFR of <30 mL/min (severe renal function). Results showed that only 6% of the patients with a moderate impaired renal function had a normal D-dimer and none of the patients with a severe renal function [Citation43].
An even larger retrospective study included 1625 patients (with a low or intermediate clinical probability). Again patients with an eGFR of <30 ml/min were not included. Results were presented applying the fixed D-dimer cutoff as well as the age-adjusted D-dimer cutoff. In patients with a normal renal function, D-dimer was negative in 46% and 52% respectively. For patients with a mild impaired renal function this was 31% and 41% respectively and in patients with a moderate impaired renal function 11% and 23% respectively [Citation45]. The sensitivity of D-dimer testing in patients with impaired renal function was excellent in all three studies [Citation43–Citation45].
Current guidelines do not provide a statement on the diagnostic value of D-dimer testing in patients with renal insufficiency [Citation11,Citation12]. However, the above discussed studies indicate that although the performance of D-dimer decreases with impaired renal function, D-dimer testing is still useful since PE can be ruled out in a substantial proportion of patients, reducing the need for imaging tests.
Guidelines consider CTPA as the method of choice for imaging in patients with suspected PE, except for patients with severe renal impairment, in whom CTPA is contraindicated due to the risks of iodinated contrast [Citation11,Citation12]. Contrast-induced nephropathy (CIN), defined as a decrease in renal clearance following injection of radiographic iodinated contrast media, is a well-known complication of CTPA. Reported incidences of CIN vary widely, depending on patient population and different definitions of CIN and acute kidney injury (AKI) [Citation46]. A prospective study on 174 patients undergoing CTPA for suspicion of PE reported a CIN incidence, defined as an increase of serum creatinine of ≥0.5 mg/dL or ≥25% within 2 to 7 days of contrast administration, of 14% (25 patients). The rate of CIN complicated by severe renal failure or death after CTPA was 1.7% (3/174) [Citation47]. Yet a retrospective study on 237 patients reported a CIN incidence, defined as an increase in serum creatinine levels over 25% or over 44 μmol/L during the first 5 days after CTPA, of 8.9% (21 patients). Only one patient in this study still suffered impaired renal function after 1 month (0.4%) [Citation48]. Patients with a severe renal function were probably not included in these studies. Still interestingly, impaired renal function (eGFR<60 mL/min or <45 mL/min) was not identified as a risk factor for CIN in both studies, although absolute numbers were low [Citation47,Citation48]. A very recent article proposed the idea of CIN rather being a marker for adverse outcomes, instead of being the mediator. Data confirming a causal relationship of CIN with adverse outcomes is lacking [Citation49]. Nevertheless, preventive care should be implemented if patients at elevated risk for CIN undergo CTPA. Ultra-low dose contrast CTPA, which does provides images of excellent diagnostic quality, may sufficiently reduce the patient’s CIN risk [Citation39,Citation50]. Importantly, preventive hydration in patients with renal disease undergoing CTPA for suspected PE can safely be withheld, as was demonstrated in a randomized study recently. This is beneficial since this causes a delay in diagnosis [Citation51]. For patients with severe renal impairment, VQ scan is still the imaging method of choice [Citation11,Citation12,Citation52].
In summary, previous studies do demonstrate that D-dimer testing is clinically useful in patients with renal insufficiency and non-high clinical probability, given that PE can be ruled out by a negative D-dimer in a substantial proportion of patients. Applying the age-adjusted D-dimer cutoff resulted in an even better yield [Citation45]. Unfortunately, it remains unclear whether D-dimer testing is also useful in patients with severe renal failure. To our knowledge, adapted D-dimer cutoffs have not been studied in this group of patients. Given the possible risks of CIN after CTPA it is essential to reduce the number of CTPA to a minimum. Therefore, we recommend the use of adjusted D-dimer cutoffs in patients with a normal to moderate impaired renal function. Regarding the risk of CIN after CTPA in patients with renal insufficiency, studies suggest that mild to even moderate renal insufficiency itself is not a very strong risk factor. Other risk factors as age, comorbidity and contrast media volume play an important role. Nevertheless, CTPA is contraindicated in patients with severe renal impairment, according to current guidelines. An option in these patients could be to perform a compression ultrasonography of the legs (CUS) before proceeding to imaging tests. A positive CUS provides an indication for anticoagulation therapy, and imaging can be avoided, although this approach will cause diagnostic delay [Citation53]. If imaging is necessary, the VQ scan is the imaging method of choice.
5. Conclusions
Pulmonary embolism is often suspected in patients with cancer, elderly patients and patients with renal insufficiency, because they comprise specific high-risk groups for venous thromboembolism. An accurate and prompt diagnosis is however difficult, since the clinical presentation can be very different and the safety and efficiency of the normally used diagnostic algorithms are partially unknown. As a consequence, imaging is often ordered at a low threshold. Nevertheless, a diagnostic strategy for patients suspected of PE based on CDRs and followed by D-dimer testing, prior to imaging, is clinically useful in these special patient populations as well. Although the number of patients that can be ruled out based on CDR and D-dimer testing alone is not as high as in the general population, it does reduce the need for CTPA. This will result in less radiation exposure, less contrast material induced complications, a reduction of potentially irrelevant sub-segmental emboli detection, as well as lower health care costs; all desirable in the setting of patients who already have comorbidity and are at increased risk for complications. Applying age-adjusted D-dimer cutoffs or D-dimer cutoffs adapted to clinical probability is preferred, since this increases the yield of the CDR/D-dimer combination considerably. The advice to use these adjusted D-dimer cutoffs is also in line with current guidelines, which state that these methods can be considered as an alternative to the fixed D-dimer cutoff. Despite all advances made, clinical questions remain. The optimal method to adjust the D-dimer cutoff (age-adjusted or adapted to clinical probability) remains to be determined, since both strategies have not been directly compared to each other. Importantly, CTPA, regarded as the method of choice, is contraindicated in patients with severe renal insufficiency. The VQ scan is the imaging method of choice in these patients, although an option could be to proceed to CUS before imaging, since a positive CUS waves the need for further imaging.
6. Expert opinion
The diagnostic approach of suspected PE in special patient populations is challenging due to the lower yield of the CDR/D-dimer combination and higher risk of contrast material induced complications. Studies in cancer patients indicated that with applying age-adjusted D-dimer cutoffs, the number of patients that can be ruled out based on CDR and D-dimer testing alone can be doubled (up to 13%). This number could even be higher when applying the D-dimer cutoff based on clinical probability. However, the failure rate of this approach in cancer patients was higher than in the general population. This could be explained by the higher prevalence of PE in this subgroup of patients. To provide a definite answer, the Hydra study is currently under way. In elderly patients, assessing clinical probability is particularly challenging, since symptoms of comorbidity can mimic the nonspecific symptoms of PE. In addition, D-dimer values are often false positive since D-dimer increases physiologically with age. The use of age-adjusted D-dimer cutoffs or cutoffs based on clinical probability are advised to overcome at least one of these problems. This advice also holds for patients with mild and moderate impaired renal function. CIN is a well-known complication of CTPA, although reported incidences vary widely. Interestingly, impaired renal function was not identified as a risk factor in these studies, although data is very limited. Nevertheless is CTPA contra-indicated in patients with severe renal failure.
Despite these challenges and limitations, diagnostic algorithms starting with the assessment of clinical probability and followed by D-dimer testing will remain the cornerstone of the diagnostic management of suspected PE in different patient populations in the future. The focus in the upcoming years however will presumably be on studying the most efficient while safe diagnostic strategy in different patient populations and comorbidity. The Hydra study, validating the YEARS algorithm in patients with cancer, is currently under way. Since this is a randomized study, the safety of the YEARS algorithm can directly be compared to the safety of CTPA alone. Furthermore will individual patient data meta-analyses contribute to tailoring diagnostic strategies for different patient populations in the nearby future, by providing large databases and enabling sufficient statistical power in these different subgroups. In addition, research shall focus on imaging techniques with a very low dose of contrast or other modalities, for instance MRI techniques, and whether these could be helpful in diagnosing patients suspected of PE while limiting the risks of unnecessary imaging tests.
Article highlights
Diagnostic algorithms based on CDRs, followed by D-dimer testing and, if necessary, imaging, are the cornerstone of the diagnostic management of suspected PE, also in special patient populations.
Awareness of the differences in the clinical presentation of elderly patients or patients with comorbidity with confirmed PE is important.
Avoidance of CTPA must be strived for (if safe) because this will result in less radiation exposure, less contrast material induced complications, a reduction of potentially irrelevant sub-segmental emboli detection, as well as lower health care costs.
The use of age-adjusted D-dimer cutoffs or cutoffs based on clinical probability is preferred over a fixed D-dimer cutoff, since the number of patients that can be ruled out based on the CDR/D-dimer combination increases considerably.
D-dimer testing is not recommended in patients with severe renal failure.
CTPA is contra-indicated in patients with severe renal failure; VQ scan is the imaging method of choice in these patients.
Declaration of interest
MV Huisman has received research grants from ZONMW- Dutch Healthcare Fund, Bayer Health Care, Pfizer-Bristol-Myers Squibb Alliance, Boehringer-Ingelheim, and Daiichi-Sankyo. FA Klok has received research grants from the Dutch Heart Foundation, Netherlands Thrombosis Foundation, Bayer Health Care, Pfizer-Bristol-Myers Squibb Alliance, Boehringer-Ingelheim, Daiichi-Sankyo, Merck-Sharpe and Dohme, and Actelion. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018 May;17(4):18028.
- Righini M, Le Gal G, Perrier A, et al. The challenge of diagnosing pulmonary embolism in elderly patients: influence of age on commonly used diagnostic tests and strategies. J Am Geriatr Soc. 2005 June;53(6):1039–1045.
- Huisman MV, Klok FA. How I diagnose acute pulmonary embolism. Blood. 2013 May 30;121(22):4443–4448.
- van Es N, van der Hulle T, Van Es J, et al. Wells rule and d-dimer testing to rule out pulmonary embolism: a systematic review and individual-patient data meta-analysis. Ann Intern Med. 2016 Aug 16;165(4):253–261.
- Pasha SM, Klok FA, Snoep JD, et al. Safety of excluding acute pulmonary embolism based on an unlikely clinical probability by the wells rule and normal D-dimer concentration: a meta-analysis. Thromb Res. 2010 Apr;125(4):e123–e127.
- Righini M, van Es J, den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014 Mar 19;311(11):1117–1124.
- Douma RA, Le Gal G, Sohne M, et al. Potential of an age adjusted D-dimer cut-off value to improve the exclusion of pulmonary embolism in older patients: a retrospective analysis of three large cohorts. BMJ. 2010 Mar 30;340:c1475.
- van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study. Lancet. 2017 July 15;390(10091):289–297.
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005 Feb 9;293(6):715–722.
- Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood. 2013 Sept 5;122(10):1712–1723.
- Konstantinides SV, Meyer G, Becattini C, et al. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2019 Aug 31; 41:543–603.
- Lim W, Le Gal G, Bates SM, et al. American society of hematology 2018 guidelines for management of venous thromboembolism: diagnosis of venous thromboembolism. Blood Adv. 2018 Nov 27;2(22):3226–3256.
- Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001 July 17;135(2):98–107.
- Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006 Feb 7;144(3):165–171.
- Douma RA, van Sluis GL, Kamphuisen PW, et al. Clinical decision rule and D-dimer have lower clinical utility to exclude pulmonary embolism in cancer patients. Explanations and potential ameliorations. Thromb Haemost. 2010 Oct; 104(4):831–836.
- Sohne M, Kruip MJ, Nijkeuter M, et al. Accuracy of clinical decision rule, D-dimer and spiral computed tomography in patients with malignancy, previous venous thromboembolism, COPD or heart failure and in older patients with suspected pulmonary embolism. J Thromb Haemost. 2006 May;4(5):1042–1046.
- Van Es J, Douma RA, Mos IC, et al. Performance of four clinical decision rules in patients with malignancy and suspected pulmonary embolism. J Thromb Haemost. 2012 Feb; 10(2):312–314.
- van Belle A, Buller HR, Huisman MV, et al. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. Jama. 2006 Jan 11;295(2):172–179.
- Wilts IT, Le Gal G, Den Exter PL, et al. Performance of the age-adjusted cut-off for D-dimer in patients with cancer and suspected pulmonary embolism. Thromb Res. 2017 Apr;152:49–51.
- Kearon C, de Wit K, Parpia S, et al. Diagnosis of pulmonary embolism with d-dimer adjusted to clinical probability. N Engl J Med. 2019 Nov 28;381(22):2125–2134.
- Qdaisat A, Yeung SJ, Variyam DE, et al. Evaluation of Cancer Patients With Suspected Pulmonary Embolism: performance of the American College of Physicians Guideline. J Am Coll Radiol. 2019 July 31;17:22–30.
- Carrier M, Klok FA. Symptomatic subsegmental pulmonary embolism: to treat or not to treat? Hematol Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):237–241.
- White RH. The epidemiology of venous thromboembolism. Circulation. 2003 June 17;107(23 Suppl 1):I4–18.
- Klok FA, Hosel V, Clemens A, et al. Prediction of bleeding events in patients with venous thromboembolism on stable anticoagulation treatment. Eur Respir J. 2016 Nov;48(5):1369–1376.
- Klok FA, Kooiman J, Huisman MV, et al. Predicting anticoagulant-related bleeding in patients with venous thromboembolism: a clinically oriented review. Eur Respir J. 2015 Jan;45(1):201–210.
- Kniffin WD Jr., Baron JA, Barrett J, et al. The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch Intern Med. 1994 Apr 25;154(8):861–866.
- Kokturk N, Oguzulgen IK, Demir N, et al. Differences in clinical presentation of pulmonary embolism in older vs younger patients. Circ J. 2005 Aug;69(8):981–986.
- Castelli R, Bergamaschini L, Sailis P, et al. The impact of an aging population on the diagnosis of pulmonary embolism: comparison of young and elderly patients. Clin Appl Thromb Hemost. 2009 Feb; 15(1):65–72.
- Righini M, Goehring C, Bounameaux H, et al. Effects of age on the performance of common diagnostic tests for pulmonary embolism. Am J Med. 2000 Oct 1;109(5):357–361.
- Harper PL, Theakston E, Ahmed J, et al. D-dimer concentration increases with age reducing the clinical value of the D-dimer assay in the elderly. Intern Med J. 2007 Sept;37(9):607–613.
- Gozalo P, Teno JM, Mitchell SL, et al. End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med. 2011 Sept 29;365(13):1212–1221.
- Siccama RN, Janssen KJ, Verheijden NA, et al. Systematic review: diagnostic accuracy of clinical decision rules for venous thromboembolism in elderly. Ageing Res Rev. 2011 Apr; 10(2):304–313.
- Di Marca S, Cilia C, Campagna A, et al. Comparison of wells and revised geneva rule to assess pretest probability of pulmonary embolism in high-risk hospitalized elderly adults. J Am Geriatr Soc. 2015 June;63(6):1091–1097.
- Ma Y, Huang J, Wang Y, et al. Comparison of the Wells score with the revised Geneva score for assessing pretest probability of pulmonary embolism in hospitalized elderly patients. Eur J Intern Med. 2016 Dec;36:e18–e9.
- Guo DJ, Zhao C, Zou YD, et al. Values of the Wells and revised Geneva scores combined with D-dimer in diagnosing elderly pulmonary embolism patients. Chin Med J (Engl). 2015 Apr 20;128(8):1052–1057.
- Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013 May 3;346:f2492.
- Polo FH, Pasciuti L, Meloni DF, et al. A higher d-dimer threshold safely rules-out pulmonary embolism in very elderly emergency department patients. Thromb Res. 2014 Mar; 133(3):380–383.
- van der Pol LM, van der Hulle T, Cheung YW, et al. No added value of the age-adjusted D-dimer cut-off to the YEARS algorithm in patients with suspected pulmonary embolism. J Thromb Haemost. 2017 Dec;15(12):2317–2324.
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004 Oct 6;44(7):1393–1399.
- Wattanakit K, Cushman M. Chronic kidney disease and venous thromboembolism: epidemiology and mechanisms. Curr Opin Pulm Med. 2009 Sept;15(5):408–412.
- Kumar G, Sakhuja A, Taneja A, et al. Pulmonary embolism in patients with CKD and ESRD. Clin J Am Soc Nephrol. 2012 Oct;7(10):1584–1590.
- Wattanakit K, Cushman M, Stehman-Breen C, et al. Chronic kidney disease increases risk for venous thromboembolism. J Am Soc Nephrol. 2008 Jan;19(1):135–140.
- Lindner G, Funk GC, Pfortmueller CA, et al. D-dimer to rule out pulmonary embolism in renal insufficiency. Am J Med. 2014 Apr;127(4):343–347.
- Karami-Djurabi R, Klok FA, Kooiman J, et al. D-dimer testing in patients with suspected pulmonary embolism and impaired renal function. Am J Med. 2009 Nov;122(11):1050–1053.
- Robert-Ebadi H, Bertoletti L, Combescure C, et al. Effects of impaired renal function on levels and performance of D-dimer in patients with suspected pulmonary embolism. Thromb Haemost. 2014 Sept 2;112(3):614–620.
- Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: a consensus report. Contrast media safety committee, European Society of Urogenital Radiology (ESUR). Eur Radiol. 1999;9(8):1602–1613.
- Mitchell AM, Jones AE, Tumlin JA, et al. Prospective study of the incidence of contrast-induced nephropathy among patients evaluated for pulmonary embolism by contrast-enhanced computed tomography. Acad Emerg Med. 2012 June;19(6):618–625.
- Kooiman J, Klok FA, Mos IC, et al. Incidence and predictors of contrast-induced nephropathy following CT-angiography for clinically suspected acute pulmonary embolism. J Thromb Haemost. 2010 Feb;8(2):409–411.
- Weisbord SD, Gallagher M. Web exclusive. Annals for hospitalists inpatient notes - preventing contrast-associated acute kidney injury-putting the issue to rest. Ann Intern Med. 2019 July 16;171(2):Ho2–ho3.
- Rajiah P, Ciancibello L, Novak R, et al. Ultra-low dose contrast CT pulmonary angiography in oncology patients using a high-pitch helical dual-source technology. Diagn Interv Radiol. 2019 May;25(3):195–203.
- Kooiman J, Sijpkens YW, van Buren M, et al. Randomised trial of no hydration vs. sodium bicarbonate hydration in patients with chronic kidney disease undergoing acute computed tomography-pulmonary ang.iography. J Thromb Haemost. 2014 Oct;12(10):1658–1666.
- Reid JH, Coche EE, Inoue T, et al. Is the lung scan alive and well? Facts and controversies in defining the role of lung scintigraphy for the diagnosis of pulmonary embolism in the era of MDCT. Eur J Nucl Med Mol Imaging. 2009 Mar;36(3):505–521.
- Righini M, Le Gal G, Aujesky D, et al. Diagnosis of pulmonary embolism by multidetector CT alone or combined with venous ultrasonography of the leg: a randomised non-inferiority trial. Lancet. 2008 Apr 19;371(9621):1343–1352.
