ABSTRACT
Introduction
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report provides guidance on effective management of chronic obstructive pulmonary disease (COPD) according to local healthcare systems. However, COPD is a heterogenous disease and certain aspects, including prevalence, disease–time course and phenotype distribution, can differ between countries. Moreover, features of clinical practice and healthcare systems for patients with COPD can vary widely, even in geographically close and economically similar countries.
Areas covered
Based on an initial workshop of respiratory physicians from eleven countries across Central and Eastern Europe (CEE) in December 2018 and subsequent discussions, this article offers region-specific insights from clinical practice and healthcare systems in CEE. Taking recommendations from the GOLD 2022 report into account, we suggest approaches to adapt these into national clinical guidelines for COPD management in CEE.
Expert opinion
Several factors should be considered when optimizing management of COPD in CEE compared with other regions, including differences in smoking status, vaccination uptake, prevalence of tuberculosis and nontuberculous mycobacteria, and variations in healthcare systems. We provide guidance and algorithms for pharmacologic and non-pharmacologic management of COPD for the following scenarios: initial and follow-up treatment, treatment of patients with frequent exacerbations, and withdrawal of inhaled corticosteroids where appropriate.
PLAIN LANGUAGE SUMMARY
Chronic obstructive pulmonary disease (COPD) is a common disease of the lungs. It causes symptoms such as breathlessness, cough, and production of phlegm. In people with COPD, these symptoms often reduce the quality of their lives. From time to time, symptoms may get worse in people with the disease. This worsening is known as ‘exacerbation’. Exacerbations of COPD can be so bad that they lead to hospital admissions. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) regularly gives advice to doctors around the world. This can help them to provide their patients with the best possible treatment for COPD. However, people with the disease and healthcare systems vary from country to country. This means that the guidance may need to be adjusted to the needs and available resources of different regions. This review looks at how COPD is treated in Central and Eastern Europe. We suggest how to adapt the GOLD recommendations to best suit the Central and Eastern European region.
Graphical Abstract
1. Introduction
Chronic obstructive pulmonary disease (COPD) is a preventable and treatable disease marked by persistent respiratory symptoms (such as breathlessness, cough, sputum production, wheezing and chest tightness) and airflow limitation that results from airway and/or alveolar abnormalities [Citation1]. An estimated 300 million people had COPD in 2017 [Citation2], and the disease burden is expected to increase in the coming years due to the aging population and continued exposure to COPD risk factors such as cigarette smoking and other environmental exposures, including air pollution and biomass fuel exposure [Citation3–5]. Of note, COPD may be significantly underdiagnosed due to the inclusion of persistent symptoms in the diagnostic criteria, which risks under-identification of asymptomatic or intermittently symptomatic patients [Citation6]. In addition to the daily symptom burden, COPD may be punctuated by periods of acute worsening of respiratory symptoms – referred to as ‘exacerbations’ – which account for the greatest proportion of total COPD burden on healthcare systems [Citation1,Citation7].
COPD is a heterogeneous disease and therefore treatment should be individualized according to patients’ different clinical characteristics and severity of their disease [Citation1]. Certain characteristics of COPD also differ from country to country, including variations in prevalence, time course of the disease and distribution of different phenotypes (such as the presence or absence of asthma, frequent exacerbators versus non-exacerbators, and the presence or absence of chronic bronchitis versus emphysema) [Citation8]. Features of clinical practice and healthcare systems for patients with COPD may also vary widely, even in geographically close and economically similar countries [Citation8,Citation9].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) strategy report [Citation1] is used worldwide as a tool for healthcare professionals to implement effective management programs for COPD according to their local healthcare system. Guidance from the GOLD report helps to inform clinical guidelines on COPD, which are then optimized by national respiratory societies according to routine clinical practice in individual countries [Citation10–16].
Across guidelines for COPD in Europe and Russia, there is general agreement for some recommendations to be used as the cornerstone of treatment; for example, treatment goals, criteria for diagnosing COPD, and use of long-acting bronchodilators (either long-acting muscarinic antagonists [LAMAs], long-acting β2-agonists [LABAs] or LAMA/LABA combinations) [Citation17]. However, differences exist between countries in other areas, such as methods for classifying disease severity, consideration of patient phenotypes, criteria for the use of inhaled corticosteroids (ICS), and recommendations for other medications in addition to bronchodilators [Citation17].
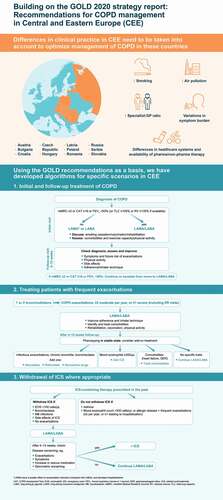
This review considers region-specific insights from clinical practice and healthcare systems across several countries of Central and Eastern Europe (CEE), including Austria, Bulgaria, Croatia, Czech Republic, Hungary, Latvia, Poland, Romania, Russia, Serbia and Slovakia, and their impact on COPD management. The recommendations of the GOLD 2022 strategy report are discussed, and approaches to adapt some of these recommendations into national clinical guidelines for COPD management in CEE are considered.
2. The situation in Central and Eastern Europe
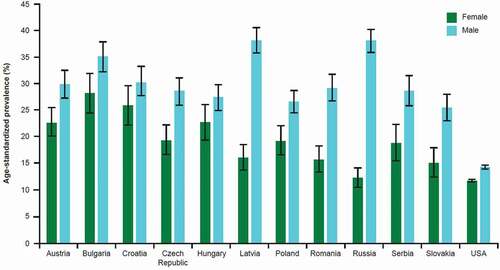
In CEE, certain region-specific features of COPD populations and healthcare systems affect the management of COPD. One of these is smoking prevalence. CEE has a high prevalence of cigarette smoking, which plays a major role in the etiology and pathogenesis of COPD. According to data from the Global Burden of Disease 2015 Tobacco Collaborators, the mean prevalence of smoking in CEE in 2015 ranged from 22–38% in men (versus 14% in USA) and 12–28% in women (versus 12% in USA; ) [Citation18]. The reduction in smoking prevalence between 2005 and 2015 in most CEE countries was less than in the USA, with little change observed in some countries (e.g. Czech Republic) and even an increase in smoking prevalence in women observed in others (e.g. Croatia and Slovakia) [Citation18]. According to 2018 data from the World Health Organization (WHO), Europe has the highest regional prevalence of smoking (regional average 29%, compared with 17% in the Americas and 24% in Western Pacific) [Citation19]. Within Europe, WHO figures show that the prevalence is particularly high in certain countries of the CEE (e.g. Russia, 41%; Serbia, 39%; Latvia, 38%; Croatia, 37%; Czech Republic, 34%) [Citation19]. These differences are important because smoking can influence the natural history of COPD and also affect the efficacy of pharmacotherapy, particularly ICS [Citation20].
Figure 1. Prevalence of smoking in Central and Eastern European countries and the USA, 2015.
It has also been consistently reported that levels of air pollution are generally higher in CEE than in Western European countries [Citation21], with increased concentrations of benzo(a)pyrene in urbanized areas and areas with high ozone levels [Citation22]. In addition, an association has been found between air pollution and the burden of COPD. For example, a Serbian study found a link between high levels of black smoke and emergency room admissions for COPD [Citation23].
Furthermore, there are differences in healthcare systems between CEE countries () and most Western European countries [Citation24], such as a higher ratio of specialists to general practitioners (GPs) in CEE countries. Indeed, in this region, COPD is largely diagnosed and treated by pulmonologists rather than GPs. For example, in a study evaluating COPD treatment patterns in Bulgaria, the proportion of patients visiting a GP was lower than those visiting specialists (66% versus 99%), whereas the number of specialist visits was significantly higher in Bulgaria than in Italy [Citation25]. Treatment by specialists versus GPs can cause variations in disease management; for example, patients under the care of their GP may be less likely to receive pharmacologic and non-pharmacologic treatments, and less likely to perform their inhalation maneuvers correctly, compared with those cared for by pneumologists [Citation26].
Table 1. Differences in the healthcare systems for COPD management in CEE countries based on estimates and available data
The burden of COPD symptoms is high across CEE. Data from a large observational cross-sectional study exploring the characteristics of patients with COPD in 11 CEE countries (the POPE study; N = 3362) found a relatively high mean COPD Assessment Test (CAT) score (17.4 ± 7.8) in patients with otherwise stable COPD. These findings are consistent with data from Western Europe and indicate that a high proportion of patients remain symptomatic despite therapy [Citation36,Citation37]. However, mean CAT scores varied significantly between countries in the CEE study, from 15.1 in Hungary to 21.2 in Bulgaria [Citation38]. These differences may be explained, at least in part, by variation in the distribution of clinical phenotypes, the proportion of frequent exacerbators, and/or differences in symptom perception [Citation38].
With respect to the use of COPD pharmacotherapy in clinical practice, data from CEE countries is similar to other cross-sectional studies [Citation39–42], with the majority of patients not receiving guideline-directed therapy and a high proportion of patients with stable COPD receiving ICS-containing regimens, including patients classified as non-exacerbators [Citation36]. Country-specific reimbursement restrictions on using combination therapies, such as LAMA/LABA, may potentially contribute to this phenomenon [Citation43]. More detailed information on the availability of pharmacotherapy for COPD patients across CEE is provided in Supplementary Table 1.
Furthermore, both the availability of and adherence to non-pharmacologic interventions for COPD patients, such as pulmonary rehabilitation and vaccination, are low in CEE (Supplementary Table 2). For example, data from a Serbian study showed that the immunization rate for seasonal influenza was 37.1% in patients with stable COPD, which is below the recommended vaccination rate and has implications for the rate of COPD exacerbations [Citation44].
Table 2. Action points for management of COPD in CEE
3. Goals of COPD treatment
According to the GOLD 2022 strategy report, the main goals of COPD treatment are reduction in both symptoms and future risk of exacerbations [Citation1]. These are supported by additional factors that contribute to these goals, including improvements in exercise tolerance and health status, prevention and treatment of exacerbations, prevention of disease progression, and a reduction in mortality [Citation1]. However, goals such as reducing mortality and preventing disease progression may be difficult to achieve, and health status is also complex to measure and treat.
Management strategies discussed in the GOLD recommendations include both pharmacologic and non-pharmacologic interventions [Citation1]. Pharmacologic therapy can be used to reduce symptoms, reduce the frequency and severity of exacerbations, improve health status and increase exercise tolerance in patients with COPD [Citation1]. Non-pharmacologic therapy is complementary and helps to supplement pharmacologic approaches [Citation1]. Each treatment for COPD should be individualized and guided by the patient’s symptoms, exacerbations, side effects, comorbidities, drug availability, cost, patient response, patient preference and their ability to use the inhalation device.
Below, we discuss a CEE perspective on the GOLD recommendations for pharmacologic and non-pharmacologic management of COPD. We refer to the following key areas: initial and follow-up treatment of COPD; treating patients with frequent exacerbations; and withdrawal of ICS where appropriate. For reference, key action points for each section are summarized in .
3.1. Initial and follow-up treatment of COPD
The GOLD strategy report places a strong emphasis on the importance of selecting the correct pharmacologic treatment for patients early in the course of their disease. For initial pharmacologic treatment of COPD, rescue medication with short-acting bronchodilators (SABAs) should be prescribed for immediate symptom relief; however, SABAs are not generally recommended for regular use. A long-acting bronchodilator is therefore most commonly recommended; initial therapy with either LAMA, LABA or LAMA/LABA is chosen according to an individualized assessment of symptoms and exacerbation risk [Citation1]. According to GOLD, LAMA is preferred over LABA in patients with a high risk of exacerbations but, for some patients, a combination treatment such as a LAMA/LABA (e.g. for patients with severe breathlessness) or LABA/ICS (for patients with a high risk of exacerbations and blood eosinophil counts >300 cells/µL) may be offered as initial treatment [Citation1]. According to the American Thoracic Society clinical practice guideline for pharmacologic treatment of COPD, however, LAMA/LABA combination treatment is preferred over LAMA or LABA monotherapy in all patients with COPD and dyspnea or exercise intolerance [Citation45].
Despite the benefits of using the current GOLD treatment recommendations to tailor treatment, assessing patients with newly diagnosed COPD according to the GOLD criteria based on exacerbations can be challenging. Firstly, at the time of COPD diagnosis, it is difficult to obtain retrospectively reliable information about past exacerbations, including their severity, frequency, etiology, and the presence of a causative link between COPD and these events. In fact, information about previous exacerbations in clinical practice is usually based on the patient’s subjective recall rather than medical records [Citation46]. Secondly, the threshold for hospitalization – and thus the classification of patients as GOLD D – may be lower in CEE due to a larger availability of hospital beds per capita compared with Western countries (3.5–8.7 per 1,000 in CEE versus 2.8 per 1,000 in USA) [Citation47,Citation48]. Thirdly, the prospective 3-year SPIROMICS study demonstrated an inconsistent exacerbation pattern (number of years both with and without exacerbations) in 41% of the population studied, particularly among patients with more severe disease (GOLD stages 3–4; 56%) [Citation49]. Thus, any treatment decision based on recollection of exacerbation history may be unreliable for guiding treatment decisions. In fact, according to a previous study within the CEE region, the majority of COPD patients are non-exacerbators [Citation36]. Finally, exacerbations may be driven by (undiagnosed) comorbidities (e.g. heart failure) rather than underlying inflammation and/or infection, thus prompting the need for in-depth diagnostic assessment of the underlying causes of episodes of acute worsening.
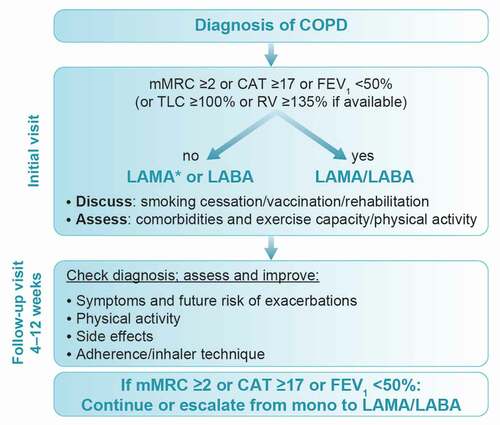
Taking into account the factors described above, and with reference to national guidelines for the management of COPD [Citation10–14,Citation50], as well as the GOLD strategy report [Citation1], we have developed a proposed algorithm for the initiation of treatment as well as considerations for follow-up (). We propose that, at the time of diagnosis, the choice of maintenance inhaler therapy should be primarily based on symptom burden and/or lung function impairment rather than exacerbation history. Since some patients may not experience symptoms to a high degree due to a sedentary lifestyle, forced expiratory volume in 1 second (FEV1) <50% predicted and/or evidence of hyperinflation (residual volume ≥135% [Citation51]) is suggested as an alternative indication for implementing dual bronchodilator therapy, even when the modified Medical Research Council (mMRC) and/or CAT scores are outside of the recommended cutoff points for grading symptoms as severe (≥2 for mMRC; ≥17 for CAT) [Citation52]. These findings are supported by the most recent pooled data from four randomized controlled trials, comparing the efficacy of LAMA/LABA versus LAMA monotherapy as maintenance therapy in patients with COPD who were not receiving maintenance treatment with long-acting bronchodilators or ICS (‘maintenance naïve’) at study entry [Citation53]. Treatment initiation with LAMA/LABA resulted in greater improvements in lung function, health status and symptom severity compared with mono-bronchodilation alone, without compromising patient safety or tolerability [Citation53]. These results support the use of dual bronchodilation as first-line maintenance treatment in patients with COPD. This should be balanced against the cost of treatment, given that reimbursement is not uniform in all CEE countries.
Figure 2. Proposed algorithm for initial and follow-up treatment of COPD in CEE.
Further support for delaying the assessment of exacerbations to the follow-up visit rather than the initial assessment stage is given by the body of data supporting first-step pharmacologic therapy (e.g. LAMAs, LABAs and LAMA/LABA) in decreasing the risk of COPD exacerbations. For example, in the 4-year UPLIFT trial, the LAMA tiotropium was superior to placebo in decreasing COPD exacerbation risk (as add-on to current therapy, which may include LABA and/or ICS) in a broad COPD population [Citation54]. Tiotropium has also shown superiority in terms of reducing moderate/severe COPD exacerbation rates over two LABAs – namely salmeterol [Citation55] and indacaterol [Citation56] – and non-inferiority to LABA/ICS (salmeterol/fluticasone) [Citation57]. In addition, LAMA/LABA (indacaterol/glycopyrronium) is more effective at preventing moderate/severe exacerbations than LABA/ICS (salmeterol/fluticasone) [Citation58]. Furthermore, real-life data from the DACCORD study recently showed that significantly fewer patients on LAMA/LABA experienced an exacerbation compared with those on triple therapy (LAMA/LABA/ICS) [Citation59]. Findings from DACCORD also demonstrated that exacerbation rates did not increase in patients on dual LAMA/LABA therapy following withdrawal of ICS compared with those continuing on triple therapy [Citation60].
Despite our suggested recommendation to use dual bronchodilation as first-line maintenance treatment in patients with COPD, we do acknowledge that any patient with COPD and concomitant asthma should be prescribed ICS in addition to bronchodilator treatment from the beginning. This is supported by the clinical benefits of ICS treatment in patients with asthma, including improvements in lung function and symptoms, and reduction in exacerbation rate [Citation61].
Beyond pharmacotherapy for COPD, all patients are advised to adhere to non-pharmacologic treatment options, such as smoking cessation, vaccination and increasing physical activity, through simple advice or formal rehabilitation or training programs. For smoking cessation, which has the greatest capacity to influence the natural history of COPD, a combination of pharmacotherapy (e.g. varenicline, bupropion and nortriptyline, as well as nicotine replacement products) and behavioral support is optimal to improve success rates [Citation1]. Behavioral support strategies which can be provided by healthcare professionals include developing a quit plan, giving practical counseling with follow-up contact, and helping the patient to receive social support [Citation1]. COVID-19 vaccination is recommended in patients with COPD, and influenza and pneumococcal vaccines can also reduce serious illness, particularly in older patients or those with comorbid conditions [Citation1]. For physical functioning, improvements have been observed in patients with COPD participating in a real-world study in CEE after 6 weeks of tiotropium/olodaterol therapy [Citation43]. Dual bronchodilator therapy may also help to promote physical activity by a variety of mechanisms in this context, such as reducing breathlessness and hyperinflation, and improving cardiac function [Citation43,Citation51].
Furthermore, choosing the right inhaler device for each patient and regular assessment of inhaler technique are important factors, both at treatment initiation and follow-up visits () [Citation62,Citation63]. The patient should be involved in the decision as to which inhaler device is best for them, taking into account ease of handling. A discussion of factors affecting inhaler choice is beyond the scope of this article but is reviewed elsewhere [Citation64–66]. It is also important to ensure that patients have the required inspiratory effort if dry powder inhalers are to be used [Citation67]. There are other options, such as pressurized metered-dose inhalers and soft mist inhalers, which are less affected by inspiratory effort [Citation68,Citation69].
Following initiation of therapy, patients should be followed up for achievement of treatment goals, and adjustments made where necessary [Citation1]. Here, we recommend a rather short-term follow-up of about 6 weeks after the initial assessment, which is believed to be the average interval between two physician visits in the CEE region after initiation of treatment. At follow-up, changes in physical activity, side effects of and adherence to inhaler therapy, and inhalation technique should be checked. If response to initial pharmacologic treatment is not sufficient, it is important to consider whether symptoms or exacerbations are the main characteristic according to the GOLD 2022 strategy report. For symptoms such as dyspnea, a long-acting bronchodilator should be added for those on LAMA or LABA monotherapy (); for exacerbations, please see the section on patients with frequent exacerbations below.
3.2. Treating patients with frequent exacerbations
Some patients with COPD are particularly susceptible to frequent exacerbations, defined as ≥2 exacerbations per year [Citation1]. Long-acting bronchodilator therapy (LAMA or LAMA/LABA) may be sufficient to prevent exacerbations in many patients, although others may require ICS therapy [Citation1]. According to the GOLD 2022 strategy report, ICS can be added to LAMA/LABA where relevant, such as in patients with frequent exacerbations who also have high blood eosinophil levels [Citation1]. However, before prescribing therapy containing ICS, it is important to assess risk factors for the development of pneumonia, such as being a current smoker or ≥55 years of age, having a history of pneumonia or a body mass index <25 kg/m2, or having a poor mMRC dyspnea grade and/or severe airflow limitation [Citation1].
Blood eosinophil counts can be used to predict the magnitude of the effect of ICS (in addition to bronchodilator regimens) in order to decrease the risk of future exacerbations. Patients with blood eosinophil counts <100 cells/µL are unlikely to benefit from ICS treatment, whereas those with counts >300 cells/µL and history of frequent exacerbations have the greatest likelihood of treatment benefit [Citation1]. However, recent findings in a healthy population suggest that median blood eosinophil counts are affected by a range of confounding factors, which may have implications for clinical practice in terms of the threshold for ICS use in the management of COPD [Citation70]. This study found that after exclusion of age ≤18 years, asthma, COPD, positive skin prick tests, current smoking, metabolic syndrome and obesity, the median blood eosinophil count was 100–120 cells/µL in healthy subjects aged >18 years, which is lower than previously regarded as normal in healthy subjects [Citation70].
In addition, blood eosinophil count, when measured once during a COPD exacerbation, may not serve as a reliable marker of the long-term inflammation phenotype (eosinophilic or susceptible to ICS) as it may be influenced by the type of infection. For example, in a prospective clinical trial, an inverse relationship was shown between blood eosinophil count and airway bacterial load during COPD exacerbations [Citation71]. Patients with COPD and bacterial infection during exacerbations had a significant decrease in absolute blood eosinophil count compared with the stable state, and no blood eosinophil count changes were observed in patients without bacterial infection [Citation71]. This suggests that blood eosinophil count may reflect the type of COPD exacerbation (bacterial or non-bacterial) rather than a robust phenotype; it must therefore be monitored over time to interpret correctly.
Data supporting the benefits of triple therapy containing ICS in patients at high risk of exacerbations come from the IMPACT and TRIBUTE studies [Citation72,Citation73]. In IMPACT, triple therapy with LAMA/LABA/ICS (umeclidinium/vilanterol/fluticasone furoate) resulted in a significantly lower rate of moderate or severe COPD exacerbations versus LABA/ICS (vilanterol/fluticasone furoate) or LAMA/LABA (umeclidinium/vilanterol) in patients with symptomatic COPD and a history of exacerbations [Citation72]. However, there was a higher incidence of pneumonia in the ICS groups versus LAMA/LABA. In TRIBUTE, LAMA/LABA/ICS (glycopyrronium/formoterol/beclometasone dipropionate) significantly reduced the rate of moderate-to-severe exacerbations compared with LAMA/LABA (glycopyrronium/indacaterol) in patients with symptomatic COPD, severe or very severe airflow limitation, and an exacerbation history despite maintenance therapy, without increasing the risk of pneumonia [Citation73]. In contrast to IMPACT, patients with concomitant asthma were excluded from TRIBUTE, and the level of benefit with triple therapy was smaller than that in IMPACT [Citation73]. In fact, rates of moderate-to-severe exacerbations were low in both study arms (0.50 [95% confidence interval (CI) 0.45–0.57] and 0.59 [95% CI 0.53–0.67] per patient per year for the triple therapy and dual bronchodilator arms, respectively), suggesting that after 52 weeks of treatment, both regimens were effective [Citation73]. The level of benefit with the triple therapy versus dual therapy was greater in patients with chronic bronchitis and those with eosinophils of at least 2% [Citation73]. Note that reduction of exacerbations should be balanced with safety (see section on ICS withdrawal below).
Criteria for who should receive an eosinophil test (outside of exacerbations, oral steroids, other causes of eosinophilia) could be better defined in future guidance. For instance, smoking status should also be taken into account when determining the cutoff value for eosinophils. Studies have shown that smoking status modifies the relationship between efficacy and blood eosinophil counts, with former smokers being more responsive to ICS at any eosinophil count than current smokers [Citation74,Citation75]. This may be particularly relevant for CEE countries with a high prevalence of smoking.
In this context, an analysis from the GLUCOLD study demonstrated that patients with COPD and chronic bronchitis had lower eosinophil counts in bronchial biopsies and higher percentages of sputum eosinophils than patients without those symptoms, suggesting a preferential distribution of eosinophils toward the airway lumen in patients with chronic bronchitis [Citation76]. The good predictivity of blood eosinophils to identify sputum eosinophilia, in turn, suggests promise for this blood biomarker as a predictive marker of response to PDE4 inhibitors in patients with COPD and chronic bronchitis already being treated with triple therapy [Citation77].
High-risk patients who have had at least three exacerbations in the previous year despite standard care may also benefit from long-term treatment with macrolide antibiotics to reduce the number of exacerbations [Citation78]. Chronic treatment with macrolides, however, may increase the occurrence of adverse events and cause a rise in the levels of macrolide-resistant bacteria [Citation79]. In this context, it is noteworthy that the composition of the lung microbiome is influenced by antibiotics [Citation80]. Furthermore, it needs to be acknowledged that there is little evidence about the interaction between long-term macrolide treatment and ICS.
Finally, we need to acknowledge that real-life predictors of triple inhaler therapy extend findings from randomized controlled trials and may include older age, current and former smoking, higher GOLD stage, the number of moderate and severe COPD exacerbations, and comorbidities such as heart failure [Citation81]. Taking into account these and other factors described above, we have developed a proposed algorithm for use with frequent exacerbators (). In addition to blood eosinophil counts, other criteria should be considered for phenotyping patients, including computed tomography (CT) scanning to rule out bronchiectasis with mucus plugs, bronchomalacia and/or emphysema, echocardiogram and/or assessment of N-terminal pro B-type natriuretic peptide, and sputum microbiology. Non-pharmacologic approaches such as vaccination and pulmonary rehabilitation should also be considered.
Figure 3. Proposed algorithm for treatment of patients with frequent COPD exacerbations in CEE.
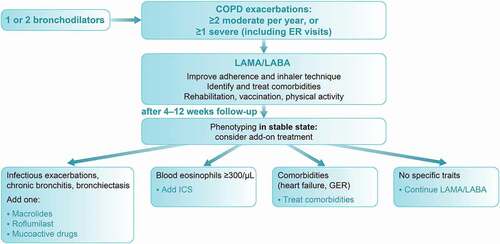
It is important that comorbidities such as cardiovascular disease, which increase exacerbation rates, are also identified and treated. In particular, heart failure (both systolic and diastolic) has an adverse impact on exacerbation frequency, morbidity and mortality in patients with COPD [Citation82]. Early identification and appropriate management of both COPD and heart failure is essential in order to reduce the morbidity and mortality of these patients and to improve their quality of life [Citation83]; however, escalation of inhaler therapy is not necessarily advised.
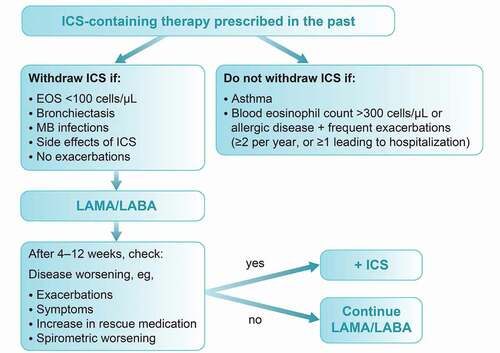
3.3. Withdrawal of ICS where appropriate
According to the GOLD 2022 strategy report, in patients treated with LAMA/LABA/ICS, ICS withdrawal can be considered if there are adverse events (such as pneumonia) or a reported lack of efficacy [Citation1]. However, a blood eosinophil count of ≥300 cells/µL identifies patients with the greatest risk of exacerbations after ICS withdrawal; these patients should be followed more closely for relapse of exacerbations [Citation1].
Results from studies of ICS withdrawal show differing results regarding impact on lung function, symptoms and exacerbations; this may reflect differences in study methodology, including use of background long-acting bronchodilators that may minimize the effect of ICS withdrawal [Citation1]. Early studies in undertreated patients with COPD demonstrated that ICS withdrawal may lead to an exacerbation [Citation84]. However, the INSTEAD and OPTIMO studies demonstrated that patients with moderate airflow limitation and low exacerbation history could be withdrawn from ICS/LABA combination if they were switched to an effective long-acting bronchodilator, with no change in lung function, exacerbation rate or patient-reported outcomes [Citation85,Citation86]. In contrast, frequent exacerbation history and high baseline eosinophils identify a subgroup of patients at high risk of exacerbations when discontinued from ICS treatment [Citation87].
Data from the POPE study showed that a large proportion of patients with COPD received ICS in CEE at the time of the study (2014–2015), often unnecessarily and contrary to the GOLD recommendations [Citation36]. Going forward, it is important to take into account evidence that long-term ICS use increases the risk of systemic side effects such as pneumonia, diabetes, osteoporosis, cataracts, skin atrophy and bruising, in addition to local side effects, such as oropharyngeal candidiasis and hoarseness [Citation88,Citation89].
In addition, there is a high prevalence of tuberculosis and nontuberculous mycobacteria in CEE, which may support the case for more restricted use of ICS where possible [Citation90–92]. A positive association has been observed between a past history of tuberculosis and the presence of chronic airflow obstruction, independent of cigarette smoking [Citation93]. Patients with COPD and prior tuberculosis have poorer lung function, more symptoms, unique features of bronchiectasis and emphysema, and a higher prevalence of exacerbations than patients without a history of tuberculosis [Citation94]; these patients also have a worse prognosis in terms of hospitalizations and survival [Citation95,Citation96]. There is an increased risk of tuberculosis development in patients with COPD associated with prior ICS use [Citation97–99], with a higher risk for fluticasone/salmeterol than budesonide/formoterol according to observational data [Citation100]. Of note, studies have shown that long-acting bronchodilation is safe and effective in patients with tuberculosis and chronic airflow obstruction [Citation101,Citation102].
Patients who do not appear to respond to ICS treatment (i.e. those who continue to experience frequent exacerbations) or those with side effects of ICS should consider a stepwise reduction and cessation of ICS. This is shown in the proposed algorithm in , and is in line with previous clinical trials and guidance [Citation103,Citation104].
4. Conclusions
Several factors need to be taken into account when optimizing management of COPD in CEE compared with other regions, including differences in smoking status, vaccination uptake, prevalence of tuberculosis and nontuberculous mycobacteria, and variations in healthcare systems.
The recommendations of the GOLD 2022 strategy report provide a solid foundation for adaptation according to the specific needs of the CEE region. Using the GOLD recommendations as a basis, we have developed algorithms for specific scenarios in CEE, including initial and follow-up treatment of COPD, treatment of patients with frequent exacerbations and ICS withdrawal. In this context, we need to acknowledge that the proposed algorithms, similar to all other guidelines, may have limitations as they refer to classes of drugs to be used rather than individual molecules with unique pharmacologic characteristics.
5. Expert opinion
The management of COPD has moved from a one-size-fits-all to a phenotype-driven therapeutic approach over the course of the past 20 years, taking into account patient-related factors such as exacerbation history, blood eosinophil data and/or the presence of chronic bronchitis symptoms. Although this has resulted in more patient-tailored treatment, global treatment algorithms such as the GOLD guidelines frequently fail to be implemented in clinical practice, as evidenced by a lack of adoption and discordant treatment behavior in a real-world setting [Citation105–107]. This may, at least in part, be due to discrepancies between patient populations from randomized controlled trials and patients in a day-to-day clinical practice environment [Citation108]. In this context, this review provides a more practical approach to the management of COPD, focusing more on the patients that represent the majority of the COPD patient population, i.e. those with dyspnea as the predominant symptom, comprising about 80% of all patients [Citation109]. We suggest a step-wise approach to diagnosis and treatment that may be easier to follow at a primary care level, with the potential for treatment escalation in the case of disease worsening, and one that takes into account important aspects beyond pharmacologic compounds, such as inhaler technique, adherence, and physical activity.
The management of COPD in the future may potentially be comparable to asthma, with primary care (GPs) and secondary care (office-based pulmonologists) physicians being responsible for diagnosis, treatment initiation, and follow-up. However, patients with severe or treatment-refractory disease (e.g. those with persistent symptoms and/or exacerbations despite maintenance inhaler therapy and rehabilitation) may be referred to specialized clinics for severe COPD, offering a wide range of treatment options for advanced-stage disease, such as noninvasive ventilation, endoscopic lung volume reduction for emphysema, or ablation technologies for chronic bronchitis. With the availability of lower-threshold CT scans and technologies such as QCT, i.e. digital quantitative analysis of CT scans, the structural aspects of COPD can now be visualized and quantified in more depth [Citation110]. Findings from CT scans may further identify specific treatable traits, such as emphysema, interstitial fibrosis, airway wall thickening and other signs of chronic bronchitis [Citation111]. In fact, endoscopically applied treatment regimens, such as valve implantation and vapor ablation for patients with severe emphysema, are already being considered standard of care in selected patient groups [Citation112]. Beyond targeting emphysema ablation, technologies such as bronchial rheoplasty and targeted lung denervation, which are currently being investigated in sham-controlled treatment trials (with results from pivotal trials expected in 2 to 3 years), may offer minimal invasive options for add-on treatment on top of inhaler therapy for patients with a chronic bronchitic phenotype [Citation113,Citation114]. Lastly, COPD disease control can often be impacted by the presence of comorbidities, such as heart failure, diabetes, and depression [Citation1]. Thus, there is an urgent need to investigate the true impact of comorbidities on symptoms and exacerbation control, as well as physicians’ prescribing behavior – beyond availability and reimbursement – on disease control in real-world settings.
In conclusion, the authors of this review expect to see further refinement in an individualized treatment approach with adoption of basic regimens for treatment initiation, and increasing use of imaging modalities for disease characteristics embedded in expert-based specialty care for advanced disease.
Article highlights
This review considers region-specific insights from clinical practice and healthcare systems across several countries of Central and Eastern Europe (CEE), including Austria, Bulgaria, Croatia, Czech Republic, Hungary, Latvia, Poland, Romania, Russia, Serbia and Slovakia, and their impact on COPD management.
Factors that should be considered when optimizing management of COPD in CEE compared with other regions include differences in smoking status, vaccination uptake, prevalence of tuberculosis and nontuberculous mycobacteria, and variations in healthcare systems.
The recommendations of the GOLD 2022 strategy report provide a solid foundation for adaptation according to the specific needs of the CEE region.
Using the GOLD recommendations as a basis, we have developed guidance and algorithms for pharmacologic and non-pharmacologic management of COPD for specific scenarios in CEE, including initial and follow-up treatment of COPD, treatment of patients with frequent exacerbations and withdrawal of inhaled corticosteroids.
Treatment of COPD is expected to become more individualized, with adoption of basic regimens for treatment initiation, and increasing use of imaging modalities embedded in specialty care for advanced disease.
Declaration of interests
A Valipour reports personal fees from AstraZeneca, Chiesi, Menarini and Novartis, and grants, personal fees and non-financial support from Boehringer Ingelheim outside the submitted work. S. Avdeev reports fees for providing scientific advice and/or lecturing from Boehringer Ingelheim, AstraZeneca, Novartis, Chiesi and Sandoz outside the submitted work. V. Koblizek reports grants and personal fees from Boehringer Ingelheim CZ, RCV and Angelini CZ, and personal fees from Novartis CZ, AstraZeneca CZ, CEE and Chiesi CZ outside the submitted work. I. Kopitovic reports fees for providing scientific advice and/or lecturing from AstraZeneca, GSK, Boehringer Ingelheim, Novartis, Menarini and Chiesi outside the submitted work. G. Lupkovics reports fees for providing scientific advice and/or lecturing from Boehringer Ingelheim outside the submitted work. M. Man reports fees for medical writing assistance from Boehringer Ingelheim outside the submitted work. M. Bukovskis reports speaker fees for speaking at the 2018 CEE Respiratory Expert Forum from Boehringer Ingelheim outside the submitted work. N. Tudoric reports personal fees from AstraZeneca, Berlin-Chemie Menarini, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis and Sandoz outside the submitted work. M. Vukoja reports fees for providing scientific advice and/or lecturing from Boehringer Ingelheim, Menarini and Novartis, and fees for providing scientific advice from AstraZeneca outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
A reviewer on this manuscript has disclosed that they are an employee of a company that works in the field of respiratory medicines. Peer reviewers on this manuscript have no other relevant financial relationships or otherwise to disclose.
Supplemental Material
Download MS Word (63.5 KB)Acknowledgments
Medical writing assistance, in the form of the preparation and revision of the manuscript, was supported financially by Boehringer Ingelheim, and provided by Cindy Macpherson of MediTech Media (London, UK) under the authors’ conceptual direction and based on feedback from the authors. V. Koblizek is supported by Charles University PROGRES Q40/08, Prague, Czech Republic and Ministry of Health Czech Republic (UHHK 00179906).
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2022 report). Available at: https://goldcopd.org/wp-content/uploads/2021/11/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf (Last accessed 2021 Dec 03).
- James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet. 2018;392(10159):1789–1858.
- Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718.
- Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–743.
- Mathers CD, Loncar D, Samet J. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- Ho T, Cusack RP, Chaudhary N, et al. Under-and over-diagnosis of COPD: a global perspective. Breathe. 2019;15(1):24–35.
- Mathioudakis AG, Janssens W, Sivapalan P, et al. Acute exacerbations of chronic obstructive pulmonary disease: in search of diagnostic biomarkers and treatable traits. Thorax. 2020;75(6):520–527.
- Zbozinkova Z, Barczyk A, Tkacova R, et al. POPE study: rationale and methodology of a study to phenotype patients with COPD in Central and Eastern Europe. Int J Chron Obstruct Pulmon Dis. 2016;11:611–622.
- Tabyshova A, Hurst JR, Soriano JB, et al. Gaps in COPD guidelines of low- and middle-income countries: a systematic scoping review. Chest. 2021;159(2):575–584.
- Aisanov Z, Avdeev S, Arkhipov V, et al. Russian guidelines for the management of COPD: algorithm of pharmacologic treatment. Int J Chron Obstruct Pulmon Dis. 2018;13:183–187.
- Vukoja M, Kopitovic I, Lazic Z, et al. Diagnosis and management of chronic obstructive pulmonary disease in Serbia: an expert group position statement. Int J Chron Obstruct Pulmon Dis. 2019;14:1993–2002.
- Zatloukal J, Brat K, Neumannova K, et al. Chronic obstructive pulmonary disease-diagnosis and management of stable disease; a personalized approach to care, using the treatable traits concept based on clinical phenotypes. Position paper of the Czech Pneumological and Phthisiological Society. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2020;164(4):325–356.
- Vogelmeier C, Buhl R, Burghuber O, et al. Guideline for the diagnosis and treatment of COPD patients-issued by the German Respiratory Society and the German Atemwegsliga in cooperation with the Austrian Society of Pneumology. Pneumologie. 2018;72(4):253–308.
- Sliwinski P, Gorecka D, Jassem E, et al. [Polish Respiratory Society guidelines for chronic obstructive pulmonary disease]. Pneumonologia I Alergologia Polska. 2014;82(3):227–263.
- Miravitlles M, Soler-Cataluna JJ. GOLD in 2017: a view from the Spanish COPD guidelines (GesCOPD). Arch Bronconeumol. 2017;53(3):89–90.
- Gunen H, Kilinc O, Polatli M, et al. Modification of the GOLD recommendations for chronic obstructive pulmonary disease to broaden their usage in Turkey. Expert Rev Respir Med. 2016;10(6):625–628.
- Miravitlles M, Vogelmeier C, Roche N, et al. A review of national guidelines for management of COPD in Europe. Eur Respir J. 2016;47(2):625–637.
- Reitsma MB, Fullman N, Ng M, et al. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: a systematic analysis from the Global Burden of Disease study 2015. Lancet. 2017;389(10082):1885–1906.
- World Health Organization. World health statistics data visualizations dashboard. SDG Target 3.a | tobacco control. Tobacco smoking. 2018. Available at: https://apps.who.int/gho/data/node.sdg.3-a-viz?lang=en (Last accessed 2021 Apr 1).
- Van Overveld F, Demkow U, Gorecka D, et al. Differences in responses upon corticosteroid therapy between smoking and non-smoking patients with COPD. J Physiol Pharmacol. 2006;57:273–282.
- European Environment Agency. Air quality in Europe - 2019 report. 2019. Available at: https://www.eea.europa.eu/publications/air-quality-in-europe-2019 (Last accessed 2021 Apr 1).
- European Environment Agency. Air pollution. 2017. Available at: https://www.eea.europa.eu/themes/air/intro (Last accessed 2021 Apr 1).
- Milutinovic S, Nikic D, Stosic L, et al. Short-term association between air pollution and emergency room admissions for chronic obstructive pulmonary disease in Nis, Serbia. Cent Eur J Public Health. 2009;17(1):8.
- Kayyali R, Odeh B, Frerichs I, et al. COPD care delivery pathways in five European Union countries: mapping and health care professionals’ perceptions. Int J Chron Obstruct Pulmon Dis. 2016;11:2831–2838.
- Kamusheva M, Dimitrova M, van Boven JF, et al. Clinical characteristics, treatment patterns, and socio-economic burden of COPD in Bulgaria. J Med Econ. 2017;20(5):503–509.
- Garcia-Aymerich J, Escarrabill J, Marrades RM, et al. Differences in COPD care among doctors who control the disease: general practitioner vs. pneumologist. Respir.Med. 2006;100(2):332–339.
- Pavlov P, Ivanov Y, Glogovska P, et al. New epidemiologic data on COPD in the region of Pleven. Thorac Medic J. 2012;4(2):44–50.
- Koblizek V, Jarkovsky J, Dusek L, et al. The Czechia COPD mortality rate declining,but total deaths increasing. Eur Respir J. 2020;56(suppl 64):434.
- Korányi National Institute of TB and Pulmonology. Korányi Bulletin. 2019. Available at: https://szakmai.koranyi.hu/bulletin/ (Last accessed 2020 May 28).
- Główny Urząd Statystyczny. [Official Journals of the Central Statistical Office]. 2021. Available at: https://dziennikigus.stat.gov.pl/dzienniki-urzedowe-gus/ (Last accessed 2021 Apr 1).
- Ulmeanu R, unpublished data. Study of COPD national prevalence in Romania 2019 - project of Romanian Society of Pneumology. Bucharest: MEDICALA.
- Cristea C, Matei E, Galan A, et al. Raport Național privind Starea de Sănătate a Populației României 2020. Available at: https://insp.gov.ro/download/cnepss/stare-de-sanatate/rapoarte_si_studii_despre_starea_de_sanatate/starea_de_sanatate/starea_de_sanatate/RAPORTUL-NATIONAL-AL-STARII-DE-SANATATE-A-POPULATIEI-%25E2%2580%2593-2020.pdf (Last accessed 2022 Jan 6).
- Republic of Serbia Ministry of Health, Institute of Public Health of Serbia. Results of the National Health Survey of the Republic of Serbia 2013. 2014. Available at: http://www.batut.org.rs/download/publikacije/2013SerbiaHealthSurvey.pdf (Last accessed 2021 Apr 1).
- Institute of Public Health of Serbia. Health statistical yearbook of Republic of Serbia 2017. 2018. Available at: http://www.batut.org.rs/download/publikacije/pub2017v026.pdf (Last accessed 2021 Apr 1).
- The World Bank. Data: total population. Available at: https://data.worldbank.org/indicator/SP.POP.TOTL (Last accessed 2021 Apr 1).
- Koblizek V, Milenkovic B, Barczyk A, et al. Phenotypes of COPD patients with a smoking history in Central and Eastern Europe: the POPE study. Eur Respir J. 2017;49(5):ii: 1601446.
- Jones PW, Brusselle G, Dal Negro RW, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35.
- Miravitlles M, Koblizek V, Esquinas C, et al. Determinants of CAT (COPD Assessment Test) scores in a population of patients with COPD in central and Eastern Europe: the POPE study. Respir Med. 2019;150:141–148.
- Bloom CI, Elkin SL, Quint JK. Changes in COPD inhaler prescriptions in the United Kingdom, 2000 to 2016. Int J Chron Obstruct Pulmon Dis. 2019;14:279–287.
- Brusselle G, Price D, Gruffydd-Jones K, et al. The inevitable drift to triple therapy in COPD: an analysis of prescribing pathways in the UK. Int J Chron Obstruct Pulmon Dis. 2015;10:2207–2217.
- Simeone JC, Luthra R, Kaila S, et al. Initiation of triple therapy maintenance treatment among patients with COPD in the US. Int J Chron Obstruct Pulmon Dis. 2017;12:73–83.
- Alonso JLI, Glez-Moro JMR. The excessive use of inhaled corticosteroids in chronic obstructive pulmonary disease. Arch Bronconeumol. 2012;48(6):207–212.
- Valipour A, Tamm M, Kocianova J, et al. Improvement in self-reported physical functioning with tiotropium/olodaterol in Central and Eastern European COPD patients. Int J Chron Obstruct Pulmon Dis. 2019;14:2343–2354.
- Ilić M, Kopitović I, Vulin A, et al. Frequency and effects of seasonal flu vaccines on exacerbations of chronic obstructive pulmonary disease in Serbia. Vojnosanit Pregl. 2019;78(2):179–185.
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An Official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69.
- Frei A, Siebeling L, Wolters C, et al. The inaccuracy of patient recall for COPD exacerbation rate estimation and its implications: results from central adjudication. Chest. 2016;150(4):860–868.
- Papanicolas I, Woskie LR, Jha AK. Health care spending in the United States and other high-income countries. JAMA. 2018;319(10):1024–1039.
- Rhodes A, Ferdinande P, Flaatten H, et al. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38(10):1647–1653.
- Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626.
- Ulmeanu R, Fildan AP, Rajnoveanu RM, et al. Romanian clinical guideline for diagnosis and treatment of COPD. J Int Med Res. 2020;48(8):300060520946907. doi:https://doi.org/10.1177/0300060520946907.
- Hohlfeld JM, Vogel-Claussen J, Biller H, et al. Effect of lung deflation with indacaterol plus glycopyrronium on ventricular filling in patients with hyperinflation and COPD (CLAIM): a double-blind, randomised, crossover, placebo-controlled, single-centre trial. Lancet Respir Med. 2018;6(5):368–378.
- Mittal R, Chhabra SK. GOLD classification of COPD: discordance in criteria for symptoms and exacerbation risk assessment. COPD. 2017;14(1):1–6.
- Buhl R, de La Hoz A, Xue W, et al. Efficacy of tiotropium/olodaterol compared with tiotropium as a first-line maintenance treatment in patients with COPD who are naive to LAMA, LABA and ICS: pooled analysis of four clinical trials. Adv Ther. 2020;37(10):4175–4189.
- Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554.
- Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364(12):1093–1103.
- Decramer ML, Chapman KR, Dahl R, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1(7):524–533.
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374(23):2222–2234.
- Buhl R, Criee CP, Kardos P, et al. Dual bronchodilation vs triple therapy in the “real-life” COPD DACCORD study. Int J Chron Obstruct Pulmon Dis. 2018;13:2557–2568.
- Vogelmeier C, Worth H, Buhl R, et al. “Real-life” inhaled corticosteroid withdrawal in COPD: a subgroup analysis of DACCORD. Int J Chron Obstruct Pulmon Dis. 2017;12:487–494.
- O’Byrne P, Fabbri LM, Pavord ID, et al. Asthma progression and mortality: the role of inhaled corticosteroids. Eur Respir J. 2019;54(1):1900491.
- Usmani OS. Choosing the right inhaler for your asthma or COPD patient. Ther Clin Risk Manag. 2019;15:461–472.
- Kaplan A, Price D. Matching inhaler devices with patients: the role of the primary care physician. Can Respir J. 2018;2018:9473051.
- Hoy H, O’Keefe L. Choosing the right inhaler for the right patient: considerations for effective management of patients with chronic obstructive pulmonary disease or asthma. J Am Assoc Nurse Pract. 2020;32(1):89–99.
- Lavorini F, Janson C, Braido F, et al. What to consider before prescribing inhaled medications: a pragmatic approach for evaluating the current inhaler landscape. Ther Adv Respir Dis. 2019;13:1753466619884532.
- Peché R, Attar-Zadeh D, Scullion J, et al. Matching the inhaler to the patient in COPD. J Clin Med. 2021;10(23):5683.
- Mahler DA. Peak inspiratory flow rate as a criterion for dry powder inhaler use in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(7):1103–1107.
- Mahler DA. The role of inspiratory flow in selection and use of inhaled therapy for patients with chronic obstructive pulmonary disease. Respir Med. 2020;161:105857.
- Mahler DA, Ludwig-Sengpiel A, Ferguson GT, et al. TRONARTO: a randomized, placebo-controlled study of tiotropium/olodaterol delivered via soft mist inhaler in COPD patients stratified by peak inspiratory flow. Int J Chron Obstruct Pulmon Dis. 2021;16:2455–2465.
- Hartl S, Breyer M-K, Burghuber OC, et al. Blood eosinophil count in the general population: typical values and potential confounders. Eur Respir J. 2020;55(5):1901874.
- Kolsum U, Donaldson GC, Singh R, et al. Blood and sputum eosinophils in COPD; relationship with bacterial load. Respir Res. 2017;18(1):88.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680.
- Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 2018;391(10125):1076–1084.
- Hinds DR, DiSantostefano RL, Le HV, et al. Identification of responders to inhaled corticosteroids in a chronic obstructive pulmonary disease population using cluster analysis. BMJ Open. 2016;6(6):e010099.
- Pascoe S, Barnes N, Brusselle G, et al. Blood eosinophils and treatment response with triple and dual combination therapy in chronic obstructive pulmonary disease: analysis of the IMPACT trial. Lancet Respir Med. 2019;7(9):745–756.
- Snoeck-Stroband JB, Lapperre TS, Gosman MM, et al. Chronic bronchitis sub-phenotype within COPD: inflammation in sputum and biopsies. Eur Respir J. 2008;31(1):70–77.
- Singh D, Watz H, Beeh KM, et al. COPD sputum eosinophils: relationship to blood eosinophils and the effect of inhaled PDE4 inhibition. Eur Respir J. 2020;56(2):2000237.
- Uzun S, Djamin RS, Kluytmans JA, et al. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (Columbus): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2(5):361–368.
- Cui Y, Luo L, Li C, et al. Long-term macrolide treatment for the prevention of acute exacerbations in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:3813.
- O’Dwyer DN, Dickson RP, Moore BB. The lung microbiome, immunity, and the pathogenesis of chronic lung disease. J Immunol. 2016;196(12):4839–4847.
- Vetrano DL, Zucchelli A, Bianchini E, et al. Triple inhaled therapy in COPD patients: determinants of prescription in primary care. Respir.Med. 2019;154:12–17.
- Axson EL, Ragutheeswaran K, Sundaram V, et al. Hospitalisation and mortality in patients with comorbid COPD and heart failure: a systematic review and meta-analysis. Respir.Res. 2020;21(1):54.
- Pirina P, Martinetti M, Spada C, et al. Prevalence and management of COPD and heart failure comorbidity in the general practitioner setting. Respir Med. 2017;131:1–5.
- Jarad NA, Wedzicha JA, Burge PS, et al. An observational study of inhaled corticosteroid withdrawal in stable chronic obstructive pulmonary disease. ISOLDE study group. Respir Med. 1999;93(3):161–166.
- Rossi A, van der Molen T, Del Olmo R, et al. INSTEAD: a randomised switch trial of indacaterol versus salmeterol/fluticasone in moderate COPD. Eur Respir J. 2014;44(6):1548–1556.
- Rossi A, Guerriero M, Corrado A. Withdrawal of inhaled corticosteroids can be safe in COPD patients at low risk of exacerbation: a real-life study on the appropriateness of treatment in moderate COPD patients (OPTIMO). Respir Res. 2014;15(1):77.
- Calverley PMA, Tetzlaff K, Vogelmeier C, et al. Eosinophilia, frequent exacerbations, and steroid response in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(9):1219–1221.
- Matera MG, Cardaci V, Cazzola M, et al. Safety of inhaled corticosteroids for treating chronic obstructive pulmonary disease. Expert Opin Drug Saf. 2015;14(4):533–541.
- Ye Q, He X-O, D’Urzo A. A review on the safety and efficacy of inhaled corticosteroids in the management of asthma. Pulm Ther. 2017;3(1):1–18.
- World Health Organization. Tuberculosis in the WHO European region. 2019. Available at: https://www.euro.who.int/__data/assets/pdf_file/0008/397484/Factsheet_WHO_WTBD_2019.pdf (Last accessed 2021 Apr 1).
- Castellana G, Castellana M, Castellana C, et al. Inhaled corticosteroids and risk of tuberculosis in patients with obstructive lung diseases: a systematic review and meta-analysis of non-randomized studies. Int J Chron Obstruct Pulmon Dis. 2019;14:2219–2227.
- Andréjak C, Nielsen R, Vø T, et al. Chronic respiratory disease, inhaled corticosteroids and risk of non-tuberculous mycobacteriosis. Thorax. 2013;68(3):256–262.
- Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration. 2013;86(1):76–85.
- Park HJ, Byun MK, Kim HJ, et al. History of pulmonary tuberculosis affects the severity and clinical outcomes of COPD. Respirology. 2018;23(1):100–106.
- Yakar HI, Gunen H, Pehlivan E, et al. The role of tuberculosis in COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:323.
- Jin J, Li S, Yu W, et al. Emphysema and bronchiectasis in COPD patients with previous pulmonary tuberculosis: computed tomography features and clinical implications. Int J Chron Obstruct Pulmon Dis. 2018;13:375.
- Lee C-M, Heo J, Han -S-S, et al. Inhaled corticosteroid-related tuberculosis in the real world among patients with asthma and COPD: a 10-year nationwide population-based study. J Allergy Clin Immunol. 2019;7(4):1197–1206. e1193.
- Kim J-H, Park J-S, Kim K-H, et al. Inhaled corticosteroid is associated with an increased risk of TB in patients with COPD. Chest. 2013;143(4):1018–1024.
- Shu -C-C, Wu H-D, Yu M-C, et al. Use of high-dose inhaled corticosteroids is associated with pulmonary tuberculosis in patients with chronic obstructive pulmonary disease. Medicine (Baltimore). 2010;89(1):53–61.
- Huang T-M, Kuo K-C, Wang Y-H, et al. Risk of active tuberculosis among COPD patients treated with fixed combinations of long-acting beta2 agonists and inhaled corticosteroids. BMC Infect Dis. 2020;20(1):1–8.
- Kim C-J, Yoon H-K, Park M-J, et al. Inhaled indacaterol for the treatment of COPD patients with destroyed lung by tuberculosis and moderate-to-severe airflow limitation: results from the randomized INFINITY study. Int J Chron Obstruct Pulmon Dis. 2017;12:1589.
- Yum H-K, Park I-N. Effect of inhaled tiotropium on spirometric parameters in patients with tuberculous destroyed lung. Tuberc Respir Dis. 2014;77:167–171.
- Avdeev S, Aisanov Z, Arkhipov V, et al. Withdrawal of inhaled corticosteroids in COPD patients: rationale and algorithms. Int J Chron Obstruct Pulmon Dis. 2019;14:1267–1280.
- Magnussen H, Disse B, Rodriguez-Roisin R, et al. Withdrawal of inhaled glucocorticoids and exacerbations of COPD. N Engl J Med. 2014;371(14):1285–1294.
- Perez X, Wisnivesky JP, Lurslurchachai L, et al. Barriers to adherence to COPD guidelines among primary care providers. Respir.Med. 2012;106(3):374–381.
- Chavez PC, Shokar NK. Diagnosis and management of chronic obstructive pulmonary disease (COPD) in a primary care clinic. COPD. 2009;6(6):446–451.
- Bourbeau J, Sebaldt RJ, Day A, et al. Practice patterns in the management of chronic obstructive pulmonary disease in primary practice: the CAGE study. Can Respir J. 2008;15(1):13–19.
- Halpin DM, Kerkhof M, Soriano JB, et al. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir.Res. 2016;17(1):120.
- Mullerova H, Lu C, Li H, et al. Prevalence and burden of breathlessness in patients with chronic obstructive pulmonary disease managed in primary care. PLoS One. 2014;9(1):e85540.
- Park J, Hobbs BD, Crapo JD, et al. Subtyping COPD by using visual and quantitative CT imaging features. Chest. 2020;157(1):47–60.
- Bodduluri S, Reinhardt JM, Hoffman EA, et al. Recent advances in computed tomography imaging in chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2018;15(3):281–289.
- Poggi C, Mantovani S, Pecoraro Y, et al. Bronchoscopic treatment of emphysema: an update. J Thorac Dis. 2018;10(11):6274.
- Valipour A, Fernandez-Bussy S, Ing AJ, et al. Bronchial rheoplasty for treatment of chronic bronchitis. Twelve-month results from a multicenter clinical trial. Am J Respir Crit Care Med. 2020;202(5):681–689.
- Slebos D-J, Shah PL, Herth FJ, et al. Safety and adverse events after targeted lung denervation for symptomatic moderate to severe chronic obstructive pulmonary disease (AIRFLOW). A multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(12):1477–1486.