KEYWORDS:
1. Introduction
Shortly after the first few breaths, the human neonatal airways must decode the environment for the first time. In the absence of a fully developed immune system, the innate recognition and elimination of pathogens at the respiratory epithelial barrier becomes a matter of life or death. In fact, a recent analysis of all-cause and cause-specific mortality from 204 countries showed that severe respiratory infections are the leading cause of death in newborns and infants, as it has been for many decades [Citation1]. In addition, infants who survive severe respiratory infections have a much higher risk of developing lifelong respiratory morbidity, such as asthma and chronic obstructive pulmonary disease [Citation2,Citation3]. Although respiratory diseases constitute a major burden on the health of newborns and infants, the mechanisms regulating airway immune responses in early life are still largely unknown. Improving our understanding of human neonatal and infant airway immunology is essential to develop novel strategies that improve respiratory health during and beyond early childhood.
Figure 1. Humans are born with an immunologically active airway epithelial barrier. In response to early exposures, newborn and infant airway epithelial cells produce high levels of antiviral (IFN type I/III) and immunomodulatory molecules (eg, TSLP). The interplay between airway epithelial and immune cell responses during infancy shapes the maturation of airway structure and adaptive immune function. This is critical for life-long risk vs. resilience to respiratory diseases.
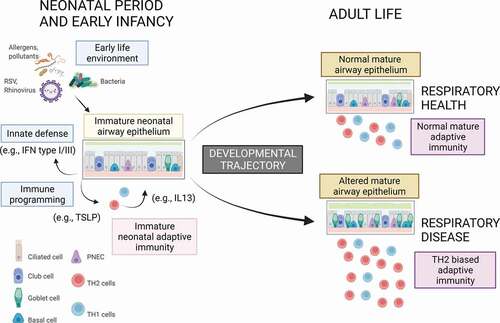
The immunology of the human neonatal airways is unique and complex. Multiple bacteria, viruses, pollutants, and allergens must be rapidly identified for the first time by naive airway epithelial cells (). These cells must orchestrate precise inflammatory and host defense responses to eliminate pathogens at the respiratory barrier. Simultaneously, innate respiratory immunity and adaptive long-term memory must be generated in response to these initial contacts. These processes need to be synchronized with stereotypical genetic and epigenetic developmental immunological programs largely established during the first months of life [Citation4]. Thus, neonatal and infant airway exposures have a dramatic and unique impact on the developmental trajectories of the respiratory tract and the immune system and will largely define individual long-term resilience or risk of respiratory diseases beyond childhood [Citation2,Citation3].
The clinical relevance of human neonatal and infant airway biology has been increasingly appreciated. New paradigms such as innate immunity training, which refers to the environmental programming of immune responses of the epithelium and innate immune cells [Citation5–8], have led to promising results in clinical trials based on specific early-life exposures to modulate airway innate responses (e.g. bacterial products) [Citation9]. However, it is necessary to enhance our mechanistic understanding of the airway epithelium, which is much more complex than was initially thought. The airway epithelium is a pseudostratified multicellular layer that develops, matures, and regenerates to adapt to the environment during health and disease [Citation10]. It is not a mere physical barrier but a complex tissue that actively initiates and regulates immune responses against viruses and other pathogens. New advances in technology (e.g. single-cell studies) have recently re-defined airway epithelial cell subtypes and functions [Citation10]. The contemporary view of the airway epithelium is that it is a heterogenous multicellular layer with dynamic interactions between basal progenitor, ciliated, secretory (club), and goblet cells as well as rare cell types such as tuft (brush) cells, ionocytes, and neuroendocrine (NE) cells [Citation10].
The heterogeneous composition of the epithelium may allow plasticity during development and explain age-related differences in structure and function. A recent study by Loske, et al. [Citation11] identified striking differences between children and adults in the airway epithelium and adjacent mucosa. Relative to adults, the nasal airway of children contained high amounts of almost each immune cell subset with an overall dominance of neutrophils [Citation11]. The airway epithelial cell structure also showed maturational differences with goblet cells decreasing and ciliated cells increasing with age. At the molecular level, pediatric airway immune cells appeared to be primed for virus sensing (e.g. higher basal expression of pattern recognition receptors such as MDA5 and RIG-I) resulting in a stronger early innate antiviral response to SARS-CoV-2 infection than in adults [Citation11]. These findings may explain, at least in part, why SARS-CoV-2 is associated with increased severity in older adults compared to children [Citation11]. How these maturational differences in the airway epithelium relate to other neonatal and infant airway diseases (e.g. viral bronchiolitis or childhood asthma) should be the focus of additional human-based studies in the future.
Early-life injuries caused by respiratory viruses during infancy (e.g. RSV and rhinovirus) are associated with a higher risk for recurrent wheezing illnesses and asthma [Citation2,Citation3]. Most studies have focused on the immunological mechanisms implicated in this association including the initiation of TH2 allergic airway inflammation. However, it is still unclear how the structure, function, and maturation of the airway epithelium are disrupted by early injuries. Importantly, a seminal study recently published by Shivaraju et al. [Citation12] demonstrates that mouse and human airway basal stem cells can differentiate into NE cells as a protective response of the epithelium against hypoxia [Citation12]. The lack of NE cells during hypoxia led to increased epithelial injury, whereas the administration of the NE cell peptide CGRP rescued this excess damage. This provides solid evidence that airway epithelial stem cells can sense hypoxia and respond to it by differentiating into NE cells that secrete a protective peptide that mitigates hypoxic injury. The findings also suggest that the NE cell hyperplasia (NEHI) seen in young children may represent, to some extent, a compensatory physiologic response and a marker of prior injuries during early development [Citation13].
The study by Shivaraju et al also opens the possibility that there are other unidentified compensatory responses (beneficial or harmful) in the airway epithelium during early development. In this regard, recent evidence suggests that the epithelial responses against viruses are influenced by the developmental stage. Our team [Citation14] recently reported that human infant airway epithelial cells respond to a virus mimic (double-stranded RNA) with robust production of thymic stromal lymphopoietin (TSLP), a cytokine linked to the generation of type 2 allergic inflammation and asthma pathogenesis in older individuals. In-vivo, we found that young children with the highest virus-induced airway TSLP production had an increased risk of recurrent viral respiratory illnesses during early childhood [Citation14]. Interestingly, the airway epithelial cells of human infants are not simply ‘polarized’ toward a type 2 allergic state. In another recent study [Citation15], we found that, compared to older children, human infants (<18 months) exhibited increased production of type III IFN-lambda, an antiviral molecule produced by the epithelium to protect against many respiratory pathogens including RSV, influenza, and SARS-CoV-2. The findings are in overall agreement with the recent notion presented by Loske, et al. [Citation11] that young children have pre-activated antiviral innate immunity in the upper airways relative to adults. Collectively, these human-based studies demonstrate the importance of the physiological responses of the airway epithelium against viral infections during early post-natal development and maturation. However, a better understanding of the structural and functional changes of the human infant airway epithelium overtime is still needed to define molecular mechanisms of disease for many respiratory disorders triggered by viruses during infancy and early childhood.
The model of early injuries and viruses altering the developmental program of the human airway epithelial barrier is likely applicable to other pathogens and chronic pediatric respiratory conditions. For instance, adenovirus is strongly associated with post-infectious bronchiolitis obliterans (PIBO), a disorder where viral infection leads to severe structural airway damage. While discussion on PIBO has mostly focused on immunological mechanisms by which adenovirus can damage the airways, novel studies demonstrating the complex plasticity and adaptability of the airway epithelium [Citation10], indicate that it is also critical to investigate how this virus (and other pathogens causing PIBO) can alter the structure and function of the pediatric airway epithelium during early post-natal development and maturation. As severe viral respiratory infections during the first years of life are also linked to prevalent conditions like asthma, future studies aiming to understand how early human airway epithelial maturation is disrupted by viral infections during infancy can provide completely novel insights into the pathogenesis of a myriad of respiratory disorders that begin in early life and may persist into adulthood.
In summary, we need to better understand how the plasticity and the adaptability of the human airway epithelium to early-life injuries may play a key role in determining the recovery or the development of sequelae after severe viral respiratory infections. Airway epithelial differences by age are present from the beginning of life as the lung and airways are not mature at birth and undergo further differentiation (septation, alveolarization, epithelialization) during postnatal development. Cellular heterogeneity has been mostly defined in proximal conducting airways (ciliated, goblet cells etc.) but the current multicellular view of the epithelium is also pertinent to distal small airways and the alveoli. In view of the complex multicellular program of the human respiratory epithelium, it is worth asking: how does this multicellular epithelial structure change during development in early human life?, is it stable or rather highly sensitive to the environment?, what role do early insults such as hypoxia or specific exposures to microbiota and viral infections play in the early airway epithelial and immunological development?. Since the answers to these questions are not yet available, we need to develop new pediatric-centered research efforts to elucidate the early developmental program of the human airway epithelium and its environmental modifiers during the first years of life. This is essential to fully understand the early origins of respiratory diseases in all age groups.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- GBD. 2019 Under-5 mortality collaborators. global, regional, and national progress towards sustainable development goal 3.2 for neonatal and child health: all-cause and cause-specific mortality findings from the global burden of disease study 2019. Lancet. 2021Sep4;398(10303):870–905. Epub 2021 Aug 17. PMID: 34416195; PMCID: PMC8429803.
- Martinez FD. Early-Life Origins of Chronic Obstructive Pulmonary Disease. N Engl J Med. 2016Sep1;375(9):871–878. PMID: 27579637. ••Comprehensive review of early life determinants of Chronic obstructive Pulmonary Disease (COPD)
- Tagiyeva N, Devereux G, Fielding S, et al. Outcomes of childhood asthma and wheezy bronchitis. A 50-year cohort study. Am J Respir Crit Care Med. 2016Jan1;193(1):23–30. PMID: 26351837; PMCID: PMC4731615.
- Olin A, Henckel E, Chen Y, et al. Stereotypic immune system development in newborn children. Cell. 2018;174(5):1277–92.e1214.••Landmark paper demonstranting convergent trajectories in immune development between premature and term infants during teh first montsh of life.
- Gutierrez MJ, Nino G, Hong X, et al. Epigenomics and early life human humoral immunity: novel paradigms and research opportunities. Front Immunol. 2020;11:1766.
- Holt P, Strickland D. Innate Immune training for prevention of recurrent wheeze in early childhood. Am J Respir Crit Care Med. 2021;204(4):392–394.
- Lynch SV, Vercelli D. Microbiota, epigenetics, and trained immunity. convergent drivers and mediators of the asthma trajectory from pregnancy to childhood. Am J Respir Crit Care Med. 2021;203:802–808.
- Nino G, Rodriguez-Martinez CE, Gutierrez MJ. Early microbial-immune interactions and innate immune training of the respiratory system during health and disease. Children (Basel). 2021May19;8(5):413. PMID: 34069319; PMCID: PMC8158711.
- Nieto A, Mazón A, Nieto M, et al. Bacterial mucosal immunotherapy with MV130 prevents recurrent wheezing in children: a randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med. 2021;204(4):462–472.
- Hewitt RJ, Lloyd CM. Regulation of immune responses by the airway epithelial cell landscape. Nat Rev Immunol. 2021Jan;13:1–16. Epub ahead of print. PMID: 33442032; PMCID: PMC7804588.••Review of recent changes in our understanding of airway epithelial biology with the advent of transcriptomic approaches.
- Loske J, Röhmel J, Lukassen S, et al. Pre-activated antiviral innate immunity in the upper airways controls early SARS-CoV-2 infection in children. Nat Biotechnol. 2021. https://doi.org/10.1038/s41587-021-01037-9.
- Shivaraju M, Chitta UK, Grange RMH, et al. Airway stem cells sense hypoxia and differentiate into protective solitary neuroendocrine cells. Science. 2021Jan1;371(6524):52–57. PMID: 33384370.
- Wang B, Cardenas M, Bedoya M, et al. Upregulation of neuropeptides and obstructive airway disorder in infancy: a review with focus on post-RSV wheezing and NEHI. Pediatr Pulmonol. 2021Jun;56(6):1297–1306. Epub 2021 Feb 1. PMID: 33524244.
- Salka K, Arroyo M, Naime S, et al. TSLP production in the human infant airway epithelium and clinical relevance during viral respiratory infections. Am J Respir Cell Mol Biol. 2020Jan;62(1):115–117. PMID: 31891308; PMCID: PMC6938137.••Study demonstrating that TSLP is the predominant innate type 2 cytokine produced by the human infant airway epithelium in response to a viral stimulus (poly I:C) or IL-1b.
- Salka K, Arroyo M, Chorvinsky E, et al. Innate IFN-lambda responses to dsRNA in the human infant airway epithelium and clinical regulatory factors during viral respiratory infections in early life. Clin Exp Allergy. 2020Sep;50(9):1044–1054. Epub 2020 Jul 26. PMID: 32623773; PMCID: PMC7484417.••Study showing that poly(I:C)-induced production of IFN-lambda in human infant airway epithelial cells is regulated by a p38-MAPK/NF-kB dependent mechanism and exposure to pro-inflammatory sugnals such as IL-1b
