1. Introduction
In his 1959 Nobel Lecture, Joshua Lederberg discusses the specific mutagen, an agent that could ‘penetrate to a given gene, recognize and modify it in a specific way’, as the ignis fatuus of genetics, a potentially unobtainable goal that one feels compelled to pursue. While several contenders emerged over the years, it was not until 2012 that Charpentier and Doudna truly realized Lederberg’s goal with their description of an easily programmable RNA-guided DNA endonuclease known as CRISPR Cas9 [Citation1], which when used in combination with a DNA donor template, opened the path to a cure for genetic diseases by precise genome editing [Citation2]. In the final months of the first decade of CRISPR, this editorial reviews the impact of CRISPR technology on respiratory medicine.
2. CRISPR in the clinic
With all new technologies, the first question is how long until it reaches the clinic? Well, in the case of CRISPR, we are already there based on clinical trial data recently published for Transthyretin Amyloidosis (TTA), a disease predominantly affecting the heart and nerves, with respiratory symptoms, such as shortness of breath. Given TTA is caused by a buildup of the misfolded TTR protein, the simplest CRISPR editing strategy, use of a single guideRNA (gRNA) to direct Cas9 to cut and disrupt the TTR gene, was used to see if this would lower the levels of circulating TTR protein [Citation3]. The gRNA and Cas9-encoding mRNA were enveloped in lipid nanoparticles (LNPs) coated with recombinant ApoE protein to target the liver, and delivered intravenously. Data from three patients at 28 days post-infusion showed >80% reduction in serum TTR levels, and initial safety data showed no adverse effects, though the authors acknowledge that participants who volunteer to receive the therapy will need to undergo long-term safety monitoring.
3. CRISPR off-target effects
One routine question about CRISPR is the potential for, and consequences of, off-target effects (OTEs); what happens if the gRNA targets the Cas9 to a target site with a similar but non-identical sequence creating an unwanted double-stranded break (DSB) at an undesirable location(s) in the genome? A huge amount of research has been performed to minimize the incidence of such OTEs, and as shown in the clinical study described above, none were detected, subject to the limits of the techniques used and tissues sampled. But what are the potential consequences of an OTE? The most recently described possibility is that some Cas9/gRNAs can cause low levels of chromothripsis (literally, chromosome shredding), and the formation of micronuclei [Citation4], reinforcing the importance of screening gRNAs to avoid potential OTEs. Reassuringly, the study found that non-dividing cells are unlikely to produce micronuclei, so other ongoing clinical trials using Cas9/gRNAs to target the retinal disorder LCA-10 (clinical trial NCT03872479) are unlikely to be affected. The authors also suggested that where ex vivo editing of dividing cells is being considered, then maintaining them in a quiescent state during editing could reduce the incidence of chromothripsis and micronuclei formation. But perhaps their final suggestion is the most pertinent; should we focus on developing editing strategies that do NOT generate DSBs!
4. CRISPR without cutting option 1 – base editing
The first major CRISPR option developed to edit without DSBs was base editing, one of the several technologies pioneered by David Liu’s lab [Citation5]. Already used in a number of preclinical studies, two recent examples of base editing have emerged for lung disease. The first tackles STAT3-hyper IgE syndrome, a dominant negative primary immunodeficiency that results in high serum IgE and lung infections [Citation6]. The dominant negative status of this disease means that cDNA-based gene addition therapy is not suitable, but two of the most common disease-causing mutations could potentially be reverted by adenine base editing (see ). In this study, they showed how one of them, c.1145G>A/p.R382Q, could be efficiently corrected for primary fibroblasts. The next step would be to evaluate in vivo with an appropriate animal model, or possibly to test it in human organoid systems derived from patient iPS cells.
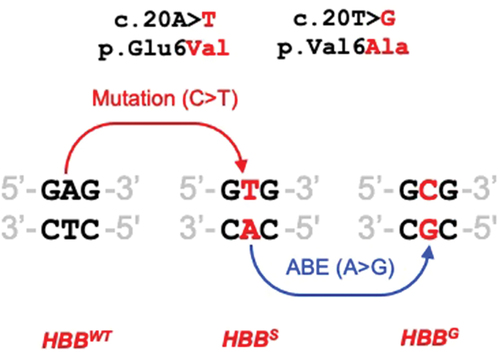
Box 1. Adenine base editing (ABE) can be targeted to either strand [Citation7]. Left – C > T mutation on top strand causes disease, ABE of A to G on bottom strand can potentially convert to WT. Right – G > A mutation on top strand causes disease, ABE of A to G on top strand can potentially convert to WT.
![Box 1. Adenine base editing (ABE) can be targeted to either strand [Citation7]. Left – C > T mutation on top strand causes disease, ABE of A to G on bottom strand can potentially convert to WT. Right – G > A mutation on top strand causes disease, ABE of A to G on top strand can potentially convert to WT.](/cms/asset/df149f7c-cce4-4500-888a-fb82f903180f/ierx_a_2056021_uf0001_oc.jpg)
In a second study, the progress with base editing is more advanced. A large proportion of people with Sickle Cell Disease (SCD) suffer significant respiratory disease. SCD is caused by the c.20A>T/p.Glu6Val mutation in both alleles of the HBB gene (HBBS/S). As shown in , it is not possible (at present) to fully correct this mutation by base editing, but adenine base editing could convert the A on the lower strand to G, which modifies codon 6 to GCG which encodes Alanine (rather than the wild-type Glutamate). Alanine at codon 6 results in the formation of HBBG which is a nonpathogenic variant known as Makassar. In a recent study [Citation7], human CD34+ hemopoietic stem progenitor cells (HSPCs) from a HBBS/S donor were edited with mRNA encoding an adenine base editor and appropriate gRNA. Twenty-four hours later, the cells were transplanted into NBSGW mice that support engraftment of human HPSCs. Four months later, almost 60% of the β-like globin was βS, and red cells derived from edited HPSC showed a five-fold reduction in sickling compared to unedited controls. Given the promise shown in a clinical trial of an ex vivo CRISPR gene editing strategy designed to up-regulate fetal hemoglobin and gamma globulin, which resulted in the first recipient being transfusion-independent and free of vaso-occlusive episodes one year later [Citation8], a similar ex vivo route could potentially be used to evaluate this CRISPR base editing strategy.
5. CRISPR without cutting option 2 – epigenetic editing
A second way to avoid double-stranded breaks and potentially treat disease is epigenetic editing. This involves the use of CRISPR to target transcriptional regulators in precise regions of the genome in order to modify gene expression. An interrogation of 18 potential regulatory regions of the CFTR gene with 133 different gRNAs and the dCas9p300 transcriptional activator identified a single guide, gRNA40, which gave a moderate increase in the levels of CFTR mRNA in wild-type cells [Citation9]. When tested on cells homozygous for the most common CF-causing mutation, F508del, the dCas9p300/gRNA40 resulted in a much larger increase in F508del CFTR mRNA. Whilst this increase in mRNA alone had no significant increase in the F508del CFTR ion channel activity, a synergistic interaction was observed with the CF modulator drug, VX809, almost doubling the short circuit current activity of the F508del CFTR ion channel relative to VX809 treatment alone. It will be interesting to see if dCas9p300/gRNA40 can upregulate mRNA expression of the other CF variants, particularly stop codon (PTC) variants where the CFTR mRNA is destabilized, and see if this leads to synergistic interactions with PTC read-through drugs in development. However, a potenital challenge in developing this approach for therapeutic application is that either long-term expression, or repeat dosing, of the dCas9p300 and gRNA would be required.
6. CRISPR and lung cancer
The classic, but relatively simple, KPGEMM mouse model of non-small-cell lung cancer (NSCLC) is made by crossing two mouse strains to generate a conditional KRas activating mutation and trigger Trp53 deletion. Using this system as the basis to model more complex cancer profiles is time consuming and requires lots of animals. A recent study [Citation10] describes two applications of CRISPR to develop models that more closely depict the genetic profile of human disease, and Reduce, Refine or Replace (3Rs) the number of animals required. First, they showed that KPGEMM mice created using CRISPR were indistinguishable from the classic KPGEMM animals. Then, they used CRISPR to install a variety of additional specific mutations onto this background, and observed different survival profiles depending on the mutations added. Their second strategy involved the use of adeno-associated virus (AAV) vectors carrying Cas9, gRNAs and editing templates to introduce variants into mice lungs on a variety of different backgrounds in 12 weeks, experiments which would take 12 months by conventional methods. It should be possible to use the same AAV vectors to install the same edits in human organoids and potentially replace some in vivo models.
In a large animal model to study lung cancer, CRISPR mini-pigs have been developed such that Cas9 is only expressed when cells are transduced with a specific AAV vector, which also encodes multiple gRNAs/donor templates to simultaneously target specific genes of interest [Citation11]. In this model, modest increases in cell proliferation rates were observed when STK1/TP53 were knocked out and the KRAS G12D mutation installed. However, a much larger increase in proliferation rates was observed when PTEN rather than STK1 was targeted. Similar to the mouse model, the fact that editing of multiple targets can be achieved with a single AAV vector allows complex gene profiles to be assessed rapidly and simply, and with far fewer animals than was previously possible.
7. CRISPR and Covid
CRISPR Cas9 can not be used to target SARS-CoV2 directly as Cas9 is a DNA nuclease, and the virus has an RNA genome. However, Cas9 has been used to identify cellular factors that this obligate intracellular parasite relies upon for its replication. Two recent studies [Citation12,Citation13] described the use of Cas9 gRNA libraries to knock out cellular genes in human cell lines, and then select for virus-resistant cells. Using this unbiased screening approach, both groups pulled out known cellular genes important for SARS-CoV2 replication such as ACE2 and CTSL, plus a common pool of other genes that constitute potential targets for antiviral therapy.
To target SARS-CoV2 directly, a panel of gRNAs targeting five conserved regions of its RNA genome were screened with the RNA-directed RNA endonuclease, Cas13a [Citation14]. Using the most effective gRNA identified from an in vitro screen, Cas13a treatment resulted in a 57% reduction in SARS-CoV-2 copy number in a hamster model of virus infection, and a statistically significant prevention of body weight reduction, providing proof-of-concept that this CRISPR strategy could alter SARS-CoV-2 pathogenesis.
8. CRISPR delivery
Clinical evaluation of CRISPR will require robust delivery systems, but there are a number of options, which have been successfully evaluated in vivo for editing systems, including nanoparticles capable of targeting mRNA to specific organs [Citation15] or DNA-loaded, mucus-penetrating particles [Citation16]. In parallel, both a number of Adeno-associated virus [Citation17] and helper-dependent Adenovirus [Citation18] vectors have also been shown to be effective at editing the lungs of animal models. Engineered virus-like particles that can be used for in vivo editing may also offer additional therapeutic options [Citation19].
9. Expert Opinion
CRISPR editing offers numerous options for the future of respiratory medicine for both inherited and acquired lung disease. The first challenge will be to sift through the growing number of CRISPR tools and delivery methods that are emerging in order to identify the strategies most amenable to clinical development, no small task considering a new CRISPR publication appears in PubMed every 60mins. As we look further ahead, the remit stretches beyond simply the science and clinical efforts to develop treatments for respiratory disease, possibly through international consortia, but also requires that these developments are translated into ethical, affordable, and accessible treatments for all.
References
- Jinek M, Chylinski K, Fonfara I, et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 2012;337(6096):816–821. Available from: https://pubmed.ncbi.nlm.nih.gov/22745249/.
- Urnov FD. Genome editing B.C. (Before CRISPR): lasting lessons from the “old testament”. CRISPR J. 2018;1(1):34–46. Available from: https://pubmed.ncbi.nlm.nih.gov/31021186/
- Gillmore JD, Gane E, Taubel J, et al. CRISPR-Cas9 in vivo gene editing for transthyretin amyloidosis. N Engl J Med. 2021;385(6):493–502. Available from: https://pubmed.ncbi.nlm.nih.gov/34215024/
- Leibowitz ML, Papathanasiou S, Doerfler PA, et al. Chromothripsis as an on-target consequence of CRISPR-Cas9 genome editing. Nat Genet. 2021;53(6):895–905. Available from: https://pubmed.ncbi.nlm.nih.gov/33846636/.
- Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38(7):824–844. Available from: https://pubmed.ncbi.nlm.nih.gov/32572269/.
- Eberherr AC, Maaske A, Wolf C, et al. Rescue of STAT3 function in hyper-IgE syndrome using adenine base editing. CRISPR J. 2021;4(2):178–190. Available from: https://pubmed.ncbi.nlm.nih.gov/33876960/.
- Newby GA, Yen JS, Woodard KJ, et al. Base editing of haematopoietic stem cells rescues sickle cell disease in mice. Nature. 2021;595(7866):295–302. Available from: https://pubmed.ncbi.nlm.nih.gov/34079130/.
- Frangoul H, Altshuler D, Domenica Cappellini M, et al. CRISPR-Cas9 Gene editing for sickle cell disease and β-thalassemia. New Engl J Med. 2021 Jan 21;384(3):252–260. Available from: https://pubmed.ncbi.nlm.nih.gov/33283989/.
- Kabadi AM, Machlin L, Dalal N, et al. Epigenome editing of the CFTR-locus for treatment of cystic fibrosis. J Cyst Fibros. 2021;S1569-1993(21): 00118–1. Available from: https://pubmed.ncbi.nlm.nih.gov/34049825/
- Hartmann O, Reissland M, Maier CR, et al. Implementation of CRISPR/Cas9 genome editing to generate murine lung cancer models that depict the mutational landscape of human disease. Front Cell Dev Biol. 2021;9:641618. Available from: https://pubmed.ncbi.nlm.nih.gov/33738287/
- Berthelsen MF, Riedel M, Cai H, et al. The CRISPR/Cas9 minipig-A transgenic minipig to produce specific mutations in designated tissues. Cancers (Basel). 2021;13(12):3024. Available from: https://pubmed.ncbi.nlm.nih.gov/34208747/.
- Biering SB, Sarnik SA, Wang E, et al. Genome-wide, bidirectional CRISPR screens identify mucins as critical host factors modulating SARS-CoV-2 infection. BioRχiv. 2021:04.22.440848. Available from: https://www.biorxiv.org/content/10.1101/2021.04.22.440848v1
- Rebendenne A, Roy P, Bonaventure B, et al. Bidirectional genome-wide CRISPR screens reveal host factors regulating SARS-CoV-2, MERS-CoV and seasonal coronaviruses. bioRχiv. 2021:2021.05.19.444823. Available from: https://pubmed.ncbi.nlm.nih.gov/34031654/
- Blanchard EL, Vanover D, Bawage SS, et al. Treatment of influenza and SARS-CoV-2 infections via mRNA-encoded Cas13a in rodents. Biotechnol. 2021;39(6):717–726. Available from: https://pubmed.ncbi.nlm.nih.gov/33536629/
- Dilliard SA, Cheng Q, Siegwart DJ. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc Natl Acad Sci U S A. 2021 Dec 28;118(52):e2109256118. Available from: https://pubmed.ncbi.nlm.nih.gov/34933999
- Kim N, Kwak G, Rodriguez J, et al. Inhaled gene therapy of preclinical muco-obstructive lung diseases by nanoparticles capable of breaching the airway mucus barrier. Thorax. 2021 Oct 25:thoraxjnl-2020-215185. Available from: https://pubmed.ncbi.nlm.nih.gov/34697091/.
- Liang SQ, Walkey CJ, Martinez AE, et al. AAV5 delivery of CRISPR-Cas9 supports effective genome editing in mouse lung airway. Mol Ther. 2022 Jan 5;30(1):238–243. Available from: https://pubmed.ncbi.nlm.nih.gov/34695545/.
- Bandara RA, Chen ZR, Hu J. Potential of helper-dependent Adenoviral vectors in CRISPR-cas9-mediated lung gene therapy. Cell Biosci. 2021 Jul 23;11(1):145. Available from: https://pubmed.ncbi.nlm.nih.gov/34301308/
- Banskota S, Raguram A, Suh, et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022 Jan 20;185(2):250–265. Available from: https://pubmed.ncbi.nlm.nih.gov/35021064/.