ABSTRACT
Introduction
Discordance between real-world prescribing patterns and global treatment guidelines for the treatment of chronic obstructive pulmonary disease (COPD) with inhaled single or dual long-acting bronchodilator maintenance therapy is increasingly being reported in the literature, particularly with regard to addition of inhaled corticosteroids (ICS). Patient-related factors, e.g. inhalation technique and inspiratory flow, are key to disease control in COPD. Treatment discordance and patient-related factors can lead to high-cost side effects and sub-optimal treatment benefit; furthermore, the COVID-19 pandemic has led to new challenges in COPD management.
Areas Covered
This article summarizes a series of presentations sponsored by Boehringer Ingelheim and delivered at the annual CHEST congress 2021 (October 17–20, 2021) that explored new insights into the optimal management of COPD.
Expert Opinion/Commentary
There is a concerning high degree of discordance with GOLD recommendations. Dual therapy without addition of ICS does not increase exacerbation risk and could reduce pneumonia risk, and unnecessary prescription of triple therapy has financial implications. Clinic-based spirometry may not reflect the home setting, and training is required; inhalers that operate independently of users’ inhalation profiles should be considered. Integration of digital healthcare solutions into clinical studies is suggested in the post-COVID setting, although further evaluation is required.
1. Introduction
Chronic obstructive pulmonary disease (COPD) is the third-leading cause of death worldwide, with an estimated 300 million cases recorded globally in 2017 [Citation1]. The disease burden is expected to increase in the coming years due to the aging population, and continued exposure to COPD risk factors [Citation2], which include tobacco smoke, occupational dusts and chemicals, biomass fuel and air pollution [Citation3]. COPD is a progressive condition characterized by airflow obstruction, dyspnea (breathlessness) and respiratory failure. Exacerbations (acute flare-ups) are a hallmark of COPD, and frequent exacerbations are associated with a deterioration in lung function, worsening quality of life, increased frequency of hospitalizations and higher healthcare costs [Citation4]. Indeed, up to 70% of COPD-related healthcare expenditures are attributable to COPD exacerbations [Citation5].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) provides evidence-based treatment recommendations for COPD based on symptoms and history of exacerbations [Citation6]. The mainstay of COPD treatment is inhaled long-acting maintenance therapy, delivered via single or dual bronchodilator therapy with long-acting muscarinic antagonists (LAMAs) and/or long-acting β2-agonists (LABAs), either alone or in combination with inhaled corticosteroids (ICS) [Citation6,Citation7]. Guidelines from the American Thoracic Society (ATS) recommend that for patients with symptomatic COPD, dual bronchodilation is the most appropriate choice for initiating maintenance therapy [Citation8]. However, there is increasing evidence to suggest discordance between GOLD and ATS recommendations and real-world prescribing patterns, revealing both under- and over-prescription of COPD maintenance therapies, which are associated with increased risks of exacerbations, pneumonia, and escalating health service costs [Citation7,Citation9,Citation10].
One aspect of the COPD treatment paradigm that has generated much debate is appropriate use of ICS. There is increasing focus within the guidelines on the use of dual bronchodilation (LAMA/LABA) versus LAMA monotherapy as first-line maintenance therapy for patients presenting with dyspnea or exercise intolerance [Citation11], with ICS recommended for patients with a history of or concomitant asthma, or those with a history of exacerbations and/or an eosinophilic COPD phenotype [Citation6,Citation11]. Some randomized clinical trials support the benefits of adding ICS to LAMA/LABA in terms of improving lung function and reducing the risk of exacerbations and mortality in patients with highly symptomatic COPD and a high risk of future exacerbations [Citation12–14]. However, there has been much debate around the methodology of these trials [Citation15–17] and the generalizability of their results given that the trial populations are not representative of the majority of patients, who have moderate COPD and are infrequent exacerbators [Citation18,Citation19]. In addition, the findings have been difficult to replicate in real-world situations [Citation20–22]. Given the risk of high-cost side effects such as pneumonia, which are associated with long-term use of ICS [Citation23,Citation24], continued research is needed to clarify which patient populations would benefit most from the addition of ICS to their bronchodilator therapy in terms of disease control [Citation25].
Equally important in achieving optimal disease control are patient-related factors such as inhalation technique and inspiratory effort and ability. Even if a patient’s inhaler contains an optimal dose of effective medication, they may still have poor clinical outcomes if their inhalation technique is not correct and their inspiratory flow is insufficient to allow optimal deposition of drug molecules in the lung for a particular inhaler type/formulation. Furthermore, assessing inspiratory ability under optimized conditions in the clinic may not accurately reflect a patient’s routine behavior in the home setting. Assessing the relationship between inhalational technique, inspiratory flow profiles and clinical outcomes is possible in a clinical trial setting, but the COVID-19 pandemic has compromised the ability of researchers to conduct face-to-face trials. As such, there is a need to explore the feasibility of conducting remote clinical trials to ensure continued evidence generation in the post-COVID world.
This article summarizes a series of presentations sponsored by Boehringer Ingelheim and delivered at the annual CHEST congress 2021 (October 17–20, 2021) that explored new insights into the optimal management of COPD. These included evaluating the clinical and health-economic consequences of guideline-discordant prescribing in the US (Palli et al.), the comparative effectiveness of dual bronchodilator therapy and ICS-containing triple therapy (Quint et al. and Palli et al.), the effect of disease severity and contextual factors on patient inspiratory flow (Wachtel et al.), as well as the potential for a new digital model to revolutionize the way we could conduct clinical trials in COPD (Giessel et al.).
2. Presentation 1: real-world impact of GOLD compliant COPD maintenance therapy on exacerbations and treatment patterns [Citation26]
Palli S, Zhou S, Shaikh A, Willey VJ
This retrospective analysis of administrative claims data from the HealthCore Integrated Research Database compared the time to COPD exacerbation and treatment change (discontinuation, switching therapies or add-on of another COPD therapy class) among patients with COPD on maintenance therapy according to compliance with the 2017 GOLD treatment guidelines in a US commercially insured/Medicare Advantage population.
Patients with COPD were identified for inclusion in the analysis using a validated claims-based predictive model [Citation27]. Those with ≥1 claim for a COPD maintenance inhaler between 1 January 2014 and 31 March 2017 (earliest fill date = index date) were included. Eligible patients were aged ≥40 years and had ≥12 months of pre-index and ≥30 days of post-index health plan enrollment. A claims-based algorithm combining exacerbation history and assessment of high/low symptoms based on treatment, medical encounters, and respiratory procedure patterns was used to classify each patient into 2017 GOLD ABCD risk groups [Citation28]. For each patient, the index maintenance therapy was compared with the GOLD-recommended maintenance therapy for their respective ABCD risk group. A patient was classified as compliant if the index maintenance therapy and corresponding treatment history were consistent with the GOLD treatment recommendations; otherwise, the patient was classified as non-compliant.
In total, 44,917 patients met the eligibility criteria. The mean age was approximately 69 years, and half of the patients were female. COPD maintenance therapy was prescribed by a primary care physician in approximately half of patients, by a pulmonologist in approximately a quarter of patients, and by a non-physician prescriber (e.g. nurse practitioners and physician assistants) in approximately a quarter of patients. Overall, 85.4% were categorized into GOLD A/B and 14.6% into GOLD C/D risk groups. Baseline demographics were comparable between the compliant and non-compliant cohorts in the GOLD A/B and C/D risk groups. Compliance with GOLD treatment regimens was observed in 32.9% of GOLD A/B patients and 58.9% of GOLD C/D patients (GOLD A: 33.5%; GOLD B: 25.7%; GOLD C: 33.6%; GOLD D: 61.5%). Among the patients receiving a non-compliant treatment regimen, 90.4% of patients from the GOLD A/B group received ICS-containing therapies, with overuse of LABA/ICS occurring most frequently (68.8%). In the GOLD C/D group, more than half (56.8%) of the non-compliant group received LABA/ICS without a prior LAMA therapy.
Patients on compliant regimens tended to have significantly fewer exacerbations (reported as mean number per patient-year) than those on non-compliant regimens ()). In addition, patients on compliant regimens had significantly fewer severe exacerbations than those on non-compliant regimens in the GOLD C/D and GOLD D groups specifically. Multivariable analysis showed that patients on compliant regimens in both the GOLD A/B and C/D groups had a significantly longer mean time to first exacerbation ()). The same was found for mean time to severe exacerbation, though this was significant for GOLD C/D only.
Figure 1. Mean number of exacerbations, time to first exacerbation and time to change in treatment in GOLD-compliant versus non-GOLD-compliant treatment regimens. (a) Mean number of any exacerbations per patient year; *P < 0.05; **P < 0.001; ***P < 0.0001 (negative binomial regression). (b) Mean time to first exacerbation among patients with ≥1 exacerbation; *P < 0.05; **P < 0.001; ***P < 0.0001 (t-test). (c) Mean time to first index therapy change; **P < 0.001; ***P < 0.0001 (t-test). (d) Mean time to discontinuation of index therapy; **P < 0.001; ***P < 0.0001 (t-test). GOLD, Global Initiative for Chronic Obstructive Lung Disease.
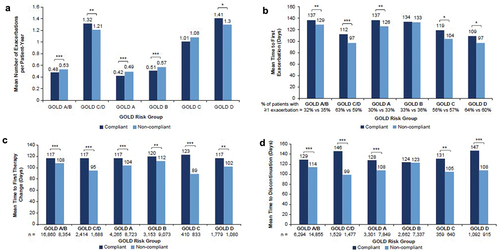
Overall, 77–86% of patients had a change in index maintenance therapy, with a higher proportion of patients on non-compliant regimens changing therapy compared with those on compliant regimens across most GOLD risk groups. These differences were significant for GOLD C/D and their respective individual GOLD risk groups, as well as for GOLD A. The time to first change in therapy was significantly longer among patients on compliant versus non-compliant regimens across all GOLD risk groups ()). Fewer patients on compliant regimens discontinued treatment versus those on non-compliant regimens; however, more patients on compliant regimens switched or escalated (added on) maintenance therapy versus those on non-compliant regimens during the 1-year follow-up period. Among those who discontinued maintenance therapy, patients on compliant regimens had a longer time to discontinuation than those on non-compliant regimens ()).
Clinical implications
Real-world treatment patterns for COPD in the US showed a high degree of discordance with GOLD treatment recommendations across all GOLD groups, particularly groups A, B and C, which represent nearly 90% of the COPD patient population on bronchodilator treatment. This is concerning since treatment regimens compliant with GOLD recommendations were associated with reduced exacerbations and improved persistence and durability of maintenance therapy compared with non-compliant regimens.
3. Presentation 2: effectiveness of COPD maintenance therapy with a LAMA/LABA versus LAMA/LABA/inhaled corticosteroids in a US claims database [Citation29]
Quint JK, Montonen J, He X, de la Hoz A, Esposito D
This retrospective cohort study compared time to a) first hospitalization for community-acquired pneumonia and b) first COPD exacerbation in patients initiating maintenance therapy with the long-acting dual bronchodilator therapy tiotropium/olodaterol versus triple therapy with LAMA/LABA/ICS.
Administrative data from the US HealthCore Integrated Research Database between January 2013 and March 2019, with up to 1-year follow-up, were evaluated. The analysis included patients who were aged ≥40 years, diagnosed with COPD (with no pre-index diagnosis of asthma), and had ≥1 inhaler prescription of fixed-dose tiotropium/olodaterol, or fixed or free triple therapy. In this study, the date of first prescription was defined as the index date. Propensity score matching and fine stratification/re-weighting were used to balance measured covariates between the cohorts. Several post hoc sensitivity analyses were also performed. In all analyses, a Cox proportional hazard regression model was used to assess the risk of outcomes (as-treated analysis).
The study population included 2,864 tiotropium/olodaterol and 24,326 triple therapy users (after reweighting: 2,785 tiotropium/olodaterol and 15,465 triple therapy users). Baseline characteristics between groups were similar, except for calendar year and season of cohort entry. No difference in prior exacerbations was observed overall (standard difference <0.1); however, a higher proportion of triple therapy users had severe exacerbations ≤1 year pre-index (32.7% versus 17.3%) and any exacerbations ≤30 days pre-index (25.8% versus 10.8%) versus tiotropium/olodaterol users.
For tiotropium/olodaterol versus triple therapy, the adjusted hazard ratio (aHR) for exacerbation in the reweighted population was 0.85 (95% confidence interval [CI] 0.76–0.96) (adjusted for calendar year and season of cohort entry). Excluding exacerbators ≤30 days pre-index balanced the cohorts both for any exacerbations (any, moderate or severe) and also for severe exacerbations specifically (standard difference <0.1). In this population, the aHR for exacerbation was 1.04 (95% CI 0.91–1.19) (adjusted for calendar year, LABA/ICS use, season of cohort entry). Results were similar when applying 1:1 greedy matching (aHR 1.02; 95% CI 0.84–1.24) and when restricting to treatment-naïve patients (no LABA, LAMA, ICS use prior to tiotropium/olodaterol or triple therapy) (aHR 1.08; 95% CI 0.81–1.44) ()). Tiotropium/olodaterol users had a numerically lower risk of pneumonia than triple therapy users in the reweighted population (aHR 0.83; 95% CI 0.64–1.07) ()).
Figure 2. (a) Risk of first COPD exacerbation. (b) Risk of first hospitalization for community-acquired pneumonia.
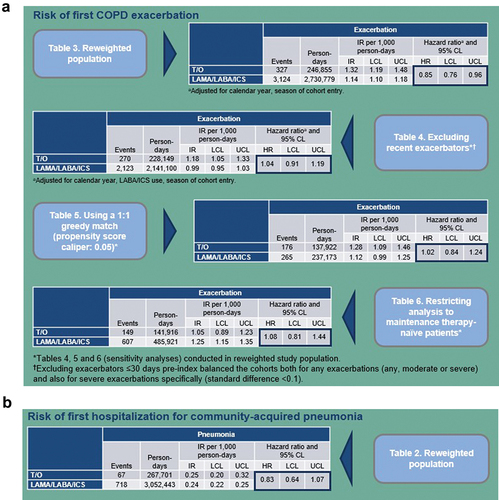
This study showed no benefit of triple therapy versus tiotropium/olodaterol in reducing exacerbation risk in the adjusted population of patients with no recent exacerbations, including treatment-naïve patients. The hazard ratio reported in our analysis was consistent with findings from previous real-world studies comparing dual therapy with triple therapy in infrequent exacerbators [Citation20–22]. In line with previous data, tiotropium/olodaterol was associated with a numerically lower risk of pneumonia versus triple therapy.
Clinical implications
In an infrequently exacerbating population, prescribing tiotropium/olodaterol in place of triple therapy appears not to increase exacerbation risk and could reduce pneumonia risk.
4. Presentation 3: annual expenditures among patients with COPD initiating tiotropium/olodaterol versus triple therapy [Citation30]
Palli S, Anderson AJ, Le L, Buikema AR, Franchino-Elder J, Frazer MS
This retrospective, non-interventional post-hoc analysis compared COPD-related costs among matched cohorts of tiotropium/olodaterol and triple therapy initiators in a US Medicare Advantage Part D (MAPD) population. The following two populations were analyzed: 1) naïve to maintenance treatment (LAMA, LABA and/or ICS), and 2) with or without prior maintenance therapy, stratified by baseline exacerbation history (proxy for COPD severity): the ‘no’ group had 0 exacerbations, the ‘single’ group had 1 moderate exacerbation, and the ‘multiple/severe’ group had ≥2 moderate or ≥1 severe exacerbation(s) in the previous year.
The analysis included COPD patients aged ≥40 years initiating tiotropium/olodaterol or triple therapy between 1 January 2014 and 31 May 2018 (treatment initiation date = index date). Patients were eligible if they had 12 months pre-index and 1-month post-index continuous medical/pharmacy coverage. Cohorts were propensity score matched 1:1 and multivariable analyses were used to estimate adjusted annualized post-index mean costs after matching.
Among patients naïve to maintenance treatment, tiotropium/olodaterol initiators had lower predicted mean COPD-related total ($9,422 versus $16,753; P < 0.001), medical ($5,845 versus $10,009; P < 0.001) and pharmacy ($3,669 versus $7,084; P < 0.001) costs than patients initiating triple therapy. In addition, Kaplan-Meier analysis revealed that tiotropium/olodaterol initiators had significantly fewer COPD-related hospitalizations than patients initiating triple therapy ()).
Figure 3. Kaplan-Meier analysis of time to COPD-related hospitalization among (a) treatment naïve MAPD patients and (b) MAPD patients with no exacerbation history. Measured from index date + 1, or discharge date + 1, if patient had inpatient visit crossing index date, until discontinuation of the index therapy. Diagnosis of interest must have occurred within an inpatient visit that began in the on-treatment period.
Figure 3. COPD, chronic obstructive pulmonary disease; MAPD, Medicare with Part D; PSM, propensity score matching; TIO+OLO, tiotropium/olodaterol; TT, triple therapy. Wald chi-square test using robust standard errors in a proportional hazard model was used for assessing equality of hazard rates.
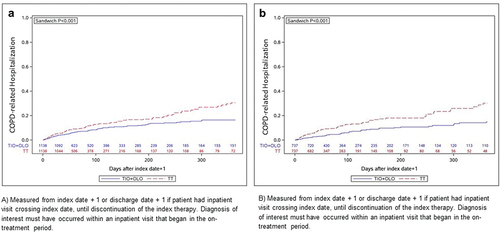
Tiotropium/olodaterol initiators with no previous exacerbations had significantly lower predicted mean COPD-related total ($7,342 versus $16,005; P < 0.001), medical ($3,675 versus $9,199; P < 0.001) and pharmacy ($3,685 versus $7,032; P < 0.001) costs versus triple therapy patients, respectively. Among patients in the ‘single’ exacerbation group, tiotropium/olodaterol patients also had lower predicted mean COPD-related total ($8,398 versus $15,058; P < 0.001), medical ($4,954 versus $8,422; P = 0.02) and pharmacy ($3,545 versus $6,912; P < 0.001) costs than triple therapy patients. Among patients in the ‘multiple/severe’ exacerbation group, tiotropium/olodaterol patients had lower predicted mean COPD-related pharmacy costs ($3,761 versus $7,249; P < 0.001) but no significant differences in COPD-related medical ($16,652 versus $15,718; P = 0.821) or total costs ($17,262 versus $22,979; P = 0.100). Kaplan-Meier analysis revealed that tiotropium/olodaterol patients with no exacerbation history had fewer COPD-related hospitalizations than patients on triple therapy (P < 0.001) ()).
In summary, patients initiating tiotropium/olodaterol had significantly lower total, medical and pharmacy costs versus those initiating triple therapy in two patient subpopulations: patients naïve to maintenance therapy, and patients with 0–1 moderate exacerbation in the year prior to initiation of tiotropium/olodaterol or triple therapy (with/without pre-index maintenance therapy).
Clinical implications
This real-world cost analysis illustrates the potential financial implications of unnecessarily prescribing triple therapy to patients with COPD who have not previously received maintenance therapy, and/or have no history of severe or frequent exacerbations.
5. Presentation 4: an investigation of factors affecting inhalation profiles of COPD patients [Citation31]
Wachtel H, Jugovic B, Singh D
This randomized, double-blind, three-period crossover study assessed how inhalation profiles vary between spontaneous maneuvers (i.e. inspiration during uncoached and normal inhaler use) and maximal maneuvers (i.e. forceful and deep inhalation from the device), and with varying inhaler inspiratory resistance (IR), in patients with moderate-to-very-severe COPD (n = 34; COPD severity: very severe [n = 10], severe [n = 12], moderate [n = 12]).
Patients with COPD aged ≥40 years with a forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ≤0.7, no exacerbation in the last 6 weeks, and no previous experience with the inhaler under study were randomized to sequential treatment with a placebo soft mist inhaler of low, medium or high IR. Following the 7-day treatment period (three periods in total, each with an inhaler of different IR, with no washout in between as no active compound was administered), the patient’s inhalation profile was assessed 30 minutes post-albuterol through the inhaler connected to a Viasys MasterScope. Both spontaneous and maximal inhalation profiles were recorded and described in terms of various spirometric parameters. Patients were assigned to one of three cohorts based on their baseline post bronchodilator % predicted FEV1 (<30%, very severe; 30%–<50%, severe; 50%–<80%, moderate).
With increasing disease severity, PIF (peak inspiratory flow) and total inhaled volume (VCin) reduced and elapsed time over which a flow of >80% of peak flow was achieved (ET80%) tended to increase (the inhalation profiles became smaller, with a lower and broader peak). Increasing inhaler flow resistance caused PIF, VCin and inhaled volume at PIF as a percentage of VCin (VPIF) to decrease and total inhalation time (Tin), time to PIF (TPIF) and ET80% to increase (inhalation profiles were smaller, with a lower and broader peak). With maneuver coaching, PIF and VCin increased, while Tin, TPIF and ET80% were reduced (inhalation profiles were larger, with a higher and narrower peak) (; ).
Figure 4. Primary endpoint results.
Figure 4. COPD, chronic obstructive pulmonary disease; ET80%, elapsed time over which a flow of >80% of peak flow was achieved; FAS, full analysis set; IR, inspiratory resistance; L, liters; PIF, peak inspiratory flow; s, seconds; SE, standard error; Tin, total inhalation time; TPIF, time to PIF; VCin, total inhaled volume; VPIF, inhaled volume at PIF as a percentage of VCin.
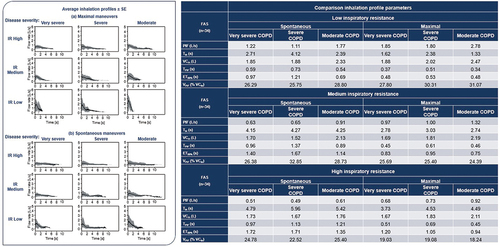
Table 1. Summary of findings
Overall, the study demonstrated that inhalation profiles vary depending upon COPD disease severity, type of inhalation maneuver (spontaneous or maximal) and internal inhaler resistance. It also showed that patients adapt their inhalation maneuvers depending upon the internal resistance of the inhaler and whether or not they have been coached.
Clinical implications
Spirometry PIF assessments in the clinic, which commonly measure maximal inhalation at minimal resistance, may not accurately reflect the inhalation profiles of patients using their inhaler (which has its own internal resistance) in the home setting. Effective inhaler therapy requires training, and inhalers that operate independently of the user’s inhalation profile could be expected to be more robust in routine use [Citation32].
6. Presentation 5: new paradigms in clinical study design: patient and sponsor perspectives from a fully remote study utilizing digital technologies [Citation33]
Giessel GM, Attick S, Franceschina, J, Harris P, Turner J, Hamilton A
ENERGITO® 2 digital health (DH) was a small exploratory study which aimed to gather patient and sponsor perspectives on the feasibility of conducting a remote COPD clinical study, with the ultimate aim of enhancing patient experience through DH technologies, identifying potential DH solutions to clinical development needs, and evaluating their integration into broader utilization. As this was an exploratory, site-specific study not designed for treatment comparison, the patients enrolled in this study were entirely independent of the main study (i.e. data collected in the DH exploratory study was analyzed separately from the data collected in the main study) [Citation34,Citation35].
In this 12-week study, COPD patients aged ≥40 years, with a smoking history of >10 pack-years, post bronchodilator FEV1 ≥30% to <80% predicted, FEV1/FVC <70%, internet access, smartphone proficiency, and prior spirometry experience were randomized and received either tiotropium/olodaterol 5/5 μg once daily or fluticasone propionate/salmeterol 250/50 μg twice daily. All patient procedures and visits were either fully remote (without site-staff contact) or involved telemedicine/e-visits (with the site-staff via video); however, e-visits could be converted to face-to-face visits as necessary.
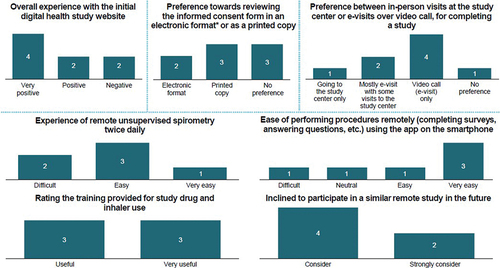
Of 18 patients screened, 11 (6 men/5 women; mean ±SD age, 66.0 ± 7.3 years) were randomized to tiotropium/olodaterol (n = 6) or fluticasone propionate/salmeterol (n = 5). Nine patients completed the study: six in the tiotropium/olodaterol group and five in the fluticasone propionate/salmeterol group. Three patients each had COPD at GOLD stages II and III (moderate to severe) [Citation2]. Overall, most patients had 80–120% treatment compliance at e-visit 3 (90.9%; n = 10) and e-visit 4 (81.8%; n = 9). Respondents varied in number (range, 3–8) across the 6 surveys; survey responses were generally favorable, although some patients experienced challenges with the invitation process, receipt of investigational medicinal product supplies, spirometer functioning and audio quality ().
Figure 5. Key findings from the survey
#Bar charts represent the number of respondents answering the survey questions; *Electronic format included video and text on asmartphone.
After completion of the study, a patient focus group was conducted, which provided valuable feedback for future patient-centric, remote clinical trial planning/conduct. The key learnings from this included: offering the flexibility of a combined in-person and virtual engagement to enhance the patient experience, increased personal connection between clinic visits, and use of supplemental information videos.
ENERGITO 2 DH demonstrated the feasibility of conducting a clinical study remotely in accordance with regulatory guidelines. Technology utilization and proactive and strategic integration of additional operational elements enabled patients to participate remotely, as confirmed by patients during the study and post-study completion.
Clinical implications
This small feasibility study provides valuable insights regarding patient experiences, supporting the potential integration of DH solutions into clinical studies and providing preliminary evidence of accurate patient use of digital spirometry in a fully remote COPD study. However, further evaluation in larger clinical studies is required, including the feasibility of remote pulmonary function testing.
7. Conclusion
To achieve tailored treatment strategies and improve clinical management for patients with COPD, many factors must be considered. Boehringer Ingelheim-sponsored research presented at the CHEST congress 2021 explored new insights into the optimal management of COPD.
Palli et al. showed discordance of real-world prescribing patterns in the US with GOLD treatment recommendations. This is concerning since compliant treatment regimens extended the time to exacerbation and treatment change. In a study aimed at addressing the ongoing debate around the clinical benefits of ICS in patients with COPD, Quint et al. showed that in an infrequently exacerbating population, the use of triple therapy did not reduce exacerbation risk versus LAMA/LABA and was associated with a higher pneumonia risk. Palli et al. also demonstrated that patients initiating tiotropium/olodaterol, who were either naïve to maintenance treatment or who had 0 or 1 moderate exacerbation prior to treatment initiation had significantly lower medical and pharmacy costs versus those initiating with triple therapy. In relation to non-pharmacologic factors, Wachtel et al. showed that patients’ inhalation profiles vary depending upon COPD disease severity, type of inhalation maneuver (spontaneous or maximal) and their inhaler’s internal resistance, illustrating the importance of context for PIF measurements. Finally, Giessel et al. investigated the use of DH technologies in the design and conduct of remote clinical studies, which has the potential to improve patient access to and experience with clinical research and self-management of COPD.
Data sharing statement
Researchers should use the https://vivli.org/ link to request access to study data and the Medical & Clinical Trials | Clinical Research | MyStudyWindow for further information.
Declaration of interest
JK Quint reports personal fees for advisory board participation or speaking fees from GlaxoSmithKline, Boehringer Ingelheim, AstraZeneca and Bayer. J Montonen, H Wachtel, S Attick and SR Palli are employees of Boehringer Ingelheim Pharmaceuticals, Inc. D Singh has received sponsorship to attend and speak at international meetings and honoraria for lecturing or attending advisory boards from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Teva, Theravance and Verona. M Frazer is an employee of Optum. VJ Willey is an employee of HealthCore, which was contracted by Boehringer Ingelheim Pharmaceuticals, Inc. to conduct the study titled ‘Real-world impact of GOLD compliant COPD maintenance therapy on exacerbations and treatment patterns’. GM Giessel received grant/research support from Boehringer Ingelheim as the principal investigator of the ENERGITO® 2 digital health study.
Acknowledgments
We wish to thank the authors included in this publication for their contributions to the development of the presentations, and to the studies that they describe. Medical writing assistance, in the form of the preparation and revision of the draft manuscript, was supported financially by Boehringer Ingelheim and provided by Francesca Lomas of MediTech Media Ltd under the authors’ conceptual direction and based on feedback from the authors. Dave Singh is supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC).
Additional information
Funding
References
- World Health Organization. The top 10 causes of death 2018 [cited 2021 Jul 7]. Available from: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2019 report) 2019. [cited 2021 Jul 7]. Available from: https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf
- World Health Organization. COPD management 2016 [cited 2021 Jul 7]. Available from: https://www.who.int/respiratory/copd/management/en/
- Wedzicha JA, Singh R, Mackay AJ. Acute COPD exacerbations. Clin Chest Med. 2014 Mar;35(1):157–163. PubMed PMID: 24507843.
- Press VG, Konetzka RT, White SR. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the hospital readmission reduction program. Curr Opin Pulm Med. 2018 Mar;24(2):138–146. PubMed PMID: 29210750; PubMed Central PMCID: PMCPMC5810972. eng.
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2021 report) 2020. [updated 2020 Nov 25]; [cited 2021 April 14]. Available from: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf
- Global Initiative for Chronic Obstructive Lung Disease. Pocket guide to COPD diagnosis, management and prevention - A guide for health care professionals (2021 report) 2020 [cited 2021 Aug 5]. Available from 2021 Aug 5: https://goldcopd.org/wp-content/uploads/2020/12/GOLD-2021-POCKET-GUIDE-v2.0-14Dec20_WMV.pdf
- Arnold MJ. Treatment of chronic obstructive pulmonary disease: guidelines from the American Thoracic Society. Am Fam Physician. 2021 Jul 1;104(1):102–103. PubMed PMID: 34264596.
- White P, Thornton H, Pinnock H, et al. Overtreatment of COPD with inhaled corticosteroids–implications for safety and costs: cross-sectional observational study. PLoS One. 2013;8(10):e75221. PubMed PMID: 24194824; PubMed Central PMCID: PMCPMC3806778.
- Make B, Dutro MP, Paulose-Ram R, et al. Undertreatment of COPD: a retrospective analysis of US managed care and Medicare patients. Int J Chron Obstruct Pulmon Dis. 2012;7:1–9. PubMed PMID: 22315517; PubMed Central PMCID: PMCPMC3273365. eng.
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020 May 1;201(9):e56–e69. PubMed PMID: 32283960; PubMed Central PMCID: PMCPMC7193862.
- Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020 Jun 15;201(12):1508–1516. PubMed PMID: 32162970; PubMed Central PMCID: PMCPMC7301738.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018 May 3;378(18):1671–1680. PubMed PMID: 29668352.
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020 Jul 2;383(1):35–48. PubMed PMID: 32579807.
- Suissa S, Ariel A. Triple therapy trials in COPD: a precision medicine opportunity. Eur Respir J. 2018 Dec;52(6):1801848. PubMed PMID: 30545959.
- Suissa S. Perplexing mortality data from triple therapy trials in COPD. Lancet Respir Med. 2021 Jul;9(7):684–685. PubMed PMID: 34126054; eng.
- Suissa S. Triple therapy in COPD: time for adaptive selection trials. COPD. 2021;1–5. DOI:https://doi.org/10.1080/15412555.2021.1982886
- Halpin DM, Kerkhof M, Soriano JB, et al. Eligibility of real-life patients with COPD for inclusion in trials of inhaled long-acting bronchodilator therapy. Respir Res. 2016 Sep 23;17(1):120. PubMed PMID: 27663386; PubMed Central PMCID: PMCPMC5034631.
- Han MK, Quibrera PM, Carretta EE, et al. Frequency of exacerbations in patients with chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2017;5(8):619–626.
- Cabrera C, Quélen C, Ouwens M, et al. Evaluating a Cox marginal structural model to assess the comparative effectiveness of inhaled corticosteroids versus no inhaled corticosteroid treatment in chronic obstructive pulmonary disease. Ann Epidemiol. 2021 Nov;67:19–28. PubMed PMID: 34798296; eng.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effects of LAMA-LABA-ICS vs LAMA-LABA for COPD: cohort study in real-world clinical practice. Chest. 2020 Apr;157(4):846–855. PubMed PMID: 31759966; eng.
- Suissa S, Dell’Aniello S, Ernst P. Triple inhaler versus dual bronchodilator therapy in COPD: real-world effectiveness on mortality. COPD. 2021;1–9. DOI:https://doi.org/10.1080/15412555.2021.1977789
- Souliotis K, Silva Miguel L, Hillas G, et al. The cost-saving switch from inhaled corticosteroid-containing treatments to dual bronchodilation: a two-country projection of epidemiological and economic burden in chronic obstructive pulmonary disease. Ther Adv Respir Dis. 2020 Jan-Dec;14:1753466620926802. PubMed PMID: 32519591; PubMed Central PMCID: PMCPMC7288795. eng.
- Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018 Dec;52(6):1801219. PubMed PMID: 30190269.
- Tashkin DP, Strange C. Inhaled corticosteroids for chronic obstructive pulmonary disease: what is their role in therapy? Int J Chron Obstruct Pulmon Dis. 2018;13:2587–2601. PubMed PMID: 30214177; PubMed Central PMCID: PMCPMC6118265. eng.
- Palli S, Zhou S, Shaikh A, et al. Real-world impact of GOLD compliant COPD maintenance therapy on exacerbations and treatment patterns. Poster presented at: CHEST Annual Meeting; 2021 Oct 17-20; Orlando, Florida.
- Palli S, Zhou S, Shaikh A, et al. Developing a claims’-based algorithm to identify US patients with COPD. Eur Respir J. 2019;54(Suppl 63):A4431.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017 Mar 1;195(5):557–582. PubMed PMID: 28128970.
- Quint JK, Montonen J, He X, et al. Effectiveness of COPD maintenance therapy with a LAMA/LABA versus LAMA/LABA/inhaled corticosteroids in a US claims database. Poster presented at: CHEST Annual Meeting; 2021 Oct 17-20; Orlando, Florida.
- Palli S, Anderson AJ, Le L, et al. Annual expenditures among patients with COPD initiating tiotropium/olodaterol versus triple therapy. Poster presented at: CHEST Annual Meeting; 2021 Oct 17-20; Orlando, Florida.
- Wachtel H, Jugovic B, Singh D. An investigation of factors affecting inhalation profiles of COPD patients. Paper presented at: CHEST Annual Meeting; 2021 Oct 17-20; Orlando, Florida.
- Mahler DA, Ludwig-Sengpiel A, Ferguson GT, et al. TRONARTO: a randomized, placebo-controlled study of tiotropium/olodaterol delivered via soft mist inhaler in COPD patients stratified by peak inspiratory flow. Int J Chron Obstruct Pulmon Dis. 2021;16:2455–2465. PubMed PMID: 34511891; PubMed Central PMCID: PMCPMC8414074. eng.
- Giessel GM, Attick S, Franceschina J, et al. New paradigms in clinical study design: patient and sponsor perspectives from a fully remote study utilizing digital technologies. Paper presented at: CHEST Annual Meeting; 2021 Oct 17-20; Orlando, Florida.
- ClinicalTrials.gov. The ENERGITO® 2 study compares 2 inhaled medicines for chronic obstructive pulmonary disease (COPD). One Medicine is a Combination of Tiotropium and Olodaterol (Stiolto®) Taken Using the Respimat® Inhaler and the Other Medicine is a Combination of Fluticasone and Salmeterol Taken Using the Diskus (NCT03240575).
- Wise R, Abrahams R, Westerman JH, et al. The lung function profile of patients with COPD receiving once-daily tiotropium/olodaterol versus twice-daily fluticasone propionate/salmeterol in ENERGITO 2 clinical trial. J Precis Respir Med. 2019;2(1):57–66.
