ABSTRACT
Introduction
Acute pulmonary embolism (PE) is a disease with a broad spectrum of clinical presentations. While some patients can be treated at home or may even be left untreated, other patients require an aggressive approach with reperfusion treatment.
Areas covered
(1) Advanced reperfusion treatment in hemodynamically stable acute PE patients considered to be at high risk of decompensation and death, (2) the treatment of subsegmental pulmonary embolism, (3) outpatient treatment for hemodynamically stable PE patients with signs of right ventricle (RV) dysfunction, and (4) the optimal approach to identify and treatpost-PE syndrome.
Expert opinion
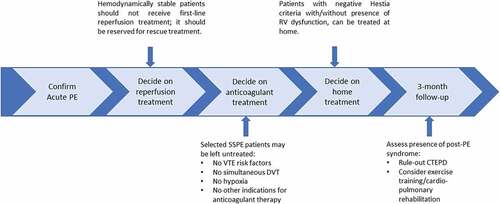
Outside clinical trials, hemodynamically stable acute PE patients should not be treated with primary reperfusion therapy. Thrombolysis and/or catheter-directed therapy are only to be considered as rescue treatment. Subsegmental PE can be left untreated in selected low-risk patients, after proximal deep vein thrombosis has been ruled out. Patients with an sPESI or Hestia score of 0 criteria can be treated at home, independent of the presence of RV overload. Finally, health-care providers should be aware of post-PE syndrome and diagnose chronic thromboembolic pulmonary disease (CTEPD) as early as possible. Persistently symptomatic patients without CTEPD benefit from exercise training and cardiopulmonary rehabilitation.
1. Introduction
Acute pulmonary embolism (PE) is a disease with a broad spectrum of clinical presentations. Important improvements in the diagnosis and treatment of acute PE have been made in recent years [Citation1]. Advanced imaging techniques have resulted in improved acute PE detection, and new risk stratification and interventional techniques have been introduced, overall resulting in a decreased PE-related mortality [Citation2,Citation3]. Important questions regarding the optimal management of acute PE remain nonetheless, especially at both extremes of the disease severity spectrum. In this review, we focus on four important but controversial aspects of acute PE management that are still subject of debate and research: (1) advanced reperfusion treatment in hemodynamically stable acute PE patients considered to be at high risk of decompensation and death, (2) the treatment of subsegmental pulmonary embolism (SSPE), (3) outpatient treatment for hemodynamically stable acute PE patients with signs of right ventricle (RV) dysfunction, and (4) the optimal approach to identify and treat post-PE syndrome in PE survivors.
2. Reperfusion therapy in stable acute PE patients
There is a general consensus that, to increase survival chances, acute PE associated with hemodynamic instability or frank obstructive shock at presentation is a clear indication for immediate reperfusion therapy [Citation4]. However, whether hemodynamically stable acute PE patients with signs of RV dysfunction and myocardial injury, who are also at increased risk of decompensation and death, referred to as intermediate-high-risk acute PE [Citation4], may also benefit from reperfusion therapy is an ongoing point of debate. This debate is fueled by the introduction of catheter-based reperfusion techniques.
The Pulmonary Embolism Thrombolysis (PEITHO) trial was designed to gain more knowledge regarding the efficacy and safety of systemic thrombolysis in intermediate-high-risk acute PE patients [Citation5]. In this trial, 1005 acute PE patients with RV dysfunction on computed tomography pulmonary angiography (CTPA) and a positive troponin test were randomized between standard anticoagulation therapy with heparin versus anticoagulation with a single-bolus injection of tenecteplase (30–50 mg depending on the body weight). Tenecteplase indeed prevented death or hemodynamic decompensation (incidence within 7 days of 2.6% in the tenecteplase group versus 5.6% in placebo group; odds ratio [OR] 0.44; 95% confidence interval [CI] 0.23 to 0.87); however, the risk for major extracranial bleeding was increased with 6.3% in the tenecteplase group versus 1.2% in the placebo group, and hemorrhagic stroke occurred 2.0% in the tenecteplase group versus 0.2% in the placebo group. Therefore, the benefits of treatment did not outweigh its risks, and the current guidelines do not recommend systemic thrombolysis in intermediate-high-risk acute PE patients as a first-line treatment option [Citation4,Citation6]. However, a post-hoc analysis of the PEITHO study showed that in intermediate-high-risk acute PE with at least two clinical criteria of severity (i.e. a systolic blood pressure ≤110 mmHg, a respiratory rate >20 breaths/min, chronic heart failure, and/or cancer), tenecteplase treatment would have resulted in an adverse event rate of 7.6% compared to 20.3% for the placebo group [Citation7]. This result suggests that further risk stratification of patients in the intermediate-high-risk category may help to select patients for whom the risk–benefit ratio of reperfusion therapy would support immediate application of the latter. While clinical signs of severity are likely important for further risk stratification, it is important to bear in mind that clot burden as a sole parameter has no beneficial role in selecting hemodynamically stable acute PE patients at risk for deterioration since a high clot burden is not associated with increased adverse events in hemodynamically stable acute PE [Citation8].
It has been proposed that reduced dose thrombolytic therapy may avoid the risk of bleeding while preserving the increased rate of thrombus resolution. Several small studies have been performed to investigate the safety and efficacy of reduced dose systemic thrombolysis. Two studies have shown that reduced systemic thrombolysis (recombinant tissue plasminogen activator at 0.5–0.6 mg/kg) is more effective than placebo in the normalization of perfusion defects and that systemic thrombolysis resulted in a reduced combined endpoint of persistent pulmonary hypertension or recurrent PE [Citation9,Citation10]. Moreover, three randomized studies suggested that a reduced dose of thrombolytic treatment (recombinant tissue plasminogen activator at 0.5–0.6 mg/kg or at 50 g per 2 hours) was equally effective as full dose in prevention of death, change in total pulmonary resistance, and residual vascular obstruction [Citation11–13]. In a network meta-analysis, low-dose thrombolysis was indeed associated with the lowest probability of dying and bleeding compared to other reperfusion options [Citation14]. The ongoing PEITHO-3 trial (NCT04430569) is formally evaluating the efficacy and safety of a reduced-dose alteplase regimen (0.6 mg/kg) with standard heparin anticoagulation in patients with intermediate-high-risk PE and at least one clinical criterion of severity (i.e. a systolic blood pressure ≤110 mmHg, a respiratory rate >20 breaths/min, and/or chronic heart failure) and will ultimately determine the role of half-dose thrombolysis in the management of intermediate-high-risk acute PE [Citation15].
Over the last decade, multiple percutaneous catheter-directed therapies (CDTs) have been introduced. CDT is a local technique aiming for thrombus resolution based on thrombus fragmentation, thrombus aspiration, rheolytic thrombectomy (i.e. disruption and removal of the thrombus using a pressure gradient or local thrombolysis), or local (ultrasound accelerated) thrombolysis [Citation16]. Studies have shown that CDT results in a decrease in RV overload compared to anticoagulation alone, along with low rates of major bleeding (ranging 0–10%) [Citation17–22]. However, evidence is limited since most studies were observational or single-arm cohort studies. There is also limited evidence on complication rates of CDT beyond major bleeding or death. Clinical studies have reported a complication rate of ~0–4% [Citation16]. The complication rates of CDT performed by inexperienced physicians are unknown, but a higher rate can be expected. The few small randomized trials performed were not designed to establish differences in clinically relevant outcomes, such as death or hemodynamic deterioration to shock. Larger randomized controlled trials are needed to prove efficacy beyond doubt, before these costly therapies become routine care for intermediate-high-risk acute PE patients. Currently ongoing trials investigating the efficacy and safety of CDT include the HI-PEITHO trial (NCT04790370) and the PEERLES study (NCT05111613) [Citation23,Citation24]. The HI-PEITHO trial randomizes intermediate-high-risk acute PE patients with at least two clinical criteria of severity (i.e. heart rate ≥100 bpm, systolic blood pressure ≤110 mmHg, respiratory rate >20/min, and/or oxygen saturation on pulse oximetry <90% on room air) to treatment with a standardized protocol of ultrasound-facilitated catheter-directed thrombolysis plus anticoagulation versus anticoagulation alone [Citation23]. The PEERLESS study randomizes intermediate-high-risk acute PE patients to mechanical thrombectomy using the FlowTriever system versus catheter-directed thrombolysis with any commercially CDT system [Citation24]. Another treatment option is surgical embolectomy, but there is little evidence on the safety and efficacy in (intermediate) high-risk acute PE since only non-randomized studies have been performed. Surgical embolectomy is therefore currently only recommended in patients with a high-risk acute PE who deteriorated after thrombolysis or have a contra-indication for thrombolysis [Citation4,Citation25]. While awaiting the results of currently ongoing clinical trials, a multidisciplinary rapid-response team, also known as PE response teams (PERT), facilitates clinical decision-making in patients with intermediate-high-risk acute PE [Citation4].
3. Treatment of subsegmental pulmonary embolism
An SSPE is an embolus located in single or multiple subsegmental pulmonary arteries [Citation1,Citation26]. It is currently debated whether SSPE is an indication for anticoagulant treatment. There are several arguments why SSPE can be left untreated. First, advances in the radiological diagnosis of PE have resulted in an increased incidence of SSPE. Because this increase in the number of PE diagnosis was associated with a decreasing trend in PE mortality, SSPE has been hypothesized to be ‘overdiagnosis’ [Citation2, Citation27–32]. The fact that imaging artifacts are often misclassified as SSPE is supportive of this concept [Citation33–36]. Second, it can be argued that the presence of small thrombi in the pulmonary system provided that proximal deep vein thrombosis (DVT) is not present may be a physiological finding as the pulmonary system might act as a filter to prevent thrombotic tissue entering the arterial system [Citation37,Citation38].
Multiple small observational studies have shown that patients with isolated SSPE may be left untreated with a low incidence of symptomatic recurrent venous thromboembolism (VTE) [Citation35, Citation39–43]. A recent large multicenter prospective cohort study showed a recurrent VTE rate of 3.1% (8 out of 266 patients; 95% CI 1.6–6.1; none of the eight recurrences observed were fatal) which led to premature stop of recruitment since the predefined inferiority stopping rule was met; the primary study hypothesis was that this recurrence rate would be below 3.0% [Citation44].
A potential explanation for the observed difference between the available studies is that, until recently, a universal SSPE diagnosis was lacking. A Delphi analysis was performed in order to establish a uniform diagnostic definition for SSPE: ”A contrast defect in a subsegmental artery, i.e. the first arterial branch division of any segmental artery independent of artery diameter, visible in at least two subsequent axial slices, using a Computed Tomography scanner with a desired maximum collimator width of ≤1 mm” [Citation26]. This universal diagnosis likely helps the reliable and reproducible identification of SSPE and should be the basis of future studies.
Another important factor in SSPE treatment is the selection of which SSPE patients can potentially be left untreated since there are multiple factors determining the risk of recurrent VTE besides location and size. SSPE patients with a malignancy or previous VTE should not be left untreated since the expected recurrence rate is higher, even when this diagnosis was incidental [Citation45–50]. Also, SSPE patients presenting with hypoxemia should not be left untreated since an isolated SSPE may become clinically relevant in patients with preexisting cardiopulmonary disease [Citation51,Citation52]. In the previously described cohort study, 435 of 749 SSPE patients (58%) were excluded from the study and treated with anticoagulants due to the presence of (among others) one of the previously described criteria [Citation44]. Finally, SSPE patients with a simultaneous DVT should not be left untreated. DVT is an important predictor for recurrent VTE and PE-related mortality and therefore requires anticoagulation [Citation52,Citation53]. For SSPE patients with concomitant DVT who receive anticoagulation for the DVT, there is no need to discuss if there is an indication for anticoagulation for the SSPE, since this treatment is already indicated based on the DVT. In the previously described cohort study, six out of 292 SSPE patients with no other risk factors for recurrent VTE were found to have (non-symptomatic) proximal DVT (2.1%) and 22 had (non-symptomatic) distal DVT (7.5%) upon bilateral compression ultrasonography, highlighting the importance of ruling out DVT in SSPE patients when considering leaving them untreated [Citation44]. The safe-SSPE trial (NCT04263038) is currently investigating the incidence of recurrent VTE, recovery of complaints, and functional performance in selected SSPE patients randomized to either placebo or rivaroxaban [Citation54].
4. Home treatment
The 2019 ESC guideline recommends classifying patients according to their risk of early (in hospital or 30-day) death and treating patients accordingly [Citation4]. The PESI score and simplified PESI (sPESI) are prediction models that can identify low-risk acute PE patients with a 30-day mortality of ~1.0% [Citation55,Citation56]. The PESI score can be used to select patients eligible for outpatient treatment since a randomized controlled trial showed non-inferiority for outpatient treatment versus hospitalization in low-risk patients according to an ad hoc decision rule in patients with PESI class I–II [Citation57]. The Hestia criteria are an alternative tool to select patients eligible for outpatient treatment. This is a pragmatic list of 11 reasons why patients would require hospitalization, e.g. need for advanced reperfusion therapy, oxygen therapy, or intravenous analgesics (). The Hestia criteria are a checklist rather than a prediction score. Patients that were negative for all 11 Hestia criteria were treated as outpatients with low molecular weight heparin (LMWH) or LMWH plus a vitamin K antagonist (VKA) in a prospective cohort study, with a 90-day overall mortality of 1.0% [Citation58,Citation59]. The Vesta study randomized patients who were negative for all Hestia criteria between direct discharge versus additional N-terminal pro–brain natriuretic peptide (NT-proBNP) assessment. Patients with an NT-proBNP below 500 ng/L were also treated at home. All patients received LMWH and VKAs. Due to the low number of adverse events, this study was unable to show incremental value of NT-proBNP testing in patients who are negative for all Hestia criteria [Citation60]. The HOME-PE trial randomized patients between Hestia and sPESI for selection for outpatient treatment with LWMH, VKAs, or directs oral anticoagulants and showed that the rate of 30-day combined end-point (i.e. recurrent VTE, bleeding, or all-cause death) for patients treated at home was low (1.3% for Hestia and 1.1% for sPESI). Moreover, in the overall population, the rate of this end-point was comparable in both groups (3.8% for Hestia versus 3.6% for sPESI), showing that both strategies are safe and effective in selecting patients for outpatient treatment [Citation61].
Notably, both Hestia and (s)PESI do not incorporate an explicit assessment of RV function (). Whether low-risk patients (according to Hestia and/or [s]PESI) with RV dysfunction can be treated as outpatients remain a point of debate. According to the 2019 ESC guidelines, assessment of RV dysfunction is obligatory before considering outpatient treatment: patients with none of the Hestia criteria, PESI I–II, or sPESI 0 but with RV dysfunction are characterized as intermediate-risk acute PE [Citation4]. Hospitalization is recommended for this patient category. This recommendation was partly based on a meta-analysis suggesting that RV dysfunction is associated with a high risk of early all-cause mortality even in selected low-risk patients according to the PESI score (OR 4.2 95% CI 1.4–12.6) [Citation62]. The HoT-PE study evaluated the safety and efficacy of early discharge (up to two nights of hospital stay were permitted) in low-risk patients (according to adapted Hestia criteria) who had no signs of RV dysfunction or intracardiac thrombi. Of the 2854 acute PE patients evaluated for study inclusion, 300 patients had negative Hestia criteria but the presence of RV dysfunction or free-floating thrombi and were therefore excluded from the trial and treated as inpatients. In the 525 patients selected for early discharge, a 0.6% incidence of recurrent non-fatal VTE and a 1.2% incidence of major bleeding were observed, suggesting that early discharge is safe in these selected low-risk patients [Citation63]. However, the studies included in the previously mentioned meta-analysis were mainly observational, and no systematic treatment decisions were made based on the (s)PESI score or signs of RV dysfunction. Therefore, we cannot simply conclude that early all-cause mortality would improve if all low-risk patients with RV dysfunction are hospitalized. In addition, patients excluded from HoT-PE due to the presence of RV dysfunction were not systematically followed, and details regarding their prognosis were unavailable.
Interestingly, an analysis of the combined Hestia and Vesta study, where RV dysfunction on CTPA was assessed post-hoc (i.e. RV/left ventricle ratio >1), showed that 30% of the patients treated at home had RV dysfunction, and the incidence of adverse events did not differ between outpatients with or without RV dysfunction (2.7% vs 2.3%, respectively) [Citation64]. Also, in the HOME-PE study, 90 of the 739 (12.2%) patients treated at home had RV dysfunction; none of these patients returned to the hospital because of hemodynamic deterioration or experienced PE recurrence of PE-related death [Citation61]. Moreover, the post-hoc assessed troponin T levels in the Vesta study showed no difference in all-cause death after 3 months for home treated patients with or without an elevated troponin T level (1.7% vs 1.7% respectively) [Citation65]. Identifying low-risk patients based on Hestia (or [s]PESI) alone—even when signs of RV dysfunction are present—seems therefore adequate for the selection of patients who are eligible for outpatient treatment. This is explained by the fact that preselection based on Hestia and/or sPESI already results in an acceptable low adverse event rate, thus diluting the additional value in the absence of RV dysfunction.
In routine Dutch clinical practice, 46% of the patients are treated at home (ranging from 13% to 83% for individual hospitals) [Citation66]. Using patient-level data of the YEARS study, health-care utilization and costs were compared between hospitalized and home-treated patients. Patients who were treated as outpatients had a mean hospitalization duration of 0.69 days compared to 4.3 days for patients who were hospitalized. This correlated with an average cost of hospitalized patients of €3,209 versus €1,512 per patient treated at home, adjusted for potential confounders, emphasizing the cost-effectiveness of treating acute PE patients as outpatients [Citation67]. More importantly, outpatient treatment results in a high level of patient satisfaction [Citation68].
Table 1. Hestia criteria and sPESI score for eligibility of home-treatment.
5. Long-term consequences after acute PE
Survivors of acute PE often report persistent symptoms, new psychosocial problems, and/or persistent limitations in their daily activities [Citation69–72]. These patients qualify as having post-pulmonary embolism syndrome (PPES) which is defined as new or progressive dyspnea, exercise intolerance, and/or impaired functional or mental status after at least 3 months of adequate anticoagulation following acute PE, which cannot be explained by other (preexisting) comorbidities [Citation73]. Up to 16–47% of the acute PE patients report persistent limitations and/or dyspnea qualifying for PPES [Citation69,Citation74,Citation75]. The exact incidence of PPES remains unclear since different criteria have been used to define the presence of PPES and PPES incidence evaluation has been performed at different time points following acute PE diagnosis. Post-PE syndrome has four largely distinct clinical presentations: (1) chronic thromboembolic pulmonary disease (CTEPD) with pulmonary hypertension, i.e. chronic thromboembolic pulmonary hypertension (CTEPH), (2) CTEPD without pulmonary hypertension, (3) post-PE cardiac dysfunction (characterized as persistent RV impairment), and (4) post-PE functional impairment [Citation73,Citation76,Citation77]. Importantly, awareness of PPES and early diagnosis of especially CTEPH will most likely lead to better health outcomes of PE survivors [Citation73,Citation78].
During follow-up of acute PE, systematic and routine evaluation of the symptom burden and quality of life (QoL) will greatly facilitate the early identification of patients who require additional treatment beyond anticoagulation. Patient reported outcome measures (PROMs) are helpful tools for this purpose, for example, by measuring dyspnea (Medical Research Council [MRC] dyspnea scale [Citation4,Citation79]) or functional limitations (Post-VTE Functional Status [PVFS] scale [Citation80,Citation81]). However, other validated tools to objectify persistent symptoms or functional limitations can also be used. An international workgroup (ICHOM) established a core set of outcome measures with matching instruments that encompass the most relevant outcomes. Implementation of this core set will help in shifting the focus [Citation82].
In patients with persistent symptoms and functional limitations, further classification of PPES should be performed. Since an early diagnosis of CTEPH will result in improved survival and better QoL, early diagnosis is of utmost importance [Citation78,Citation83,Citation84]. A CTEPH diagnosis is confirmed by mismatched perfusion defects in ventilation-perfusion (V/Q) scan in combination with a mean pulmonary artery pressure of ≥20 mmHg, pulmonary capillary wedge pressure of ≤15 mmHg, and pulmonary vascular resistance of >2 woods-units measured with right heart catheterization (RHC) [Citation85,Citation86]. There are several strategies to select patients who should be subjected to V/Q scan and RHC. The ESC guidelines recommend performing echocardiography in all patients with persistent dyspnea, functional limitations, or risk factors for CTEPH. Patients with intermediate to high probability of pulmonary hypertension on echocardiography require further evaluation [Citation4,Citation85]. A strategy to limit the number of patients referred for echocardiography is the InShape II algorithm, which consists of a CTEPH prediction score and the CTEPH rule-out criteria [Citation87–91]. Moreover, there are several radiological signs on CTPA that are highly specific for CTEPH and can contribute in early identification of patients who require focused diagnostic evaluation early in the course of disease [Citation79,Citation92–95].
Decreased daily physical activity after a PE diagnosis, anxiety, and post-thrombotic panic syndrome, as well as fear for recurrences or complications all result in deconditioning with persistent symptoms and functional limitations as a result; these patients are referred to as having post-PE functional impairment [Citation69–72,Citation74,Citation96–98]. Exercise treatment or cardiopulmonary rehabilitation is a potential treatment option for these patients. A Dutch study showed that in patients with persistent moderate-to-severe dyspnea >3 months after acute PE, a 12-week rehabilitation program resulted in significant improvement in training intensity and PE-specific QoL [Citation99]. An Austrian study showed that a 6-week rehabilitation course initiated after a median of 19 weeks following an acute PE diagnosis resulted in improvement in the 6-minute walk test and self-reported health [Citation100]. While rehabilitation seems effective in the treatment of PPES, it has been suggested that exercise training early after PE diagnosis may prevent deconditioning and resulting loss of QoL. Several studies have shown that exercise training is safe in acute PE patients [Citation100–105]. Two studies randomized acute PE patients to early initiation of exercise training versus no exercise training [Citation102,Citation106]. The first study showed significant improvement of estimated VO2max, RV/left ventricle ratio, and health-related QoL in the exercise training group, while no improvement was found in the control group [Citation106]. The second study showed a greater improvement in incremental Shuttle Walk Test and PE-specific QoL for the exercise group compared to the control group. However, group differences were small.[Citation102] A potential explanation for the less than convincing findings of these two studies was that unselected post-PE patients without considering persistent symptoms were included, potentially diluting the effects of early exercise training. The currently ongoing PE@HOME study (Dutch trial register NL9615) is randomizing acute PE patients with persistent symptoms and function limitation after 2–3 weeks (i.e. MRC ≥2 and PVFS ≥ 2) to an 8-week home-based exercise program versus no exercise program. This study will provide more knowledge on optimal patient counseling regarding prevention of post-PE syndrome.
6. Expert opinion
We have discussed four important aspects of acute PE management that are still subject of debate and research (). When treating a patient with acute PE, the first step should be the assessment of the need for reperfusion treatment. We argue that the first-line treatment of intermediate-high-risk PE outside clinical trials remains anticoagulant treatment. Full-dose systemic thrombolysis is associated with a too high risk of major bleeding to be considered as primary treatment in this patient category; CDT cannot be recommended yet as randomized studies, using relevant clinical outcomes, are lacking. Only if intermediate-high-risk patients show progress to hemodynamic instability or obstructive shock despite adequate anticoagulant treatment, systemic thrombolytic treatment or CDT should be considered as rescue treatment [Citation4,Citation16]. Decisions regarding rescue treatment are best discussed in a PERT to facilitate consistent decision-making. Reduced dose systemic thrombolysis, catheter-directed thrombolysis, and mechanical thrombectomy are currently being evaluated in large, randomized studies. Results from these trials will provide us with more information regarding the future role of primary reperfusion treatment for hemodynamically stable acute intermediate-high PE patients.
In those patients not requiring reperfusion treatment, the need for anticoagulant treatment should be weighed. There are several arguments as to why SSPE may potentially be left untreated. When considering not starting anticoagulant treatment in an SSPE patient, the following should be considered (1) the universal SSPE definition should be used, confirmed by an experienced radiologist, (2) patients with risk factors for recurrent VTE (e.g. pregnancy, cancer, trauma, recent surgery, prior VTE, and antiphospholipid syndrome), or patients presenting with hypoxemia should receive treatment if the bleeding risk is acceptable, and (3) SSPE patients with a simultaneous DVT should receive anticoagulation as well. Excluding non-symptomatic DVT in SSPE patients using the same diagnostic strategy to exclude symptomatic DVT in a patient without SSPE is therefore advised. There is no evidence for the additional value of venography or ultrasonography of pelvic veins in SSPE patients. However, since compression ultrasonography is the cornerstone of DVT diagnosis in patients without SSPS, we also advise performing a bilateral compression ultrasonography to exclude DVT in SSPE patients. The currently ongoing safe-SSPE study will hopefully provide more precise guidance in the management of SSPE patients [Citation54].
After confirmation of the indication for anticoagulant treatment, the need for hospitalization should be determined. Outpatient treatment of acute PE is safe, cost-effective, and results in a high level of patient satisfaction. When selecting eligible patients for outpatient treatment, the Hestia criteria or sPESI can be used, with or without assessment of RV dysfunction. In our practice, we apply the Hestia criteria. sPESI is an alternative clinical decision rule, although it was designed as a prediction score for all-cause death rather than a clinical tool to evaluate potential home-treatment. In the HOME-PE trial 28.5% of the patients with an sPESI of 0 were ultimately hospitalized based on overruling by the treating physicians, highlighting that sPESI therefore should always be combined with other clinical (Hestia like) criteria to evaluate the feasibility of home treatment.
Finally, there is increased awareness of all aspects of the prognosis of PE patients. The ICHOM standard set of outcome measures can help to assess all important patient outcomes. Patients with persistent symptoms and/or functional limitations qualify as PPES. If so, the first priority is to evaluate the presence of CTEPD. For patients with post-PE impairment, dedicated exercise training likely improves QoL and functional abilities. The ongoing PE@HOME studywill give us more insight into the role of exercise training initiated shortly after PE diagnosis in the prevention of PPES. There is currently no evidence on the relationship between different types of anticoagulant treatment or treatment adherence and the development of PPES.
Article highlights
Hemodynamically stable acute PE patients should not receive reperfusion therapy as primary treatment.
Subsegmental PE may be left untreated in selected low risk patients, after proximal deep vein thrombosis has been ruled out.
Patients with a Hestia score or sPESI score of 0, can be treated at home, without explicit evaluation of the RV function.
Chronic thromboembolic pulmonary disease (CTEPD) should be diagnosed as early as possible.
Patients with post-PE syndrome without CTEPD benefit from exercise training or cardiopulmonary rehabilitation.
Declaration of interest
FA Klok reports grants or contracts from Bayer, BMS, BSCI, MSD, Leo Pharma, Actelion, Pharm-X, The Netherlands Organisation for Health Research and Development, The Dutch Thrombosis Association, The Dutch Heart Foundation, and the Horizon Europe Program, all unrelated to this work and paid to his institution. MV Huisman reports grants from The Netherlands Organisation for Health Research and Development (ZonMW) and Dutch Heart Foundation, grants from Boehringer-Ingelheim, grants from Pfizer-BMS, and grants from Bayer Health Care all outside the submitted work. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Huisman MV, Barco S, Cannegieter SC, et al. Pulmonary embolism. Nat Rev Dis Primers. 2018 May 17;4(1):18028.
- Barco S, Mahmoudpour SH, Valerio L, et al. Trends in mortality related to pulmonary embolism in the European Region, 2000–15: analysis of vital registration data from the WHO Mortality Database. Lancet Respir Med. 2020 Mar;8(3):277–287.
- Dronkers CE, Klok FA, Huisman MV. Current and future perspectives in imaging of venous thromboembolism. J Thromb Haemost. 2016 Sep;14(9):1696–1710.
- Konstantinides SV, Meyer G, Becattini C, et al. ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Task Force for the diag manage of acute pulm embol of the Eur Heart J (ESC). 2019;1901647
- Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370(15):1402–1411.
- Ortel TL, Neumann I, Ageno W, et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020 Oct 13;4(19):4693–4738.
- Barco S, Vicaut E, Klok FA, et al. Improved identification of thrombolysis candidates amongst intermediate-risk pulmonary embolism patients: implications for future trials. Eur Respir J. 2018;51(1):1701775.
- Hariharan P, Dudzinski DM, Rosovsky R, et al. Relation among clot burden, right-sided heart strain, and adverse events after acute pulmonary embolism. Am J Cardiol. 2016 Nov 15;118(10):1568–1573.
- Levine M, Hirsh J, Weitz J, et al. A randomized trial of a single bolus dosage regimen of recombinant tissue plasminogen activator in patients with acute pulmonary embolism. Chest. 1990 Dec;98(6):1473–1479.
- Sharifi M, Bay C, Skrocki L, et al. Moderate pulmonary embolism treated with thrombolysis (from the “MOPETT” Trial). Am J Cardiol. 2013 Jan 15;111(2):273–277.
- Goldhaber SZ, Agnelli G, Levine MN. Reduced dose bolus alteplase vs conventional alteplase infusion for pulmonary embolism thrombolysis: an international multicenter randomized trial. Chest. 1994 Sep 01;106(3):718–724.
- Sors H, Pacouret G, Azarian R, et al. Hemodynamic effects of bolus vs 2-h infusion of alteplase in acute massive pulmonary embolism. A randomized controlled multicenter trial. Chest. 1994 Sep;106(3):712–717.
- Wang C, Zhai Z, Yang Y, et al. Efficacy and safety of low dose recombinant tissue-type plasminogen activator for the treatment of acute pulmonary thromboembolism: a randomized, multicenter, controlled trial. Chest. 2010 Feb;137(2):254–262.
- Jimenez D, Martin-Saborido C, Muriel A, et al. Efficacy and safety outcomes of recanalisation procedures in patients with acute symptomatic pulmonary embolism: systematic review and network meta-analysis. Thorax. 2018 May;73(5):464–471.
- Sanchez O, Charles-Nelson A, Ageno W, et al. Reduced-dose intravenous thrombolysis for acute intermediate-high-risk pulmonary embolism: rationale and design of the pulmonary embolism international Thrombolysis (PEITHO)-3 trial. Thromb Haemost. 2022 May;122(5):857–866.
- Pruszczyk P, Klok FA, Kucher N, et al. Percutaneous treatment options for acute pulmonary embolism: a clinical consensus statement by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function and the European Association of Percutaneous Cardiovascular Interventions. EuroIntervention. 2022 Oct 7;18(8):e623–e638.
- Kucher N, Boekstegers P, Müller OJ, et al. Randomized, controlled trial of ultrasound-assisted catheter-directed thrombolysis for acute intermediate-risk pulmonary embolism. Circulation. 2014 Jan 28;129(4):479–486.
- Piazza G, Hohlfelder B, Jaff MR, et al. A prospective, single-arm, multicenter trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submassive pulmonary embolism: the Seattle II study. JACC Cardiovasc Interv. 2015 Aug 24;8(10):1382–1392.
- Tapson VF, Sterling K, Jones N, et al. A Randomized trial of the optimum duration of acoustic pulse thrombolysis procedure in acute intermediate-risk pulmonary embolism: the OPTALYSE PE trial. JACC Cardiovasc Interv. 2018 Jul 23;11(14):1401–1410.
- Avgerinos ED, Jaber W, Lacomis J, et al. Randomized trial comparing standard versus ultrasound-assisted thrombolysis for submassive pulmonary embolism: the SUNSET sPE trial. JACC Cardiovasc Interv. 2021 Jun 28;14(12):1364–1373.
- Sista AK, Horowitz JM, Tapson VF, et al. Indigo aspiration system for treatment of pulmonary embolism: results of the EXTRACT-PE trial. JACC Cardiovasc Interv. 2021 Feb 8;14(3):319–329.
- Tu T, Toma C, Tapson VF, et al. A prospective, single-arm, multicenter trial of catheter-directed mechanical thrombectomy for intermediate-risk acute pulmonary embolism: the FLARE study. JACC Cardiovasc Interv. 2019 May 13;12(9):859–869.
- Klok FA, Piazza G, Sharp ASP, et al. Ultrasound-facilitated, catheter-directed thrombolysis vs anticoagulation alone for acute intermediate-high-risk pulmonary embolism: rationale and design of the HI-PEITHO study. Am Heart J. 2022 Sep;251:43–53.
- The PEERLESS Study. https://ClinicalTrials.gov/show/NCT05111613.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest. 2006 Apr;129(4):1043–1050.
- den Exter PL, Kroft LJM, Gonsalves C, et al. Establishing diagnostic criteria and treatment of subsegmental pulmonary embolism: a Delphi analysis of experts. Res Pract Thromb Haemost. 2020 Nov;4(8):1251–1261.
- Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011 May 9;171(9):831–837.
- Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. Bmj. 2013 Jul 2;347(jul02 2):f3368.
- Carrier M, Klok FA. Symptomatic subsegmental pulmonary embolism: to treat or not to treat? Hematol Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):237–241.
- den Exter PL, van Es J, Klok FA, et al. Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism. Blood. 2013 Aug 15;122(7):1144–1149. quiz 1329
- Swan D, Hitchen S, Klok FA, et al. The problem of under-diagnosis and over-diagnosis of pulmonary embolism. Thromb Res. 2019 May;177:122–129.
- van der Pol LM, Bistervels IM, van Mens TE, et al. Lower prevalence of subsegmental pulmonary embolism after application of the YEARS diagnostic algorithm. Br J Haematol. 2018 Nov;183(4):629–635.
- Goodman LR. Small pulmonary emboli: what do we know? Radiology. 2005 Mar;234(3):654–658.
- Ghanima W, Nielssen BE, Holmen LO, et al. Multidetector computed tomography (MDCT) in the diagnosis of pulmonary embolism: interobserver agreement among radiologists with varied levels of experience. Acta Radiol. 2007 Mar;48(2):165–170.
- Pena E, Kimpton M, Dennie C, et al. Difference in interpretation of computed tomography pulmonary angiography diagnosis of subsegmental thrombosis in patients with suspected pulmonary embolism. J Thromb Haemost. 2012 Mar;10(3):496–498.
- Miller WT Jr., Marinari LA, Barbosa E Jr., et al. Small pulmonary artery defects are not reliable indicators of pulmonary embolism. Ann Am Thorac Soc. 2015 Jul;12(7):1022–1029.
- Gurney JW. No fooling around: direct visualization of pulmonary embolism. Radiology. 1993 Sep;188(3):618–619.
- Schoepf UJ, Costello P. CT angiography for diagnosis of pulmonary embolism: state of the art. Radiology. 2004 Feb;230(2):329–337.
- Goy J, Lee J, Levine O, et al. Sub-segmental pulmonary embolism in three academic teaching hospitals: a review of management and outcomes. J Thromb Haemost. 2015 Feb;13(2):214–218.
- Mehta D, Barnett M, Zhou L, et al. Management and outcomes of single subsegmental pulmonary embolus: a retrospective audit at North Shore Hospital, New Zealand. Intern Med J. 2014 Sep;44(9):872–876.
- Donato AA, Khoche S, Santora J, et al. Clinical outcomes in patients with isolated subsegmental pulmonary emboli diagnosed by multidetector CT pulmonary angiography. Thromb Res. 2010 Oct;126(4):e266–70.
- Cha SI, Shin KM, Lee JW, et al. Clinical characteristics of patients with peripheral pulmonary embolism. Respiration. 2010;80(6):500–508.
- Eyer BA, Goodman LR, Washington L. Clinicians’ response to radiologists’ reports of isolated subsegmental pulmonary embolism or inconclusive interpretation of pulmonary embolism using MDCT. AJR Am J Roentgenol. 2005 Feb;184(2):623–628.
- Le Gal G, Kovacs MJ, Bertoletti L, et al. Risk for recurrent venous thromboembolism in patients with subsegmental pulmonary embolism managed without anticoagulation. Ann Intern Med. 2021 [2022 Jan 18];175(1):29–35.
- van der Hulle T, den Exter PL, Planquette B, et al. Risk of recurrent venous thromboembolism and major hemorrhage in cancer-associated incidental pulmonary embolism among treated and untreated patients: a pooled analysis of 926 patients. J Thromb Haemost. 2016 Jan;14(1):105–113.
- Prandoni P, Noventa F, Ghirarduzzi A, et al. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica. 2007 Feb;92(2):199–205.
- Iorio A, Kearon C, Filippucci E, et al. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Arch Intern Med. 2010 Oct 25;170(19):1710–1716.
- den Exter PL, Kroft LJ, van der Hulle T, et al. Embolic burden of incidental pulmonary embolism diagnosed on routinely performed contrast-enhanced computed tomography imaging in cancer patients. J Thromb Haemost. 2013 Aug;11(8):1620–1622.
- den Exter PL, van der Hulle T, Hartmann IJ, et al. Reliability of diagnosing incidental pulmonary embolism in cancer patients. Thromb Res. 2015 Sep;136(3):531–534.
- Klok FA, Huisman MV. Management of incidental pulmonary embolism. Eur Respir J. 2017 Jun;49(6):1700275.
- Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016 Feb;149(2):315–352.
- Stein PD, Goodman LR, Hull RD, et al. Diagnosis and management of isolated subsegmental pulmonary embolism: review and assessment of the options. Clin Appl Thromb Hemost. 2012 January-February;18(1):20–26.
- Jiménez D, Aujesky D, Díaz G, et al. Prognostic significance of deep vein thrombosis in patients presenting with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2010 May 1;181(9):983–991.
- Baumgartner C, Klok FA, Carrier M, et al. Clinical Surveillance vs. Anticoagulation For low-risk patiEnts with isolated SubSegmental Pulmonary Embolism: protocol for a multicentre randomised placebo-controlled non-inferiority trial (SAFE-SSPE). BMJ Open. 2020;10(11):e040151.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med. 2005 Oct 15;172(8):1041–1046.
- Jiménez D, Aujesky D, Moores L, et al. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med. 2010 Aug 9;170(15):1383–1389.
- Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open-label, randomised, non-inferiority trial. Lancet. 2011 Jul 2;378(9785):41–48.
- Zondag W, Hiddinga BI, Crobach MJT, et al. Hestia criteria can discriminate high- from low-risk patients with pulmonary embolism. Eur Respir J. 2013;41(3):588–592.
- Zondag W, Mos IC, Creemers-Schild D, et al. Outpatient treatment in patients with acute pulmonary embolism: the Hestia Study. J Thromb Haemost. 2011 Aug;9(8):1500–1507.
- den Exter PL, Zondag W, Klok FA, et al. Efficacy and safety of outpatient treatment based on the Hestia clinical decision rule with or without N-terminal pro-brain natriuretic peptide testing in patients with acute pulmonary embolism. A randomized clinical trial. Am J Respir Crit Care Med. 2016 Oct 15;194(8):998–1006.
- Roy PM, Penaloza A, Hugli O, et al. Triaging acute pulmonary embolism for home treatment by Hestia or simplified PESI criteria: the HOME-PE randomized trial. Eur Heart J. 2021 Aug 31;42(33):3146–3157.
- Barco S, Mahmoudpour SH, Planquette B, et al. Prognostic value of right ventricular dysfunction or elevated cardiac biomarkers in patients with low-risk pulmonary embolism: a systematic review and meta-analysis. Eur Heart J. 2019 Mar 14;40(11):902–910.
- Barco S, et al. 2020. Early discharge and home treatment of patients with low-risk pulmonary embolism with the oral factor Xa inhibitor rivaroxaban: an international multicentre single-arm clinical trial. Eur Heart J. 41(4):509–518. doi:10.1093/eurheartj/ehz367.
- Hendriks SV, Klok FA, den Exter PL, et al. Right ventricle-to-left ventricle diameter ratio measurement seems to have no role in low-risk patients with pulmonary embolism treated at home triaged by Hestia criteria. Am J Respir Crit Care Med. 2020 Jul 1;202(1):138–141.
- Hendriks SV, Lankeit M, den Exter PL, et al. Uncertain value of high-sensitive troponin T for selecting patients with acute pulmonary embolism for outpatient treatment by Hestia criteria. Acad Emerg Med. 2020 Oct;27(10):1043–1046.
- Hendriks SV, Bavalia R, van Bemmel T, et al. Current practice patterns of outpatient management of acute pulmonary embolism: a post-hoc analysis of the YEARS study. Thromb Res. 2020 Sep;193:60–65.
- Hendriks SV, van den Hout WB, van Bemmel T, et al. Home treatment compared to initial hospitalization in normotensive patients with acute pulmonary embolism in the Netherlands: a cost analysis. Thromb Haemost. 2022 Mar;122(3):427–433.
- Bledsoe JR, Woller SC, Stevens SM, et al. Management of low-risk pulmonary embolism patients without hospitalization: the low-risk pulmonary embolism prospective management study. Chest. 2018 Aug;154(2):249–256.
- Sista AK, Miller LE, Kahn SR, et al. Persistent right ventricular dysfunction, functional capacity limitation, exercise intolerance, and quality of life impairment following pulmonary embolism: systematic review with meta-analysis. Vasc Med. 2017 Feb;22(1):37–43.
- Klok FA, Barco S. Follow-up after acute pulmonary embolism. Hamostaseologie. 2018 Feb;38(1):22–32.
- Klok FA, van der Hulle T, den Exter PL, et al. The post-PE syndrome: a new concept for chronic complications of pulmonary embolism. Blood Rev. 2014 Nov;28(6):221–226.
- Boon GJAM, Huisman MV, Klok FA. Determinants and management of the post-pulmonary embolism syndrome. Semin Respir Crit Care Med. 2021 Feb 6;42(2):299–307.
- Klok FA, Ageno W, Ay C, et al. Optimal follow-up after acute pulmonary embolism: a position paper of the European Society of Cardiology Working Group on Pulmonary Circulation and Right Ventricular Function, in collaboration with the European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology, endorsed by the European Respiratory Society. Eur Heart J. 2022 Jan 25;43(3):183–189.
- Kahn SR, Hirsch AM, Akaberi A, et al. Functional and exercise limitations after a first episode of pulmonary embolism: results of the ELOPE prospective cohort study. Chest. 2017 May;151(5):1058–1068.
- Klok FA, van Kralingen KW, van Dijk AP, et al. Prevalence and potential determinants of exertional dyspnea after acute pulmonary embolism. Respir Med. 2010 Nov;104(11):1744–1749.
- Le Gal GCM, Castellucci LA, Castellucci LA, et al. for the ISTHCDE Task Force. Development and implementation of common data elements for venous thromboembolism research: officialCommunication from the SSC of the ISTH. J Thromb Haemost. 2021;19(1):297–303.
- Boon G, Bogaard HJ, Klok FA. Essential aspects of the follow-up after acute pulmonary embolism: an illustrated review. Res Pract Thromb Haemost. 2020 Aug;4(6):958–968.
- Klok FA, Barco S, Konstantinides SV, et al. Determinants of diagnostic delay in chronic thromboembolic pulmonary hypertension: results from the European CTEPH Registry. Eur Respir J. 2018 Dec;52(6):1801687.
- Guérin L, Couturaud F, Parent F, et al. Prevalence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Prevalence of CTEPH after pulmonary embolism. Thromb Haemost. 2014 Sep 2;112(3):598–605.
- Klok FA, Barco S, Siegerink B. Measuring functional limitations after venous thromboembolism: a call to action. Thromb Res. 2019 Jun;178:59–62.
- Boon GJAM, Barco S, Bertoletti L, et al. Measuring functional limitations after venous thromboembolism: optimization of the Post-VTE Functional Status (PVFS) Scale. Thromb Res. 2020 Jun;190:45–51.
- Gwozdz AM, de Jong CMM, Fialho LS, et al. Development of an international standard set of outcome measures for patients with venous thromboembolism: an International Consortium for Health Outcomes Measurement consensus recommendation. Lancet Haematol. 2022 Sep 01;9(9):e698–e706.
- Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2020 Dec 17;57(6):2002828.
- Boon GJAM, van den Hout WB, Barco S, et al. A model for estimating the health economic impact of earlier diagnosis of chronic thromboembolic pulmonary hypertension. ERJ Open Res. 2021;7(3):00719–2020.
- Humbert M, Kovacs G, Hoeper MM, et al. ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: developed by the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by the International Society for Heart and Lung Transplantation (ISHLT) and the European Reference Network on rare respiratory diseases (ERN-LUNG). Eur Heart J. 2022;43(38):3618–3731.
- Delcroix M, Torbicki A, Gopalan D, et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021 Jun;57(6):2002828.
- Klok FA, Surie S, Kempf T, et al. A simple non-invasive diagnostic algorithm for ruling out chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Thromb Res. 2011 Jul;128(1):21–26.
- Klok FA, Dzikowska‐Diduch O, Kostrubiec M, et al. Derivation of a clinical prediction score for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. J Thromb Haemost. 2016 Jan;14(1):121–128.
- Boon G, Ende-Verhaar YM, Bavalia R, et al. Non-invasive early exclusion of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: the InShape II study. Thorax. Published Online First 2021 Mar 23.
- Ende-Verhaar YM, Huisman MV, Klok FA. To screen or not to screen for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. Thromb Res. 2017 Mar;151:1–7.
- Ende-Verhaar YM, Ruigrok D, Bogaard HJ, et al. Sensitivity of a simple noninvasive screening algorithm for chronic thromboembolic pulmonary hypertension after acute pulmonary embolism. TH Open. 2018 Jan;2(1):e89–e95.
- Ende-Verhaar YM, Meijboom LJ, Kroft LJM, et al. Usefulness of standard computed tomography pulmonary angiography performed for acute pulmonary embolism for identification of chronic thromboembolic pulmonary hypertension: results of the InShape III study. J Heart Lung Transplant. 2019 Jul;38(7):731–738.
- Boon G, Jairam PM, Groot GMC, et al. Identification of chronic thromboembolic pulmonary hypertension on CTPAs performed for diagnosing acute pulmonary embolism depending on level of expertise. Eur J Intern Med. 2021 Jul;93:64–70.
- Boon G, Ende-Verhaar YM, Beenen LFM, et al. Prediction of chronic thromboembolic pulmonary hypertension with standardised evaluation of initial computed tomography pulmonary angiography performed for suspected acute pulmonary embolism. Eur Radiol. 2022 Apr;32(4):2178–2187.
- Braams NJ, Boon G, de Man FS, et al. Evolution of CT findings after anticoagulant treatment for acute pulmonary embolism in patients with and without an ultimate diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2021 Dec;58(6):2100699.
- Albaghdadi MS, Dudzinski DM, Giordano N, et al. Cardiopulmonary exercise testing in patients following massive and submassive pulmonary embolism. J Am Heart Assoc. 2018 Mar 3;7(5). DOI:10.1161/JAHA.117.006841.
- Kirchberger I, Ruile S, Linseisen J, et al. The lived experience with pulmonary embolism: a qualitative study using focus groups. Respir Med. 2020 Jun;167:105978.
- Danielsbacka JS, Rostberg L, Olsén MF, et al. “Whole life changed” - experiences of how symptoms derived from acute pulmonary embolism affects life. A qualitative interview study. Thromb Res. 2021 Sep;205:56–62.
- Boon GJAM, Janssen SMJ, Barco S, et al. Efficacy and safety of a 12-week outpatient pulmonary rehabilitation program in Post-PE Syndrome. Thromb Res. 2021 Aug 17;206:66–75.
- Nopp S, Klok FA, Moik F, et al. Outpatient pulmonary rehabilitation in patients with persisting symptoms after pulmonary embolism. J Clin Med. 2020 Jun 10;9(6):1811.
- Cires-Drouet RS, Mayorga-Carlin M, Toursavadkohi S, et al. Safety of exercise therapy after acute pulmonary embolism. Phlebology. 2020 Jul 27;35(10):824–832.
- Rolving N, Brocki BC, Bloch-Nielsen JR, et al. Effect of a physiotherapist-guided home-based exercise intervention on physical capacity and patient-reported outcomes among patients with acute pulmonary embolism: a randomized clinical trial. JAMA network open. 2020 Feb 5;3(2):e200064.
- Amoury M, Noack F, Kleeberg K, et al. Prognosis of patients with pulmonary embolism after rehabilitation. Vasc Health Risk Manag. 2018;14:183–187.
- Noack F, Schmidt B, Amoury M, et al. Feasibility and safety of rehabilitation after venous thromboembolism. Vasc Health Risk Manag. 2015;11:397–401.
- Lakoski SG, Savage PD, Berkman AM, et al. The safety and efficacy of early-initiation exercise training after acute venous thromboembolism: a randomized clinical trial. J Thromb Haemost. 2015 Jul;13(7):1238–1244.
- Ghram A, Jenab Y, Soori R, et al. High-intensity interval training in patients with pulmonary embolism: a randomized controlled trial. Med Sci Sports Exerc. 2021;53(10):2037–2044.