Abstract
Hospitalizations for acute heart failure syndromes (AHFS) are associated with high post-discharge morbidity and mortality. The potential role of vasopressin antagonists (VA) in AHFS was presented at the 2008 European Society of Cardiology Working Group on Acute Cardiac Care Meeting held in Versailles, France from 25–28 October 2008. This report represents a summary of the presentation at this meeting.
Acute heart failure syndromes (AHFS) resulting in hospitalization are associated with a very high post-discharge mortality and re-hospitalization for heart failure (HF) in spite of the available medical therapies Citation[1]. The potential role of vasopressin antagonists (VA) in AHFS was discussed at the 2008 European Society of Cardiology Working Group on Acute Cardiac Care Meeting held in Versailles, France from 25–28 October 2008. This report represents a summary of the presentation at this meeting.
Vasopressin in heart failure
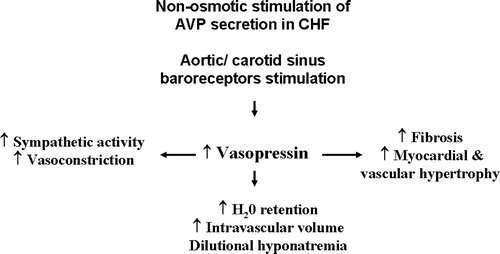
HF is associated with abnormal levels of circulating neurohormones that may contribute to its progression. One of such circulating neurohormones is vasopressin, which is known to be elevated in acute and chronic HF. This elevation is proportional to the severity of HF Citation[2]. High levels of vasopressin may contribute to cardiac remodeling and that negatively affects prognosis in HF (). In addition, elevated levels of vasopressin are also responsible for water retention and dilutional hyponatremia Citation[3].
Rationale for developing vasopressin antagonists in AHFS
There are three important reasons for studying and developing VA for the management of AHFS. These are related to congestion, the limitation of therapy with loop diuretics, and hyponatremia.
Congestion
Congestion and not low cardiac output is the reason for admission and re-admission.Citation[4] Patients with AHFS commonly present with signs and symptoms of both systemic and pulmonary congestion at admission Citation[4]. Hemodynamic congestion (i.e. elevated left ventricular (LV) pressures) which may be present several days before clinical congestion (dyspnea, edema) develops, leads to subendocardial ischemia, alterations in LV morphology (increased sphericity) resulting in secondary mitral insufficiency and diastolic dysfunction as a result of a decreased lymphatic drainage into the right atrium. The available data suggest that many patients admitted for AHFS are discharged with congestive symptoms and often have little or no weight loss during hospitalization Citation[5], Citation[6].
Loop Diuretics
Although loop diuretics are the mainstay of treatment for congestion, they are associated with side effects which limit their use. Non-potassium sparing diuretics have the ability to provide a rapid improvement in symptoms and decrease volume overload However, they are associated with undesirable effects, such as further stimulation of neurohormones, electrolyte disturbances, worsening renal function and possibly increasing hospitalization and mortality, particularly when higher doses are prescribed Citation[7]. This increase in event rate may be related to worsening renal function in response to high doses of diuretics, which in effect promotes fluid retention and further progression of HF.
Hyponatremia
Mild hyponatremia is common (approximately 25%) in patients admitted with AHFS irrespective of systolic function. Hyponatremia is usually not corrected during the hospital stay and is associated with longer hospital stays and higher in-hospital and early post-discharge mortality Citation[8]. This is in spite of the fact that these patients have similar clinical and hemodynamic improvements during hospitalization when compared to normonatremic patients Citation[9].
Vasopressin (AVP, ADH)
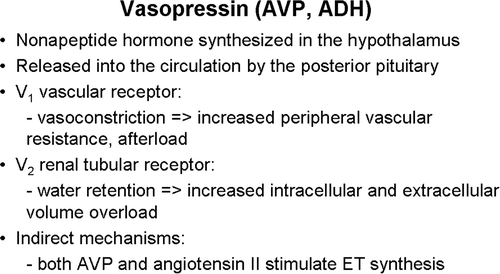
Vasopressin is a hormone synthesized in the hypothalamus and released into the circulation by the posterior pituitary. Vasopressin V1 vascular receptors are responsible for vasoconstriction and an increase in afterload and V2 renal tubular receptors are associated with water retention and subsequent volume overload Citation[3] ().
Contemporary vasopressin antagonists:
There are more than 10 VA in different stages of development Citation[10–12]. Among them the three VA that have been used more in clinical trials are Citation[1] conivaptan, an intravenous dual V1/V2 receptor antagonist, Citation[2] tolvaptan, an oral primarily V2 receptor antagonist and Citation[3] lixivaptan, an even more potent V2 receptor inhibitor.
Hemodynamics
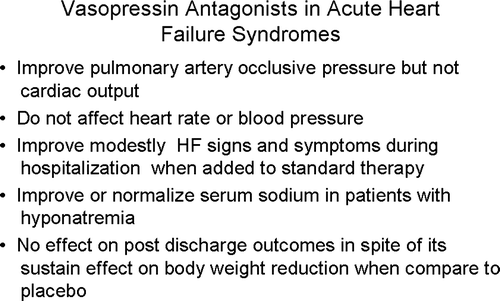
Conivaptan has been associated with a minor reduction in pulmonary capillary wedge pressure (PCWP) Citation[13]. The effect of tolvaptan on hemodynamic parameters in subjects with heart failure (ECLIPSE) trial also revealed a similar reduction in PCWP Citation[14]. However, both conivaptan and tolvaptan did not affect cardiac output, blood pressure or heart rate. The use of tolvaptan in ECLIPSE was also associated with an increase in serum osmolality and an increase in urine output.
Hyponatremia
Ghali et al observed that oral conivaptan successfully corrects serum sodium in patients with euvolemic or hypervolemic hyponatremia Citation[15]. In another study, Zeltser et al. assessed the efficacy and safety of intravenous conivaptan in a similar population and also found that its use was associated with an increase in serum sodium without significant adverse effects.Citation[16] Tolvaptan has also been shown to correct effectively and safely hyponatremia more effectively than fluid restriction Citation[6], Citation[17], Citation[18]. The SALT I and II (study of ascending levels of tolvaptan in hyponatremia) trials observed that tolvaptan improved serum sodium and hyponatremia on day four and at the end of 30 days of therapy for many conditions, such as chronic HF, cirrhosis, or the syndrome of inappropriate antidiuretic hormone secretion (SIADH) in association with hyponatremia Citation[18]. VA, particularly tolvaptan, appears to be a very effective therapy for improvement and normalization of serum sodium in those patients with hyponatremia of various etiologies. However, to date, this improvement of hyponatremia has not been translated into improvement in long-term clinical outcomes.
Body weight
In healthy individuals, VA do not typically change body weight, but tolvaptan has been associated with weight loss in patients with mild HF Citation[17], Citation[19]. Gheorghiade et al. observed that the reduction in weight loss was not associated with changes in blood pressure, heart rate or renal function Citation[19].
Myocardial remodeling
The METEOR (multicenter, randomized, double-blind, placebo controlled, efficacy study on the effects of tolvaptan on left ventricular dilatation in congestive heart failure patients) study examined the effects of tolvaptan on LV remodeling. The investigators enrolled HF patients with reduced systolic function and monitored changes in LV volumes over time. The authors found no significant effect of tolvaptan therapy on LV volumes observed during one year of therapy despite the theoretical disadvantage of the unopposed V1 receptor activation. However, in retrospect, in this study, tolvaptan appeared to reduce in the combined endpoint of mortality and HF hospitalization Citation[20].
Acute and Chronic heart failure
The acute and chronic therapeutic impact of a vasopressin antagonist in congestive heart failure (ACTIV in CHF) trial enrolled 319 patients with LV ejection fraction of less than 40% and hospitalized for HF with persistent signs and symptoms of systemic congestion despite standard therapy in order to evaluate the short and intermediate-term effects of tolvaptan Citation[19]. The investigators demonstrated non-statistically significant improvements in dyspnea, jugular venous distention and edema between day one of hospitalization and discharge as well as a significant decrease in weight during hospitalization without causing changes in heart rate, blood pressure, potassium level and renal function. No differences were found in worsening HF at 60 days follow-up between the tolvaptan and placebo groups. However, in retrospect, it appeared that tolvaptan was associated with a lower mortality in patients with hyponatremia, renal insufficiency and severe congestion.
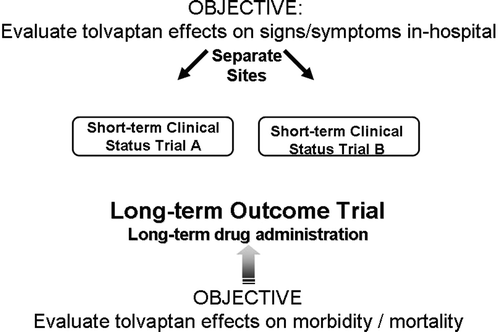
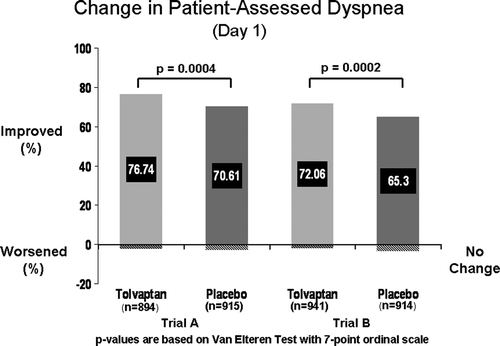
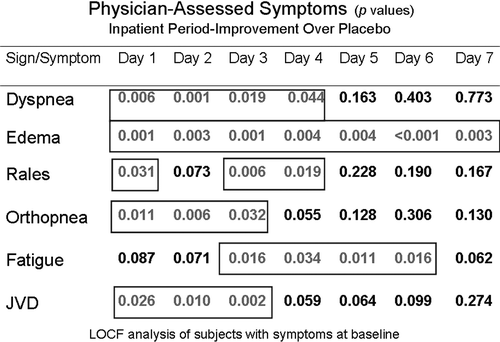
The EVEREST (effects of oral tolvaptan in patients hospitalized for worsening heart failure) trials were event-driven, randomized, double-blind, placebo-controlled studies, which were designed to assess the effects of tolvaptan on patients hospitalized for HF with the primary endpoints being post-discharge all-cause mortality and cardiovascular death or HF hospitalization (). There were also several secondary endpoints including changes in dyspnea, body weight and edema. The study essentially consisted of three trials, with the main study being an event-driven trial conducted post-discharge that evaluated the two primary endpoints Citation[21]. There were also two identical short-term trials (trials A and B) designed to assess the change from baseline as a composite end point in patient-assessed global clinical status and body weight changes at day seven or discharge (if earlier) Citation[22]. The study group consisted of patients hospitalized for worsening HF with a reduced LV ejection fraction (≤40%) and two or more signs of congestion at the time of randomization (jugular venous distention, pitting edema > + 1 or dyspnea) despite initial medical therapy for worsening HF. An important feature of the population is that less than 8% had hyponatremia, which is smaller than other studies and HF registries. The investigators found that patients treated with tolvaptan exhibited improved physician and patient assessed symptoms (a and b), increased serum sodium levels and reductions in body weight and furosemide use during hospitalization without serious adverse effects Citation[22]. However, in spite a post-discharge reduction in body weight and improvement in serum sodium, tolvaptan had no effect on long-term mortality and rehospitalization Citation[21].
In a recently published study, Goldsmith et al reported that in patients admitted with worsening HF conivaptan, a dual vasopressin V1A/V2-receptor antagonist, increased urine output significantly without affecting significantly body weight or symptoms Citation[23].
The therapy with tolvaptan appeared to be relatively safe; however, it was associated with increased thirst, polyuria and a slight increase in creatinine and a decrease in blood urea nitrogen. In addition, rapid correction of severe hyponatremia with VA may potentially cause central pontine myelinolysis.
Conclusions
It appears that VA, particularly tolvaptan, significantly decrease body weight in acute and chronic HF without affecting blood pressure, heart rate, or renal function when added to standard therapy. Tolvaptan when added to standard therapy modestly improves signs and symptoms in patients with reduced ejection fraction admitted for worsening HF. This improvement is short-lived, lasting for only 2-3 days in spite of the fact that tolvaptan continues to decrease body weight and improve serum sodium in hyponatremic patients. In addition, VA appear to effectively improve and normalize serum sodium in hyponatremia of diverse etiology. It is somewhat disappointing that the decrease in body weight and improvement in serum sodium have not translated so far into an improvement in long-term outcomes (mortality, hospitalization) (). These somewhat disappointing results with tolvaptan results may be related to: Citation[1] lack of titration during follow-up (fixed dose); Citation[2] its selectively for specific receptors (i.e. V2 receptor); Citation[3] it not being tested against a diuretic; and or Citation[4] it not being tested in patients who are more likely to respond (i.e. those with hyponatremia). Furthermore, tolvaptan was not tested in patients with HF and preserved systolic function, who represent nearly half of the HF population.
Acknowledgements
Declaration of interest: Mihai Gheorghiade Disclosure: Consultant: Otsuko, Solvay Pharma, Norvartis, Bayer, Sigma Tau, Debiopharm, Medtronic, Merck, Astellas, Cytokinetics, CorThera Inc, Pericor Therapeutics, GlaxoSmithKline, Johnson & Johnson, Abbott, Errekappa Therapeutic, Protein Design Laboratories, Astra Zeneca, Sanofi Aventis. Matthew E. Harinstein and Gerasimos S. Flippatos have no conflicts of interest.
References
- Gheorghiade M, Pang PS. Acute heart failure syndromes. J Am Coll Cardiol. 2009; 53: 557–73
- Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, et al. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (SOLVD). Circulation 1990; 82: 1724–9
- Goldsmith SR, Gheorghiade M. Vasopressin antagonism in heart failure. J Am Coll Cardiol. 2005; 46: 1785–91
- Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006; 119(Suppl 1)S3–S10
- Fonarow GC. The Acute Decompensated Heart Failure National Registry (ADHERE): opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev Cardiovasc Med. 2003; 4(Suppl 7)S21–30
- Gheorghiade M, Gottlieb SS, Udelson JE, Konstam MA, Czerwiec F, Ouyang J, et al. Vasopressin v(2) receptor blockade with tolvaptan versus fluid restriction in the treatment of hyponatremia. Am J Cardiol. 2006; 97: 1064–7
- Yamokoski LM, Hasselblad V, Moser DK, Binanay C, Conway GA, Glotzer JM, et al. Prediction of rehospitalization and death in severe heart failure by physicians and nurses of the ESCAPE trial. J Card Fail. 2007; 13: 8–13
- Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007; 28: 980–8
- Gheorghiade M, Rossi JS, Cotts W, Shin DD, Hellkamp AS, Pina IL, et al. Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial. Arch Intern Med. 2007; 167: 1998–2005
- Farmakis D, Filippatos G, Kremastinos DT, Gheorghiade M. Vasopressin and vasopressin antagonists in heart failure and hyponatremia. Curr Heart Fail Rep. 2008; 5: 91–6
- Farmakis D, Filippatos G, Parissis J, Kremastinos DT, Gheorghiade M. Hyponatremia in heart failure. Heart Fail Rev. 2009; 14: 59–63
- Filippatos G, Parissis JT. Vasopressin antagonists for the treatment of acute decompensated heart failure: When, for whom, for how long, and on what standard therapy?. J Card Fail. 2008; 14: 648–50
- Udelson JE, Smith WB, Hendrix GH, Painchaud CA, Ghazzi M, Thomas I, et al. Acute hemodynamic effects of conivaptan, a dual V(1A) and V(2) vasopressin receptor antagonist, in patients with advanced heart failure. Circulation 2001; 104: 2417–23
- Udelson JE, Orlandi C, Ouyang J, Krasa H, Zimmer CA, Frivold G, et al. Acute hemodynamic effects of tolvaptan, a vasopressin V2 receptor blocker, in patients with symptomatic heart failure and systolic dysfunction: an international, multicenter, randomized, placebo-controlled trial. J Am Coll Cardiol. 2008; 52: 1540–5
- Ghali JK, Koren MJ, Taylor JR, Brooks-Asplund E, Fan K, Long WA, et al. Efficacy and safety of oral conivaptan: a V1A/V2 vasopressin receptor antagonist, assessed in a randomized, placebo-controlled trial in patients with euvolemic or hypervolemic hyponatremia. J Clin Endocrinol Metab. 2006; 91: 2145–52
- Zeltser D, Rosansky S, van Rensburg H, Verbalis JG, Smith N. Assessment of the efficacy and safety of intravenous conivaptan in euvolemic and hypervolemic hyponatremia. Am J Nephrol. 2007; 27: 447–57
- Gheorghiade M, Niazi I, Ouyang J, Czerwiec F, Kambayashi J, Zampino M, et al. Vasopressin V2-receptor blockade with tolvaptan in patients with chronic heart failure: results from a double-blind, randomized trial. Circulation 2003; 107: 2690–6
- Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006; 355: 2099–112
- Gheorghiade, M, Gattis, WA, O'Connor, CM, Adams, KF,Jr, Elkayam, U, Barbagelata, A, , et al Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004;291:1963–71.
- Udelson JE, McGrew FA, Flores E, Ibrahim H, Katz S, Koshkarian G, et al. Multicenter, randomized, double-blind, placebo-controlled study on the effect of oral tolvaptan on left ventricular dilation and function in patients with heart failure and systolic dysfunction. J Am Coll Cardiol. 2007; 49: 2151–9
- Konstam, MA, Gheorghiade, M, Burnett, JC,Jr, Grinfeld, L, Maggioni, AP, Swedberg, K, , et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31.
- Gheorghiade, M, Konstam, MA, Burnett, JC,Jr, Grinfeld, L, Maggioni, AP, Swedberg, K, , et al Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43.
- Goldsmith SR, Elkayam U, Haught WH, Barve A, He W. Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: A dose-ranging pilot study. J Card Fail. 2008; 14: 641–7