Abstract
Purpose
Movement repetition is known to play a key role in promoting functional improvements or maintaining functional levels in post-stroke hemiparetic patients. However, repetitive movements tend to be monotonous, making it challenging for patients to continue. Here, we developed a new gamified system to allow patients perform repetitive movements with enjoyment. The present study aimed to examine the usability of the system in subacute stroke patients.
Method
The exercise system comprised an electromyography-controlled operating system that enabled users to play a virtual game by repetitive finger and wrist movements on the affected side. A total of 13 patients with upper-limb hemiparesis underwent a single bout of exercise using the system and assessed its usability, satisfactoriness, enjoyability, etc. using the System Usability Scale (SUS), Quebec User Evaluation of Satisfaction with assistive Technology (QUEST)-like questionnaire, and numerical rating scale (NRS).
Results
All the participants, who had a wide range of paretic levels, were able to perform the exercise using the system. Participants scored the system a median of 85.0 for SUS and 4.2 for the QUEST-like questionnaire, with an “excellent” in usability and “satisfied” in user satisfaction with the system. The median NRS scores for enjoyability, potential for continuous use, and effectiveness were 8.0, 9.0, and 9.0, respectively, which were greater than the scores for usual rehabilitation training for the upper extremity.
Conclusions
The novel electromyography-controlled gamified exercise system may have sufficient usability and enjoyability to motivate patients with a wide range of paretic levels to perform repetitive finger and wrist movements.
The electromyography-controlled gamified exercise system had overall positive perspectives on the usability of the system.
This exercise system could help motivate patients with a wide range of paretic levels to perform repetitive finger and wrist movements.
IMPLICATIONS FOR REHABILITATION
Introduction
Stroke is a major cause of death and disability, with a global prevalence of approximately 1% [Citation1]. Stroke survivors suffer from a variety of conditions such as motor paralysis, urinary incontinence, dysphagia, and impairments in consciousness and cognitive functions [Citation2]. Hemiparesis in the upper extremity is a serious impairment affecting approximately 80% of post-stroke patients. Patients with upper extremity hemiparesis require assistance in daily living [Citation2], which results in a decrease in their quality of life [Citation3]. The reluctance to use the impaired upper extremity in daily living is common in paretic patients, which is often described as “learned non-use” [Citation4,Citation5]. The increased non-use of the impaired upper extremity and the increased use of the non-paretic side leads to the continued disuse of the impaired upper extremity. Moreover, the decreased use of the upper extremity results in musculoskeletal atrophy, which could trigger the degradation of motor function and further impair the use of the extremity. Therefore, approaching the non-use and functional decline of the upper extremity is important to prevent this negative cycle [Citation6].
Previous studies have shown that intensive and repetitive training are key elements to improving motor impairments [Citation7,Citation8]. Studies in animal models that investigated neural plasticity underlying motor improvement or post-stroke functional recovery required the animals to engage in hundreds of repetitions of movement practice [Citation9,Citation10]. In clinical settings, however, the amount of practice provided during post-stroke rehabilitation is small compared to that in animal models [Citation11]. Although daily self-exercises can help to compensate for the shortage in the amount of training during supervised training with a therapist, it has been shown that only about 30% of patients adhere to the self-exercise program because of the repetitive nature of such programs and the lack of feedback during exercise [Citation12,Citation13].
In recent years, adding game elements ("gamification”) to rehabilitation training has attracted attention as a means of motivating patients to continuously perform exercises. Integrating gaming with training, such as balance exercises, can increase the patient’s enjoyment of the exercise [Citation14]. Enjoyment is related to an intrinsic motivation that can be sustained over time [Citation14,Citation15]. Therefore, the feeling of enjoyment during rehabilitation could be an essential factor that would increase the chances of patients to continuously perform repetitive exercises. Gamified rehabilitation has been rated as excellent for eliciting user motivation, which contributes to continuous active training [Citation16–18]. Indeed, previous studies showed that gamified rehabilitation is suitable for enhancing the repetition of exercise [Citation18] and this, in turn, has greater effects on the improvement of upper extremity function after stroke compared to conventional rehabilitation training [Citation18,Citation19]. However, since most gamified rehabilitation systems are operated via the kinematic information of users (e.g., visible movements) [Citation20], a certain level of upper extremity motor function is required to adapt. This makes it difficult for patients with severe impairments, who cannot exert a sufficient degree of kinematic movements, to operate and benefit from gamified exercises.
To address this issue, we developed a new gamified exercise system that depended on electromyography (EMG)-controlled manoeuvres (i.e., not requiring obvious kinematic movements). The EMG-controlled system was adopted to enable severely paralysed patients, who can only exert a certain level of muscle activity but not sufficient kinematic movements to engage in a gamified rehabilitation exercise. The developed system utilised EMG signals from the forearm muscles on the affected side to achieve game-controlling manoeuvres. The implemented game was designed to promote the repetition of simple movements in the affected fingers and wrist joint. The present study aimed to evaluate the usability of this system in subacute stroke patients. Participants underwent a single bout of training using the system and then answered psychometric questionnaires regarding the usability, satisfactoriness, enjoyability, potential for continuous use, and potential effectiveness of the system as a tool for rehabilitation exercise.
Materials and methods
Participants
Thirteen post-stroke patients (10 men and 3 women; mean age 67.3 ± 12.4 years) were recruited from a rehabilitation ward in Fujita Health University Hospital between June and July 2020. All participants provided written informed consent prior to joining the study. The study protocol was approved by the Committee of Ethics Review at Fujita Health University (approval No. HM19-231), and the experiment was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013. The inclusion criteria were as follows: (1) diagnosis of ischaemic or haemorrhagic stroke; (2) cognitive ability to follow instructions and understand the rules of the task; (3) ability to sit independently; and (4) finger function in Stroke Impairment Assessment Set (SIAS-FF) [Citation21,Citation22] of no less than 1 A, minimal voluntary movement, or mass flexion.
Elements of the developed gamified rehabilitation exercise system
shows an overview of the developed system. Surface EMG signals from the forearm muscles were used to control the actions in a game. The EMG signals were analysed for patterns based on a mathematical model to estimate the types of finger and wrist movements attempted while playing the game ().
Figure 1. An overview of the electromyography-controlled gamified exercise system. A. (i) Muscle activities were measured from the forearm via 12 channels of surface electromyography (EMG). (ii) Attempted movements were estimated by EMG activity patterns based on a mathematical model. (iii) The information of the estimated movements was used to play the game. B. The sleeve-type electrodes. C. Screenshots of the game. The game consists of six stages, from 0 to 5, which have different levels of difficulties. Stage 5 is the most difficult stage.
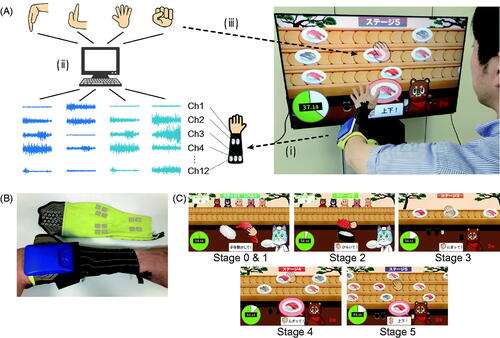
Electromyography measurements
A custom-made elastic sleeve was developed to capture surface EMG signals from the forearm (SEIREN Co., LTD, Fukui, Japan) (). The sleeve was made of interknitted fabric comprising polyurethane elastic yarns and nylon yarns integrated with 12 pairs of wireless surface EMG electrodes (size: 1 cm × 1 cm, SMK Co., Ltd, Tokyo, Japan). The electrodes were placed at equal intervals around the forearm, four pairs at around one-quarter of the length of the forearm from the wrist position, and the remaining 8 pairs at around three-quarters of the aforementioned length. In other words, the location of the EMG electrodes did not necessarily match the belly of specific muscles. Conductive silver-coated textile and silver-based inks were used for the electrodes and wiring.
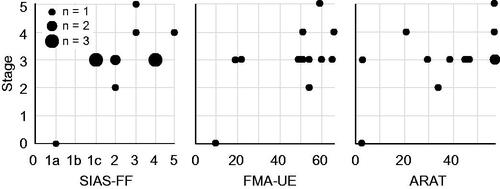
Figure 2. Relationship between the highest playable stage and individual motor impairments or function. SIAS-FF: Stroke Impairment Assessment Set for finger function; FMA-UE: Fugl-Meyer Assessment for upper extremity; ARAT: Action Research Arm Test.
We recorded EMG at 1,000 Hz with a bandpass filter of 20–500 Hz. The EMG was amplified approximately 4,000 times and then transformed to integrated EMG (iEMG) with a down-sampling frequency of 20 Hz by full-wave rectification of the EMG signals that passed through the bandpass filter. The iEMG data was wirelessly transferred to a laptop computer. From the iEMG data, we calculated synergy patterns using non-negative matrix factorisation, and discriminated the attempted finger and wrist movements via k-nearest neighbour density estimation from the synergy pattern [Citation23], such as whole-finger flexion or extension.
Game stages with different degree of difficulties
We developed a computer graphic game in which a user controls a character for making, serving, grabbing, or eating sushi. The actions of a character are controlled by the muscle activity exerted during certain finger and wrist movements. The game is composed of six stages, each requiring users to repeat different sets of movements. The finger and wrist movements required in each stage were designed to accommodate a wide range of hemiparesis severity levels ().
Table 1. The finger and wrist movements required in each stage.
In stages 1 and 2, players were required to repeatedly attempt a set of movements, including whole-finger flexion (stage 1) or extension (stage 2) followed by relaxation, to make and serve sushi. These were intended to facilitate an isolated muscle activity pattern that differed from that of relaxation. Stage 3 required a set of movements of whole-finger flexion followed by extension to grab and eat sushi, with an intention to facilitate a smooth transition between agonistic and antagonistic muscle activities. Stages 4 and 5 were extensions of stage 3, which required wrist flexion and extension to control the position of a virtual hand in addition to a set of the whole-finger flexion and extension as in stage 3. The wrist flexion and extension movements corresponded to changes in the position of the virtual hand to upward and downward, respectively. The number of aiming positions (i.e., lanes, ) was two for stage 4 and three for stage 5, which gradually increased the complexity of game control from stage 3. These stages were intended to promote the execution of isolated muscle activity patterns that differed from each other and to enable a smooth transition between these patterns. If a player was unable to exert reproducible EMG patterns and thus the pattern classification algorithm did not work well even in stage 1, we prepared stage 0, where any discernible EMG signal, decoded as one of the above-mentioned movements, could be used to make and serve sushi as in stage 1.
In each stage, the playing time was set at 60 s and the number of successful game actions was fed back to the participants. Verbal encouragement was provided to participants to repeat the required actions as many times as possible during the playing time.
Evaluation of the exercise system
Participants performed a single bout of the training using the developed system for about 15 min and then answered psychometric questionnaires.
Participants sat on a wheelchair or a chair with a backrest at 1.5 m away from a display. The affected arm was placed on a cushion to place the forearm in a horizontal position. The electrode sleeve was placed on the affected forearm after the electrodes were thoroughly moistened. Before starting the exercise, we performed pre-settings for each individual participant as follows: we recorded EMG activities at rest and those exerted during the four movements – whole-finger flexion and extension, and wrist flexion and extension. The individual EMG patterns were specified (see Electromyography measurements above) which were used as reference models to estimate the attempted movements. We then qualitatively evaluated the accuracy and stability of the estimated movements while participants attempted the four movements. Based on this qualitative evaluation, we determined playable game stages for each participant. For instance, if the dissociation of EMG patterns between whole-finger flexion and extension was poor, the playable game stages for that participant were set at stages 1 and 2. Similarly, if the dissociation of EMG patterns between wrist flexion or extension movements was poor, the playable game stages were set at stages 1, 2, and 3. In contrast, if the quality of dissociation was good, playable game stages were set at stages 1 to 4. Whether to finally play stage 5 or not was determined by the qualitative evaluation of the controllability during stage 4. After completing these pre-settings, participants performed the gamified exercise step by step from stage 1 until the highest playable stage, and finally performed their preferred stage at the end of training.
After performing the exercise, the usability, satisfactoriness, enjoyability, potential for continuous use, and potential effectiveness of the system were evaluated using psychometric questionnaires. For the global evaluation of usability, we used the System Usability Scale (SUS), which is a reliable tool for measuring the usability of a wide variety of products and services [Citation24]. The SUS is a 10-item scale (with a score from 0 to 100) that allows participants to rate their agreement or disagreement on a five-point Likert scale from strongly agree to strongly disagree. Higher scores indicate greater acceptance and usability (ease of use, ease of learning, and confidence in using the product). A score of ≥50 indicates that a user considers the system “OK”, a score of 70 and above indicates “good” usability, and a score of ≥85 indicates “excellent” usability [Citation24].
We further evaluated user satisfaction for each element of the system based on the Quebec User Evaluation of Satisfaction with Assistive Technology (QUEST) questionnaire [Citation25]. We modified the original questionnaire to better evaluate the present system. The following items were included: 1) weight of the elastic sleeve, 2) ease of adjustment of the sleeve, 3) ease of understanding the game rules, 4) sensitivity of the game control system, 5) difficulty of the game, and 6) playing time. The following five-point Likert scale was used for grading: 1, very unsatisfied; 2, unsatisfied; 3, moderately satisfied; 4, satisfied; and 5, very satisfied. The average value of all the items were taken as the overall satisfaction rating of the system, which was interpreted in the same way as the grades explained above.
Subjective enjoyability, potential for continuous use, and potential effectiveness were also assessed using the numerical rating scale (NRS). Participants were asked to assign a score from 0 to 10 by a step of 0.5 for each item, with a referring anchor of “5” set for usual rehabilitation exercise for the upper limb.
To investigate whether the prepared game stages matched the level of impairment and/or function, we performed correlation analysis between the highest playable game stage and impairment (SIAS-FF, Fugl-Meyer Assessment for the upper extremity: FMA-UE [Citation26]), or function (Action Research Arm Test: ARAT [Citation27]). We computed Spearman’s rank-order correlation coefficient (rs) to evaluate the strength of the relationships using SPSS (version 26; IBM, Armonk, NY, USA).
Results
Participants
The characteristics of the participants are presented in . The participants had a wide range of motor impairment and dysfunction in the finger (1 A–5 in SIAS-FF) and upper extremity (10–66 in FMA-UE; 3–57 in ARAT).
Table 2. Participants’ characteristics.
Playable game stage
All the participants were able to play at least one stage using EMG signals associated with finger and/or wrist movements on the affected side (). Although patient No. 1 had difficulty playing stage 1, she/he was able to play stage 0. The highest stage the participants were able to play seemed to match the individual level of motor impairment, especially in the fingers (SIAS-FF, rs = .54, p = .06; FMA-UE, rs = .46, p = .11; ARAT, rs = .45, p = .12, ).
Table 3. Outcomes of subjective assessments and the highest playable stage.
Subjective assessments
A detailed account of the outcomes is shown in . The median SUS score among participants was 85.0 (range: 45.0–100.0). Even with some extent of individual variability, the median score indicated “excellent” usability of the system. Regarding satisfaction, the QUEST-like questionnaire showed a median of 4.2 (range: 3.3–5.0), resulting in a “satisfied” rating. A detailed examination of the sub-items showed a median of 4.0 for all items, demonstrating the absence of obvious flaws in the system. The median NRS score was 8.0 for enjoyability, 9.0 for the potential for continuous use, and 9.0 for potential effectiveness, demonstrating greater scores than that for usual rehabilitation exercise for the upper extremity.
Discussion
We developed an EMG-controlled gamified exercise system that aimed to provide people with motor impairment and dysfunction with opportunities to perform repetitions of movements in the distal upper extremity with enjoyment. The present study evaluated the feasibility of the system in subacute post-stroke patients using subjective assessments. The results revealed that the newly developed system can be operated by patients with a variety of paretic levels, with good usability, satisfaction, and enjoyment. Furthermore, we found that participants had a positive perspective on the potential for the continuous use of the system and its effectiveness on motor function.
One of the important features of the developed system is its manoeuvrability. We implemented EMG-controlled operations so that a participant who could exert a certain level of muscle activity but not obvious kinematic changes could benefit from gamified rehabilitation exercises. Although the number of participants with severe paralysis (FMA-UE≤ 19 points [Citation28]) in the present study was relatively small (n = 2), we confirmed that all participants, including the severely impaired, were able to play at least one stage of the game exercise. Compared with conventional gamified exercises controlled by kinematic movements (FMA-UE range: 42–65 [Citation29], 23–56 [Citation30], and 17–56 [Citation31]), it seems likely that the present system can be adopted as a means of training patients with more severely impairments. The median score of 4.0 per 5.0 for one of the sub-items regarding the sensitivity of the control system in the QUEST-like questionnaire suggests the practical use of the EMG-controlled operation.
At the individual level, however, some variability existed in scores for sensitivity. We assumed that this could be attributed to unstable muscle control with less coordinated muscle activities during exercise. We found, via qualitative observation, that those with relatively lower scores tended to exhibit co-contractions – concurrent activities in the agonist and antagonist muscles. Although quantitative evaluations for EMG patterns were not systematically performed in the present study, this individual observation indicates that EMG-controlled operation may be difficult to adapt well in certain participants who could not exert a stable muscle activity pattern.
The results of subjective assessments demonstrated sufficient satisfaction, usability, and enjoyment with the developed exercise system, indicating positive perspectives on the system. Although the items in the questionnaire were not sufficient to further investigate the underlying factors leading to these positive results, we speculate that some of the following characteristics may have contributed to the results: 1) the rules of the game are simple and easy to understand; 2) the sleeve-type electrodes are easy to put on and take off; 3) the game has friendly and uplifting computer graphics and sounds; and 4) the difficulty of game stages was set properly to fit well with the individual severity of hemiparesis.
Importantly, the NRS score regarding the potential for continuous use was high, indicating that the system is attractive enough to sustain the interest of participants in performing repetitions of even simple movements. One plausible explanation for this is that using the system provokes enough pleasure. In the assessment of the system’s enjoyability, 12 out of the 13 participants assigned higher scores than noted for usual rehabilitation exercise for the upper extremity. Enjoyability has been linked to intrinsic motivation [Citation14,Citation15], defined as the willingness to perform an activity for intrinsic satisfaction, such as interest, enjoyment, and challenge, rather than for a result or reward [Citation32]. Therefore, we assume that a feeling of fun during the exercise may have contributed to the high score regarding the willingness to continue. This high score may also be attributed to a positive perspective on the potential positive effect on motor function. In addition to enjoyment, a positive impact on health effects has been shown to affect motivation to exercise in older people [Citation33]. Previous work has reported that varying the difficulty of the task to suit the disability has positive effects on motivating users and improving their upper extremity function [Citation34]. Since the sub-item in the QUEST-like questionnaire regarding the difficulty of the game showed reasonable value, it is also plausible that appropriate difficulties set for each stage led to a positive perspective on the potential for continuation.
Despite our findings, our study has some limitations. First, the interpretation of the present results requires caution given the sex bias in the recruited participants (10 male and 3 female). Previous work has demonstrated that the factors affecting motivation for participation in physical activity differ across sexes [Citation35]. Therefore, the present result, specifically the high score on the potential for continuous use, is likely to have been biased by the uneven population. Second, the present study did not clarify whether participants would actively engage in exercise using the system when allowed to use it daily. In addition, it remains unclear how much the participants’ positive perspective (i.e., high scores for enjoyability and the potential for continuation) would persist while using the system repetitively. Furthermore, the study did not address whether repetitive training using the present system prevents the decreased use of the affected side, or degradation of motor function. This is only a usability study and the effectiveness of the rehabilitative treatment provided is still lacking. These questions should be addressed in future studies that investigate day-to-day changes in subjective scores while participants perform rehabilitation training using the system.
In conclusion, the present investigation of subacute post-stroke participants demonstrated that users of the EMG-controlled gamified exercise system had overall positive perspectives on the usability of the system. These findings suggest the feasibility of the system in motivating post-stroke patients with a wide range of paretic levels to perform repetitive movements with enjoyment.
Acknowledgements
We would like to thank Mr. Kosuke Otsubo, a software engineer, and Ms. Yui Minemura and Mr. Shinji Kato, computer graphic designers in SPEED Inc., for their significant contribution to developing game elements in the developed system.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Johnson CO, Nguyen M, Roth GA, et al. Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(5):439–458.
- Lawrence ES, Coshall C, Dundas R, et al. Estimates of the prevalence of acute stroke impairments and disability in a multiethnic population. Stroke. 2001;32(6):1279–1284.
- Gunaydin R, Karatepe AG, Kaya T, et al. Determinants of quality of life (QoL) in elderly stroke patients: a short-term follow-up study. Arch Gerontol Geriatr. 2011;53(1):19–23.
- Taub E, Uswatte G, Elbert T. New treatments in neurorehabilitation founded on basic research. Nat Rev Neurosci. 2002;3(3):228–236.
- Taub E, Uswatte G, Mark VW, et al. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42(3):241–256.
- Wolf SL, Winstein CJ, Miller JP, et al. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the EXCITE randomized clinical trial. JAMA. 2006;296(17):2095–2104.
- Arya KN, Pandian S, Verma R, et al. Movement therapy induced neural reorganization and motor recovery in stroke: a review. J Bodyw Mov Ther. 2011;15(4):528–537.
- Takeuchi N, Izumi S. Rehabilitation with poststroke motor recovery: a review with a focus on neural plasticity. Stroke Res Treat. 2013;2013:128641.
- Nudo RJ, Milliken GW, Jenkins WM, et al. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807.
- Nudo RJ, Wise BM, SiFuentes F, et al. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791–1794.
- Lang CE, Macdonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698.
- Hung YX, Huang PC, Chen KT, et al. What do stroke patients look for in game-based rehabilitation: a survey study. Medicine (Baltimore). 2016;95(11):e3032.
- Shaughnessy M, Resnick BM, Macko RF. Testing a model of post-stroke exercise behavior. Rehabil Nurs. 2006;31(1):15–21.
- van der Kooij K, van Dijsseldonk R, van Veen M, et al. Gamification as a sustainable source of enjoyment during balance and gait exercises. Front Psychol. 2019;10:294.
- Vansteenkiste M, Lens W, Deci EL. Intrinsic versus extrinsic goal contents in self-determination theory: another look at the quality of academic motivation. Educ Psychol. 2006;41(1):19–31.
- Lohse K, Shirzad N, Verster A, et al. Video games and rehabilitation: using design principles to enhance engagement in physical therapy. J Neurol Phys Ther. 2013;37(4):166–175.
- Swanson LR, Whittinghill DM. Intrinsic or extrinsic? Using videogames to motivate stroke survivors: a systematic review. Games Health J. 2015;4(3):253–258.
- Karamians R, Proffitt R, Kline D, et al. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. 2020;101(5):885–896.
- Lohse KR, Hilderman CG, Cheung KL, et al. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLoS One. 2014;9(3):e93318.
- Koutsiana E, Ladakis I, Fotopoulos D, et al. Serious gaming technology in upper extremity rehabilitation: scoping review. JMIR Serious Games. 2020;8(4):e19071.
- Chino N, Sonoda S, Domen K, et al. Stroke Impairment Assessment Set (SIAS). A new evaluation instrument for stroke patients. Jpn J Rehabil Med. 1994;31(2):119–125.
- Abdullahi A. Effects of number of repetitions and number of hours of shaping practice during constraint-induced movement therapy: a randomized controlled trial. Neurol Res Int. 2018;2018:1–9.
- Kim Y, Stapornchaisit S, Kambara H, et al. Muscle synergy and musculoskeletal model-based continuous multi-dimensional estimation of wrist and hand motions. J Healthc Eng. 2020;2020:5451219.
- Bangor A, Kortum P, Miller J. Determining what individual sus scores mean: adding an adjective rating scale. J Usability Stud. 2009;4(3):114–123.
- Demers L, Lambrou R, Ska B. The Quebec user evaluation of satisfaction with assistive technology (QUEST 2.0)- an overview and recent progress. Technol Disabil. 2002;14(3):101–105.
- Fugl-Meyer AR, Jaasko L, Leyman I, et al. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31.
- Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res. 1981;4(4):483–492.
- Woodbury ML, Velozo CA, Richards LG, et al. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch Phys Med Rehabil. 2013;94(8):1527–1533.
- Vanbellingen T, Filius SJ, Nyffeler T, et al. Usability of videogame-based dexterity training in the early rehabilitation phase of stroke patients: a pilot study. Front Neurol. 2017;8:654.
- Dodakian L, McKenzie AL, Le V, et al. A home-based telerehabilitation program for patients with stroke. Neurorehabil Neural Repair. 2017;31(10–11):923–933.
- Putrino D, Zanders H, Hamilton T, et al. Patient engagement is related to impairment reduction during digital game-based therapy in stroke. Games Health J. 2017;6(5):295–302.
- Ryan RM, Deci EL. Intrinsic and extrinsic motivations: classic definitions and new directions. Contemp Educ Psychol. 2000;25(1):54–67.
- Subramanian S, Dahl Y, Skjaeret Maroni N, et al. Assessing motivational differences between young and older adults when playing an exergame. Games Health J. 2020;9(1):24–30.
- Colombo R, Pisano F, Mazzone A, et al. Design strategies to improve patient motivation during robot-aided rehabilitation. J Neuroeng Rehab. 2007;4:3.
- Molanorouzi K, Khoo S, Morris T. Motives for adult participation in physical activity: type of activity, age, and gender. BMC Public Health. 2015;15:66.
