The coronavirus disease 2019 (COVID-19) reached pandemic proportions in March 2020 with unexpectedly rapid spread and serious health and social impacts. In the middle of August, the population of infected patients worldwide has risen to more than 20 million with 750 000 deaths. Number of severely affected patients in some countries overwhelms the capacities of hospitals and places insurmountable demands on healthcare systems. Acute respiratory distress syndrome (ARDS), as the most frequent serious complication, has been under thorough investigation since the beginning of the pandemic. Yet, cardiovascular consequences, the other commonly occurring complication contributing to a poor prognosis, has received much less attention and it remains still poorly defined [Citation1].
1. Cardiovascular injury in COVID-19 and potential underlying pathomechanisms
Cardiovascular disorders related to COVID-19 may be considered from different views: cardiovascular disease as a preexisting comorbidity in infected patients and cardiac damage resulting from SARS-CoV-19-related pathologic alterations. Both conditions worsen prognosis [Citation1,Citation2]. The confirmed number of identified cardiovascular comorbidities in confirmed COVID-19 cases varied from 4,2% to 40% and the incidence of acute cardiac injury in the course of the disease ranged from 12% to 23% according to illness severity of COVID-19-patients investigated [Citation1,Citation2]. Moreover, COVID-19 may result in long-term cardiovascular complications such as cardiomyopathy or heart failure due to unrecognized acute heart damage, delayed interventions, suboptimal medical therapy or drug toxicity.
‘Acute myocardial injury’ is characterized by a markedly elevated serum level of high-sensitive cardiac troponin (hs-cTn) or brain natriuretic peptide (BNP)/N-terminal proBNP, while acute myocarditis, acute coronary syndrome, dysrhythmias, and heart failure are presumed to be the underlying pathologies [Citation2–4].
Several pathomechanisms underlie heart damage in SARS-CoV-2 infection. A massive inflammatory cell activation with a damaging cytokine storm leads to acute lung injury in terms of inflammation, increased capillary permeability, edema and hyaline membrane formation resulting in ARDS [Citation5]. Likewise, both the virus-induced cytokines and the direct viral cytotoxic effect may participate in COVID-19-related cardiac impairment [Citation3]. Moreover, oxygen supply-demand imbalance due to infection-stimulated hemodynamic activation, severe respiratory dysfunction with hypoxemia, and inflammation-induced plaque instability with coronary hypoperfusion could result in ischemic heart damage [Citation1,Citation3].
SARS-CoV-2 attaches to a cell membrane-bound peptidase angiotensin converting enzyme 2 (ACE2), expressed abundantly in the lungs, heart, and kidneys, which serves as an entrance receptor for the virus [Citation4]. In the heart, ACE2 is localized in cardiomyocytes, endothelial and smooth muscle cells. Bearing in mind both deleterious and protective effects of the renin angiotensin system, it is conceivable that the observed downregulation of ACE2 by SARS-CoV-2 may participate at heart damage [Citation4]. Ang II stimulates vasoconstriction, thrombogenesis and proliferation via angiotensin type 1 receptor (AT1). However, a portion of the Ang II is converted to Ang 1–7 by ACE2, which exerts counterregulatory actions via the Mas-receptor in terms of vasodilation, antiinflammatory and antifibrotic action. Thus, ACE2 tissue downregulation by SARS-CoV-2 through endocytosis and proteolytic processing may result in the promotion of the deleterious action of Ang II [Citation3,Citation4], which may contribute to heart injury via local harmful effects or increased hemodynamic burden.
2. Melatonin as a potential treatment of myocardial injury in COVID-19
2.1. Protective effects of melatonin
Numerous therapeutic approaches to modulate the course of COVID-19 and potentially to reduce the extent of heart damage have been proposed including antimicrobials, corticosteroids, antibody- and cell-based therapies [Citation3]; however, their effects need to be verified in clinical trials. Although cardiovascular complications represent a life-threatening danger in SARS-CoV-2 infection, their management remains insufficiently defined.
Melatonin, an endogenously-produced molecule with protective effects on cardiomyocytes [Citation6,Citation7], could be a significant player in therapeutic approaches to damaged heart in COVID-19. Melatonin is known primarily for its circadian synthesis and release by the vertebrate pineal gland, but it is also widely distributed in non-vertebrates, which lack a pineal gland, and in plants [Citation8]. Recent evidence suggests that melatonin may be produced in the mitochondria of all cells. Mitochondria-derived melatonin has local autocrine and paracrine actions but is not released into the blood [Citation9]. This molecule has prominent antioxidant and anti-inflammatory functions [Citation10]. Moreover, melatonin may act as a counterregulatory signal of the activated sympathetic nervous system resulting in the dominance of the parasympathetic system [Citation11]. The hemodynamic effect in terms of bradycardia, attenuation of the uncoupling catecholamine effect, as well as antioxidative mitochondrial protection helps to preserve energy metabolism and ATP storage [Citation9,Citation11].
Melatonin is a versatile molecule exerting cellular protection in a context-specific manner. Melatonin has been shown to protect both animals and plants from a variety of viral infections [Citation12]. Although melatonin is not directly virucidal, it can attenuate tissue damage due to exaggerated immune response to a microbial infection. Given melatonin’s direct free radical scavenging activity, indirect stimulatory effect on antioxidative enzymes [Citation9], its ability to curb macrophage activation to the proinflammatory phenotype along with the suppression of the inflammasome [Citation10], makes melatonin a potential molecule to be considered as a treatment for SARS-CoV-19 infection [Citation13,Citation14].
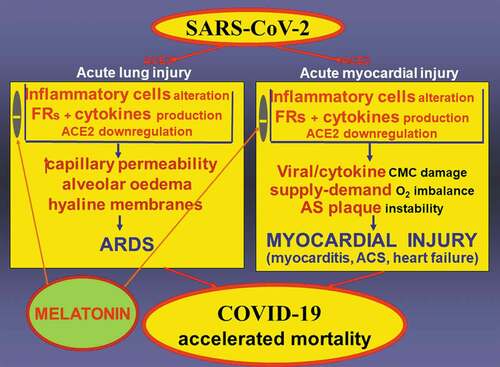
We propose that melatonin may also protect against myocardial injury during a SARS-CoV-19 infection ().
Figure 1. SARS-CoV-2 binds to a cell membrane-bound peptidase angiotensin converting enzyme 2 (ACE2), expressed abundantly in the lungs, heart and kidneys, and thus serving as an entrance receptor for the virus. SARS-CoV-2 alters inflammatory cells with subsequent excessive production of cytokines and free radicals (FRs). Binding SARS-CoV-2 to ACE2 associated with ACE2 downregulation, may shift the angiotensin II (Ang) II – Ang 1–7 balance toward the deleterious action of Ang II. Increased capillary permeability, pulmonary edema and hyaline membrane formation in the lungs result in the development of acute respiratory distress syndrome (ARDS). In the heart, direct virus damage or cytokine-induced cardiomyocyte (CMC) injury, hemodynamic stress with an oxygen supply-demand imbalance and inflammation-induced atherosclerotic (AS) plaque instability result in myocardial injury. Both ARDS and myocardial injury contribute to mortality incidence in COVID-19. Melatonin has the potential to reduce oxidative stress, modulate inflammatory cell activation and cytokine release, attenuate endothelial dysfunction and AS plaque instability and improve myocardial energetic metabolism, thereby reducing myocardial injury and fatal outcomes in COVID-19
2.2. Melatonin´s interactions with cardiovascular pathologies
There are reports documenting that melatonin provides protection in myocarditis and other myocardial pathologies. Indeed, melatonin attenuates inflammatory cell infiltration, interstitial edema and apoptosis and autophagy in coxsackievirus B3-induced myocarditis in mice [Citation15]. In myocarditis with cardiac dysfunction, melatonin reduces mitochondrial dysfunction via restricting mitochondrial permeability transition pore opening, attenuating caspase-9-related apoptosis, reducing lactate dehydrogenase release and improving heart contractile function [Citation16]. Intraperitoneal pre-treatment of rats with melatonin prevents the intense inflammatory reaction, myocyte necrosis, and fibrosis and vasculitis development in single-dose, radiation-induced heart damage in rats [Citation17]. Melatonin treatment of chronic heart damage in Chagas disease in rats, reduced the level of nitric oxide and TNF-alpha and increased the concentration of the protective IL-10, resulting in lowered heart weight, inflammatory foci number and creatine-phosphokinase-MB level [Citation18]. Moreover, in several models of hypertensive heart induced by L-NAME, continuous 24-hour light, lactacystine treatment or in spontaneously hypertensive rats, melatonin reduced left ventricular concentration of insoluble collagen with abundant cross-linkage contributing to LV stiffness [Citation19]. In rat models of heart failure induced by isoproterenol, melatonin decreased the level of oxidative stress, improved left ventricular function, reduced collagen content and concentration and improved survival [Citation20]. Furthermore, melatonin enhances bioavailability of nitric oxide and improves endothelial function at various levels, alleviates intraplaque inflammation and protects ischemic cardiomyocytes via receptor and non-receptor pathways potentially mitigating the risk of acute coronary syndrome [Citation6], and reperfusion damage [Citation7].
Additionally, the elderly and/or the population with cardiovascular diseases or risk factors such as hypertension, or diabetes mellitus type 2, have a high susceptibility to SARS-CoV-2 with poor prognosis. Interestingly, in a number of these pathologies, depressed nighttime melatonin levels were observed. It has been suggested that melatonin deficiency, potentially associated with an immunological imbalance, might be a unifying pathomechanism in the high-risk population and that patients with cardiovascular afflictions could benefit from supplementation of melatonin during the COVID-19 pandemic [Citation21].
The collective findings indicate that melatonin depresses inflammation and oxidative stress, induces antiapoptotic and antifibrotic actions in the damaged myocardium, and favors parasympathetic system dominance sparing energy stores. As such, it seems justified to assume that this indoleamine may alleviate the myocardial injury occurring in COVID-19 in terms of myocarditis, ischemic damage and heart failure.
3. Future research perspectives of melatonin in COVID-19
The following principle questions regarding the role of melatonin in COVID-19-related myocardial injury should be examined:
Is the level of melatonin decreased in patients with excessive cytokine release during a severe course of COVID-19?
Does the concentration of specific melatonin receptors change in patients with ARDS and myocardial injury in the lung and left ventricle?
Define the specific mechanism by which melatonin works to forestall or prevent the cardiomyocyte damage resulting from a SARS-CoV-19 infection.
What is the relationship between melatonin and ACE2, which serves as the binding site for SARS-CoV-2 and to the renin-angiotensin system in general in patients with severe COVID-19 disease and cardiovascular injury?
The bulk of melatonin´s protective actions in cardiovascular pathologies were obtained in animal experiments or phase 2 early clinical studies and larger phase 3 randomized interventional studies are needed [Citation22]. Furthermore, the correct dosage and times of administration are to be elucidated in the clinical setting. Recently, a potential therapeutic algorithm for the use of melatonin in patients with COVID-19 has been suggested, being 3 to 10 mg for high-risk and elderly patients and up to 40 mg for health care workers, preferably 30 to 60 min before bedtime [Citation23].
Although a number of questions remain unanswered at the moment, the high accessibility and minimal side effects favor melatonin as part of adjunctive therapy in patients with severe SARS-Cov-2 disease burden and increased probability of severe lung or cardiovascular involvement. As such, it seems reasonable to assume that melatonin may alleviate the heart damage occurring in SARS-CoV-2-infected patients and clinical trials in patients with COVID-19 and myocardial injury are warranted.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties
Reviewer declarations
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Additional information
Funding
References
- Madjid M, Safavi-Naeini P, Solomon SD, et al. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27;5:831.
- Wu L, O’Kane AM, Peng H, et al. SARS-CoV-2 and cardiovascular complications: from molecular mechanisms to pharmaceutical management. Biochem Pharmacol. 2020;178:114114.
- Akhmerov A, Marbán E. COVID-19 and the heart. Circ Res. 2020;126(10):1443–1455.
- Hanff TC, Harhay MO, Brown TS, et al. Is there an association between COVID-19 mortality and the renin-angiotensin system-a call for epidemiologic investigations. Clin Infect Dis. 2020;71:870–874.
- Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506.
- Fu Z, Jiao Y, Wang J, et al. Cardioprotective role of melatonin in acute myocardial infarction. Front Physiol. 2020;11:366. eCollection 2020.
- Lochner A, Marais E, Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: what’s new? A review. J Pineal Res. 2018;65(1):e12490.
- Reiter RJ, Tan DX, Rosales-Corral S, et al. The universal nature, unequal distribution and antioxidant functions of melatonin and its derivatives. Mini Rev Med Chem. 2013;13:373–384.
- Reiter RJ, Ma Q, Sharma R. Melatonin in mitochondria: mitigating clear and present dangers. Physiology (Bethesda). 2020;35:86–95.
- Hardeland R. Aging, melatonin, and the pro- and anti-inflammatory networks. Int J Mol Sci. 2019;20:1223.
- Simko F, Baka T, Paulis L, et al. Elevated heart rate and nondipping heart rate as potential targets for melatonin: a review. J Pineal Res. 2016;61:127–137.
- Reiter RJ, Ma Q, Sharma M. Treatment of ebola and other infectious diseases: melatonin “goes viral”. Melat Res. 2020;3:43–57.
- Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583.
- Anderson G, Reiter RJ. Melatonin: roles in influenza, Covid-19, and other viral infections. Rev Med Virol. 2020;30(3):e2109.
- Sang Y, Gu X, Pan L, et al. Melatonin ameliorates coxsackievirus B3-induced myocarditis by regulating apoptosis and autophagy. Front Pharmacol. 2018;9:1384.
- Ouyang H, Zhong J, Lu J, et al. Inhibitory effect of melatonin on Mst1 ameliorates myocarditis through attenuating ER stress and mitochondrial dysfunction. J Mol Histol. 2019;50:405–415.
- Gürses I, Özeren M, Serin M, et al. Histopathological evaluation of melatonin as a protective agent in heart injury induced by radiation in a rat model. Pathol Res Pract. 2014;210:863–871.
- Oliveira LG, Kuehn CC, Dos Santos CD, et al. Protective actions of melatonin against heart damage during chronic Chagas disease. Acta Trop. 2013;128:652–658.
- Simko F, Paulis L. Antifibrotic effect of melatonin-perspective protection in hypertensive heart disease. Int J Cardiol. 2013;3:2876–2877.
- Simko F, Bednarova-Repova K, Krajcirovicova K, et al. Melatonin reduces cardiac remodeling and improves survival in rats with isoproterenol-induced heart failure. J Pineal Res. 2014;57:177–184.
- Simko F, Reiter RJ. Is melatonin deficiency a unifying pathomechanism of high risk patients with COVID-19? Life Sci. 2020;11790. DOI: https://doi.org/10.1016/j.lfs.2020.117902.
- Baltatu OC, Senar S, Campos LA, et al. Cardioprotective melatonin: translating from proof-of-concept studies to therapeutic use. Int J Mol Sci. 2019;20(18):4342. .
- Reiter RJ, Abreu-Gonzalez P, Marik PE, et al. Therapeutic algorithm for use of melatonin in patients with COVID-19. Front Med (Lausanne). 2020 May 15;7:226. eCollection 2020.
