ABSTRACT
Introduction: Healthcare providers (HCPs) see many patients with obesity-related complications and are therefore well placed to help treat obesity itself. However, limited collated information exists to help HCPs with the practical use of anti-obesity medications (AOMs). We focus on the initiation and maintenance of a glucagon-like peptide-1 receptor agonist (GLP-1 RA) for weight management, liraglutide 3.0 mg. Literature search was conducted between 25–28 November 2019 on PubMed and ClinicalTrials.gov.
Areas covered: Clinical trial and real-world data describing weight-loss efficacy, cardiometabolic risk factors, incidence of adverse events (AEs), and persistence are presented to assist HCPs with patient discussions. Practical considerations to overcome barriers to optimal use are provided, equipping HCPs with the information required to aid with adherence to and persistence with AOMs. The use of other GLP-1- RA therapies in obesity is discussed in light of the recent US Food and Drug Administration approval of semaglutide 2.4 mg for weight management.
Expert opinion: Liraglutide 3.0 mg provides benefits regarding weight loss and improvements in cardiometabolic risk factors. Promising areas of future research in the field of obesity include dual receptor agonists and the combination of glucagon-like peptide-1 receptor agonists with other molecules.
1. Introduction
Obesity, defined as ‘abnormal or excessive fat accumulation that presents a risk to health,’ is a growing problem, globally [Citation1]. Weight-related complications include metabolic syndrome, type 2 diabetes (T2D), dyslipidemia, hypertension, cardiovascular disease, nonalcoholic fatty liver disease, osteoarthritis, Alzheimer’s disease, obstructive sleep apnea, infertility, and certain cancers [Citation2–9]. Obesity is recognized by national organizations, including the American Medical Association, as a chronic disease in its own right [Citation10–16].
However, obesity (defined as a body mass index [BMI] of >30 kg/m2) remains underdiagnosed and undertreated. In a survey of people with obesity in the USA, 71% had discussed their weight with a healthcare provider (HCP) and only 55% were diagnosed with obesity [Citation15]. HCPs play an important role in the treatment of obesity-related chronic diseases, as well as the disease itself [Citation17]. Therefore, it is important that they understand the benefits and side effects of available weight-management treatments.
National and international guidelines define meaningful weight loss as 5–10% of initial weight, at which point an improvement in cardiovascular risk factors is observed [Citation18–20]. Obesity treatment guidelines typically follow a three-tier pathway, moving from lifestyle interventions, to pharmacotherapy, to metabolic surgery [Citation6,Citation8,Citation21]. While lifestyle intervention remains the first line treatment for obesity, its efficacy is limited and weight regain is common [Citation22]. Metabolic surgery is highly efficacious, but it is invasive, and, owing to barriers to reimbursement and access, as few as 1% of eligible patients undergo this treatment [Citation23–25]. Pharmacotherapy, being less invasive than surgery and more efficacious than lifestyle modifications alone, can bridge the treatment gap.
Currently, US Food and Drug Administration (FDA)-approved anti-obesity medications (AOMs) intended for long-term use, include lipase inhibitor, glucagon-like peptide-1 receptor agonist (GLP-1 RA), and neurotransmitter agonists/antagonists and reuptake inhibitor drug classes () [Citation26,Citation27]. While GLP-1 RAs are well established for treating T2D, they have also been demonstrated to be very effective in treating obesity. However, there is currently limited collated information to help HCPs with the practical use of AOMs and how to optimize treatment.
Figure 1. Mechanisms of action of US FDA-approved anti-obesity medications
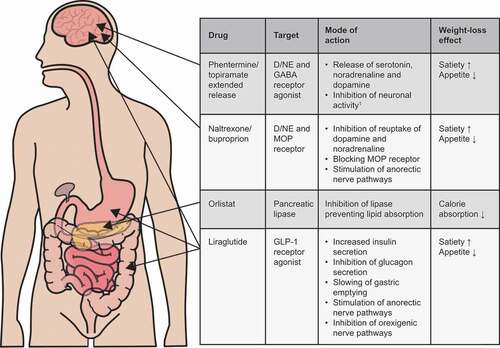
This review seeks to address this deficit and focus on the initiation and maintenance of the GLP-1 RA liraglutide 3.0 mg for weight management, with the aim of equipping HCPs with the information needed to optimize patients’ use of this AOM.
A literature search was conducted between 25–28 November 2019. The following terms were searched on PubMed and ClinicalTrials.gov: ‘antihypertensive medication pharmacists’; ‘obesity global prevalence’; ‘GLP-1 RA pharmacist’; ‘GLP-1 RA pharmacy safety’; ‘GLP-1’; ‘liraglutide approval obesity’; ‘GLP-1 RA’; ‘liraglutide mechanism’; ‘liraglutide half-life’; ‘liraglutide’; ‘semaglutide.’
1.1. Understanding appetite, obesity, and the mode of action of GLP-1 and its receptor agonists
A prolonged imbalance between energy intake and expenditure leads to obesity. The etiology of obesity is multifactorial; however, the underlying bases originate in the brain where the appetite regulation system resides [Citation28]. Hunger and appetite are driven by a complex interaction between the environment and a person’s biology, controlled by homeostatic, hedonic, and cognitive regulatory pathways located in the central nervous system [Citation28–31]. Homeostatic regulation of appetite relies on the stimulation of anorexigenic and orexigenic neurons, which decrease and increase appetite, respectively, in response to peripheral signals [Citation30]. The hedonic regulation of appetite operates even in the presence of satiety signals, resulting in food consumption irrespective of metabolic need [Citation28].
Several AOMs have been developed that target different aspects of energy intake and appetite regulation. The lipase inhibitor orlistat reduces the absorption of fat, resulting in energy wastage, whereas drugs that modify neurotransmission, such as bupropion/naltrexone, reduce energy intake through appetite suppression [Citation8,Citation26,Citation27] (). For GLP-1 RAs, instead of acting on one physiological system, both gastrointestinal (GI) and neurological pathways are targeted.
GLP-1 is one of the incretin peptide hormones, and is rapidly secreted from the small intestine [Citation32] into the circulation following food ingestion [Citation33]. It signals via the GLP-1 receptor located in various organs, of which the brain, pancreas, and GI tract [Citation34,Citation35] are of pharmacological interest in the context of this review. GLP-1 receptors in the pancreas act in a glucose-dependent manner to stimulate insulin secretion and inhibit glucagon release [Citation36–38]. In addition, GLP-1 increases satiety and reduces hunger via stimulation of anorexigenic and inhibition of orexigenic neurons [Citation30,Citation39], and GLP-1 also likely regulates food intake partly through direct effects on vagal afferent neurons in the GI tract [Citation40]. All of these actions can help with weight loss. Indeed, after metabolic surgery, postprandial GLP-1 levels rise, which may contribute to improved glucose metabolism and weight loss [Citation41].
1.2. GLP-1 RAs for weight loss
In its native form, GLP-1 has a very short half-life of 1.5 minutes when delivered intravenously, and 1.5 hours when delivered subcutaneously (s.c.) [Citation42]. Different pharmaceutical companies had varied approaches to extend its short half-life, resulting in several GLP-1 RAs being approved for treating T2D. Exenatide (twice daily), exenatide extended-release (once weekly), and lixisenatide (once daily) are based on the exendin-4 molecule and have ~50% sequence similarity to native GLP-1. Liraglutide (once daily), dulaglutide (once weekly), and semaglutide (available as a once-weekly s.c. formulation and a once-daily oral formulation) are classed as GLP-1 analogs with >90% sequence similarity to human GLP-1 [Citation43–50]. While all GLP-1 RAs have proven efficacy in reducing glucose levels [Citation43–49], their weight-loss efficacies vary, possibly due to their molecular size, affinity for the GLP-1 receptor sequence and similarity to native GLP-1 [Citation50,Citation51].
The GLP-1 RA liraglutide 3.0 mg (Saxenda®, Novo Nordisk A/S, Bagsværd, Denmark) is approved for treating obesity in many countries [Citation52–54]. Liraglutide is delivered as a once-daily s.c. injection and has 97% sequence similarity to native GLP-1. To extend its half-life, liraglutide is acylated to allow reversible binding to serum albumin and protect it from degradation [Citation42]. The concentration of liraglutide in blood peaks 11 hours after injection, and its elimination half-life is 13 hours [Citation52,Citation54].
A higher dose of liraglutide is approved for the treatment of obesity than for T2D (3.0 mg; Saxenda® versus 1.8 mg; Victoza®, Novo Nordisk A/S, Bagsværd, Denmark, respectively), based on the results of a phase 2 study showing that liraglutide 3.0 mg was the optimal dose for weight management [Citation55].
1.3. Trial data
The safety and efficacy of liraglutide 3.0 mg for weight management were assessed in four phase 3a trials (SCALE trial program), which included 5,339 participants with overweight or obesity [Citation56–60]. In each trial, all patients received lifestyle modification counseling and were instructed to undertake a 500 Kcal/day deficit diet and ≥150 minutes of brisk physical activity/week [Citation56–60]. The SCALE Obesity and Prediabetes trial was the largest and longest in the SCALE program [Citation59]. As well as examining the efficacy of liraglutide 3.0 mg for weight management over 56 weeks, delay of the onset of T2D over 160 weeks was assessed in patients also diagnosed with prediabetes at randomization [Citation58]. The 56-week SCALE Diabetes trial investigated the efficacy of liraglutide 3.0 mg for weight management in patients with overweight or obesity and T2D [Citation57]. The SCALE Maintenance trial assessed the efficacy of liraglutide 3.0 mg in maintaining the weight loss achieved during a low-calorie diet (1200–1400 Kcal/day) in patients without diabetes over 56 weeks [Citation60]. The SCALE Sleep Apnea trial enrolled adults with obesity and moderate or severe obstructive sleep apnea, but without diabetes, and assessed the change in the apnea–hypopnea index (AHI) after 32 weeks of treatment [Citation56].
The safety and efficacy of liraglutide 3.0 mg was further investigated in two additional phase 3b trials [Citation61,Citation62]. The SCALE IBT trial compared the effect of combining intensive behavioral therapy (IBT) and liraglutide 3.0 mg with IBT and placebo on weight loss in adults with obesity, in a primary care setting. The IBT program consisted of 23 brief counseling sessions with registered dieticians [Citation61]. The SCALE Insulin trial investigated the efficacy and safety of liraglutide 3.0 mg in patients with overweight or obesity and T2D, treated with basal insulin and up to two oral antidiabetic drugs [Citation62].
Data relating to the 3.0 mg dose of liraglutide, including change in body weight, body composition, cardiometabolic risk factors, persistence with treatment and incidence of adverse events (AEs), are included here, to equip HCPs with the information needed in patient discussions (, [Citation52,Citation54,Citation56–62]).
Figure 2. Mean weight loss over time in the phase 3a SCALE clinical trial program
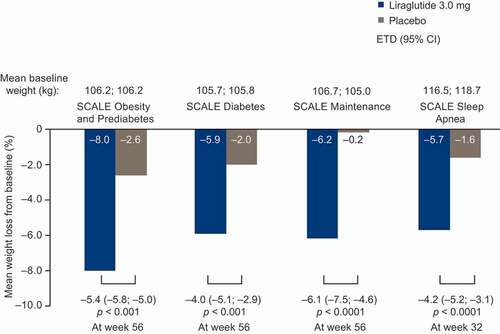
Figure 3. Gastrointestinal adverse events reported in ≥5% of patients in the phase 3a SCALE clinical trials
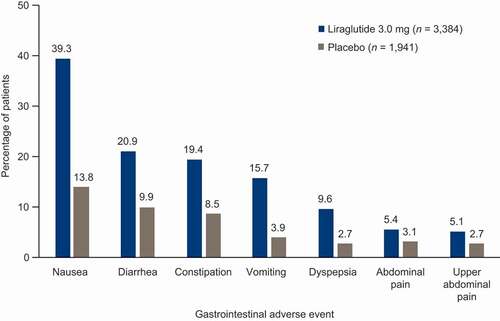
Table 1. Weight loss from baseline with liraglutide 3.0 mg in the phase 3a SCALE clinical program.a.
Table 2. Change from baseline to primary endpointa in cardiometabolic risk factors in the phase 3a SCALE clinical trial program
1.3.1. Efficacy
Across all SCALE trials, significantly greater mean and categorical weight loss was achieved with liraglutide 3.0 mg versus placebo [Citation56–62]. In the phase 3a SCALE trials, a reduction from initial body weight of 6%–8% was achieved with liraglutide 3.0 mg versus 0%–2% weight loss with placebo (, ) [Citation56–60,Citation62]. Similar findings were observed in the SCALE phase 3b trials. In the SCALE IBT trial, the mean weight reduction was 7.5% for liraglutide 3.0 mg plus IBT versus 4.0% for placebo plus IBT at 56 weeks [Citation61]. This is consistent with a previous trial that found that the addition of liraglutide 3.0 mg to IBT significantly increased weight loss compared with IBT alone (P = 0.005) [Citation63]. In the SCALE Insulin trial, significantly greater weight loss was observed with liraglutide 3.0 mg versus placebo (−5.8% versus −1.5%, respectively; P < 0.0001) [Citation62].
Significantly more patients treated with liraglutide 3.0 mg also achieved ≥5% or >10% weight loss across all of the phase 3a SCALE trials versus placebo; 46%–63% of patients achieved ≥5% weight loss, and 23%–33% lost at least 10% of their initial weight in the liraglutide 3.0 mg arms, versus 14%–27% and 2%–11%, respectively, of patients in the placebo arms ( [Citation56–62]). This was consistent with the SCALE IBT and SCALE Insulin trials. In SCALE IBT, significantly more patients treated with liraglutide versus placebo achieved ≥5% (61.5% versus 38.8%, respectively; P = 0.0003) or >10% weight loss (30.5% versus 19.8%, respectively; P = 0.0469). The respective values in the SCALE Insulin trial were 51.8% versus 24.9% and 22.8% versus 6.6% (P = 0.0001 for both) [Citation61,Citation62].
Patients receiving treatment in the SCALE Maintenance study who had already achieved ≥5% weight loss induced by a low-calorie diet achieved an additional mean 6.2% (6.0 kg) weight loss. Furthermore, the liraglutide 3.0 mg group reported an additional ≥5% weight loss in 51% of patients, an additional >10% weight loss in 26% of patients, and maintained diet-induced weight loss in 81% of patients [Citation60].
The effect of liraglutide on body composition was assessed in a 24-week cohort study in which doses up to 3.0 mg were used [Citation64]. It was found that weight loss caused by liraglutide was primarily from reduction in fat mass (–2.01 kg; P = 0.015) rather than fat free mass (–0.39 kg; P = 0.407) [Citation64].
Significant improvements in some cardiometabolic risk factors were observed with liraglutide 3.0 mg in addition to clinically meaningful weight loss. Across all of the phase 3a SCALE trials, improvements in systolic blood pressure, waist circumference, glycated hemoglobin, high-sensitivity C-reactive protein, total cholesterol, and triglycerides were observed with liraglutide 3.0 mg versus placebo () [Citation56–60]. Additionally, in the SCALE Insulin trial, significant improvement in glycemic control and a reduced need for basal insulin were also demonstrated [Citation62]. A reduction in AHI for patients receiving liraglutide 3.0 mg was observed in the SCALE Sleep Apnea trial, alongside improvement in cardiovascular risk factors, in line with evidence that sleep apnea is associated with increased incidence and progression of cardiovascular disease [Citation56,Citation65].
In addition, a real-world study that collected data from an obesity clinic database showed effectiveness for liraglutide 3.0 mg compared with orlistat [Citation66]. Here, significant weight loss was observed after ~7 months of treatment (−7.7 kg with liraglutide versus −3.3 kg with orlistat [crude mean difference −2.5 kg, P < 0.0001]; P < 0.0001 from baseline for both), with 65% of patients losing ≥5% of their initial weight with liraglutide versus 27% with orlistat. Additional improvements from baseline observed with liraglutide versus orlistat included reduced fasting plasma glucose (−0.4 mmol/L; P < 0.0001 versus −0.2 mmol/L; P = 0.003), systolic blood pressure (−3.6 mmHg; P = 0.029 versus −4.2; P < 0.0001), and low‐density lipoprotein‐cholesterol (−0.20 mmol/L; P = 0.004 versus −0.23; P < 0.0001) [Citation66]. A similar magnitude of weight loss was observed in another real-world setting, determined using a database of electronic medical records from multiple weight-loss clinics in Canada [Citation67].
1.3.2. Persistence with treatment
Persistence with AOM is important since obesity is a chronic disease and weight reduction is not typically sustained once the AOM is discontinued. Persistence with liraglutide 3.0 mg in a real-world setting has been shown to be significantly greater than with other AOMs [Citation68]. Using market data collected in the USA, it was found that 41.8% of patients persisted with liraglutide 3.0 mg treatment for 6 months, versus 18.1% with naltrexone/bupropion and 27.3% with phentermine/topiramate (P < 0.001 for all) [Citation68]. Older age (>65 years), having hyperlipidemia, and being male were found to be associated with higher persistence, while effectiveness and cost (all patients were insured) did not factor [Citation68]. However, it is important to note that discontinuation of liraglutide 3.0 mg is common. In another real-world effectiveness study conducted in Canada, persistence with liraglutide 3.0 mg was approximately 6.3 months on average [Citation69].
1.3.3. Safety
Liraglutide 3.0 mg is contraindicated for people with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia syndrome type 2, hypersensitivity to liraglutide or any product components, and in pregnancy [Citation52,Citation54]. There are limited data surrounding neoplasms in patients with obesity treated with liraglutide 3.0 mg but, to date, no causal link has been established between neoplasms and liraglutide 3.0 mg [Citation54]. A link in this area has been suggested in animals exposed to doses between two and 43 times that approved for use in humans with obesity [Citation54], but the relevance of this to humans is unknown and has not been shown in clinical trials [Citation70].
Across all SCALE trials, common AEs with liraglutide 3.0 mg tended to be mild and transient in nature, resolving within a few days or weeks without requiring discontinuation of treatment [Citation56–60,Citation71]. Treatment discontinuation due to AEs occurred in 9.8% of patients treated with liraglutide 3.0 mg and 4.3% of patients treated with placebo [Citation54]. GI AEs were the most frequently reported AE with liraglutide 3.0 mg, with the most common being nausea, diarrhea, constipation, and vomiting [Citation54]. In the SCALE program, 16%–39% of patients treated with liraglutide 3.0 mg experienced these AEs, compared with 4%–14% of placebo recipients [Citation54] (). Only a small proportion of patients experienced ongoing nausea by the end of the 56-week treatment with liraglutide 3.0 mg (2.5%–5.5%) versus placebo (1.4%–1.7%) [Citation57,Citation59].
As with most pharmacological treatments, some AEs of special interest were identified. In post-marketing reports, acute pancreatitis has been observed in patients treated with liraglutide 3.0 mg. Across the SCALE program, pancreatitis was observed in 0.3% of patients with liraglutide 3.0 mg and 0.1% of placebo groups [Citation54]. Based on 3,720 patient-years of exposure to any dose of liraglutide, the risk of pancreatitis associated with active treatment is very low [Citation72]. The risk to patients with a history of pancreatitis is unknown, as these patients were excluded from clinical trials. Gallbladder-related events, including acute gallbladder disease, were more common with liraglutide 3.0 mg versus placebo, respectively, in SCALE (cholelithiasis: 2.2% versus 0.8%; cholecystitis: 0.8% versus 0.4%) [Citation54,Citation56–60]. As liraglutide 3.0 mg acts centrally to some extent, neuropsychiatric events were of special interest. In SCALE, suicidal ideation was reported in 0.3% of patients treated with liraglutide 3.0 mg versus 0.1% of patients treated with placebo [Citation56–60].
Hypoglycemia in patients taking antidiabetic medication with liraglutide was also of special interest [Citation54]. In the SCALE Insulin trial, fewer hypoglycemic episodes were reported with liraglutide 3.0 mg than with placebo (742.3 versus 937.9 events/100 patient-years for hypoglycemia, defined using American Diabetes Association criteria; 336.1 versus 441.7 events/100 patient-years for documented symptomatic hypoglycemia), and no new safety or tolerability issues were observed [Citation62]. Other AEs of special interest in the SCALE program were dehydration and renal impairment as a consequence of nausea, vomiting, or diarrhea leading to volume depletion, and hypersensitivity reactions. Across the phase 3a SCALE trials, the rates of these AEs of special interest were comparable between liraglutide and placebo, and no new safety signals were identified.
1.3.4. Cardiovascular outcomes
The SCALE trials were not powered sufficiently to determine any cardiovascular benefit for liraglutide 3.0 mg; however, alongside the measurement of cardiometabolic risk factors as described in the efficacy section above, data regarding incidence of major adverse cardiovascular events (MACEs) were collected. These SCALE data were pooled and analyzed post hoc (n = 5908); the overall risk of cardiovascular events was numerically smaller with liraglutide 3.0 mg versus placebo (events/1000 person-years 1.54 versus 3.83; P = 0.07) [Citation73]. Furthermore, in a dedicated placebo-controlled cardiovascular outcomes trial with liraglutide 1.8 mg in patients with T2D and at high risk of cardiovascular events (LEADER), treatment with liraglutide was associated with a significant reduction in the risk of MACEs (first occurrence of death, non-fatal myocardial infarction, or non-fatal stroke) versus placebo (13.0% versus 14.9%, respectively; P = 0.01) [Citation74]. The LEADER trial is considered relevant to liraglutide 3.0 mg in relation to safety findings given the overlap in baseline characteristics and similar safety profiles observed between the 1.8 mg and 3.0 mg doses. As such, results from LEADER have been added to the liraglutide 3.0 mg product label by the European Medicines Agency and the US FDA [Citation52,Citation54]. It is important to note, however, that the patient population in the LEADER trial consisted of patients with T2D who were at higher risk of CVD than those in the SCALE trials. Further data are therefore required to establish a cardiovascular benefit of liraglutide 3.0 mg in people with obesity. A potential barrier in obtaining these data from CVOTs is the high treatment discontinuation rates seen with AOMs. Further investigation is also required to assess whether the cardiovascular benefit of liraglutide 1.8 mg seen in the LEADER trial is a direct effect of GLP-1 RAs or an indirect effect of weight loss and improved glycemic control.
1.4. Liraglutide for adolescents with obesity
In April 2020, the US FDA approved liraglutide 3.0 mg for chronic weight management in adolescents (aged >12 years) with obesity [Citation75], based on a recent randomized, phase 3 trial that investigated the efficacy and safety of liraglutide 3.0 mg versus placebo [Citation76]. The trial consisted of a 56-week treatment period and a 26-week follow-up period. A total of 251 participants (aged 12–<18 years) were randomized. The trial focused on the change from baseline in the BMI standard deviation score at week 56 and incidences of AEs.
Treatment with liraglutide 3.0 mg resulted in a larger reduction in the BMI standard deviation score from baseline compared with placebo, with an estimated treatment difference of −0.22 BMI standard deviation score (95% confidence interval [CI] −0.37; −0.08; P = 0.002). A numerically higher number of participants achieved ≥5% or >10% reduction in BMI with liraglutide 3.0 mg versus placebo (43.3% versus 18.7%, or 26.1% versus 8.1%, respectively) [Citation76]. The percentages of adolescents who reported AEs during the treatment period were similar between the liraglutide and placebo group (88.8% versus 84.9%). Like the SCALE trials, most of the common AEs were mild/moderate and transient. GI AEs were the most frequently reported events with liraglutide 3.0 mg versus placebo (64.8% versus 36.5%; P < 0.001). AEs that led to discontinuation of treatment occurred in 13 participants in the liraglutide group (of which 10 were GI AEs) versus none in the placebo group (10.4% versus 0%; P < 0.001).
2. Practical considerations
HCPs have regular patient contact, particularly with patients with obesity-related complications, and, as such, are well placed to help patients initiating GLP-1 RAs, and to facilitate long-term adherence to and persistence with it. Specific barriers to optimal use of liraglutide 3.0 mg include AEs (particularly GI), initial dose escalation, injection technique, prescription and insurance nuances, and polypharmacy.
2.1. Dose escalation and tolerability
The dose-escalation period for GLP-1 RAs is intended to make treatment more tolerable. GI side effects, such as nausea, are commonly reported upon initiation of GLP-1 RAs [Citation39]. To minimize these, it is recommended that the dose of liraglutide is escalated over a 4-week period, starting with 0.6 mg for the first week and escalating by 0.6 mg per week for each of the following 3 weeks, to a maintenance dose of 3.0 mg [Citation52,Citation54]. If patients do not tolerate an increased dose, it is recommended to delay escalation for 1 additional week. The prescribing information recommends discontinuing liraglutide if the 3.0 mg dose cannot be tolerated [Citation54]. If a dose is missed, the once-daily regimen should be resumed as prescribed with the next scheduled dose, without taking an extra dose or increase in dose to make up for the missed dose. If more than 3 days have passed since the last liraglutide dose, patients should reinitiate treatment at 0.6 mg daily and follow the dose-escalation schedule as used for treatment initiation [Citation54]. It is important to note that dose escalation does not fully mitigate GI side effects, as these AEs were still commonly observed across the SCALE trials despite patients following a dose-escalation schedule [Citation55,Citation58,Citation60].
2.2. Side effects
Although GI AEs were common in patients receiving liraglutide 3.0 mg in clinical trials, AEs were mild or moderate, transient, occurred when initiating treatment, and were unlikely to cause discontinuation [Citation54,Citation71]. It is useful to remind patients that they may experience mild-to-moderate nausea, diarrhea, constipation, and/or vomiting, especially during the dose-escalation phase [Citation54]. It is also important to note that, while most patients will experience only transient GI side effects, a small proportion of patients may experience ongoing symptoms, such as nausea. Patients who experience GI side effects should be advised to drink plenty of fluids to prevent dehydration and consequently potential kidney failure [Citation52]. Because GLP-1 RAs reduce body weight by lowering appetite and promoting earlier feelings of fullness when eating [Citation39], GI AEs may be minimized by encouraging patients to eat smaller meals and avoid foods high in fat [Citation77,Citation78].
Patients taking liraglutide 3.0 mg should be recommended to seek advice from a HCP if they experience signs and/or symptoms of a thyroid tumor (lump in the neck, hoarseness, dysphagia, or dyspnea), symptoms of acute pancreatitis (persistent severe abdominal pain and sometimes vomiting [liraglutide should be promptly discontinued if acute pancreatitis is suspected]), cholelithiasis, or a sustained period of increased heart rate while at rest [Citation54]. Patients receiving medication for T2D, such as sulfonylureas, should be advised to monitor blood glucose and report any symptoms of hypoglycemia, as the dose of antidiabetic medication may need to be lowered while liraglutide 3.0 mg is also being taken.
2.3. Injection technique
Liraglutide is delivered via a pre-loaded injection pen that contains 3 mL of solution, equivalent to 18 mg of liraglutide (6 mg/mL). Patients can select doses of 0.6, 1.2, 1.8, 2.4, or 3.0 mg by dialing a dose on the pen [Citation54]. The patient ‘information for use’ section of the prescribing information provides details of the technique to be used, with diagrams. If a patient is uncertain, it may be worthwhile spending a few minutes with them and these diagrams until they are comfortable with the injection procedure [Citation54].
2.4. Prescription and insurance nuances
The decision to cover AOMs is made at the employer level and the employer must opt in for such coverage for commercially insured patients. Several patient prescription assistance programs exist, for example on the American Association of Clinical Endocrinologists website (http://prescriptionhelp.aace.com/), and HCPs can help patients access such programs. HCPs can help patients navigate through patient-assistance programs and savings cards to improve coverage and affordability.
In some states in the USA, and potentially in other countries, the pen and the pen needle require separate prescriptions [Citation79]; therefore, two prescriptions may be required to take liraglutide 3.0 mg. In addition, there are two needle sizes that can be used with the liraglutide pen: 5 mm x 32 G (Novotwist®, Novo Nordisk A/S, Bagsværd, Denmark) and 6 mm x 32 G (Novofine®, Novo Nordisk A/S, Bagsværd, Denmark) [Citation79,Citation80]. Patients’ HCPs select the optimal size for each patient, and should be able to help patients with this when the pens and needles are provided.
Patients’ insurance companies may cover a limited number of obesity-related visits with their HCPs. As they initiate liraglutide 3.0 mg, HCPs should consider when patients will be seeing the prescribing HCP again, as it may be more beneficial for a pharmacist to perform the initial follow-up during the dose-escalation phase of treatment. Collaborative practice agreements (CPAs) may be established among HCPs for coordinated drug therapy management in states where legislation permits; CPA guidance on this has been produced by the US Centers for Disease Control and Prevention [Citation81]. CPAs can expand the scope of a pharmacist’s role with authorization to adjust medication or dosage, helping with adherence, reducing the number of visits a patient has to make, reducing the workload for overscheduled primary care doctors, and lowering costs [Citation82].
The liraglutide 3.0 mg product label in the USA states that change in body weight should be evaluated 16 weeks post-initiation, at which point liraglutide should be discontinued if ≥4% weight loss is not achieved. This is because, in this scenario, it is unlikely that patients will achieve and sustain clinically meaningful weight loss with continued treatment [Citation54]. Product discontinuation information is based upon an early-responder analysis of patients in SCALE demonstrating that most patients (93.4%) who did not achieve this goal also did not achieve ≥10% weight loss after 1 year of treatment [Citation52]. HCPs should also be aware that some insurance companies will not cover costs for liraglutide 3.0 mg after 16 weeks for patients who do not achieve ≥4%–5% weight loss. As such, these 16 weeks of liraglutide initiation are critical for the patient and healthcare team. Therefore, it is important for HCPs to encourage persistence with liraglutide 3.0 mg in this early period, particularly given the positive correlation between medication adherence and weight loss with liraglutide [Citation83].
2.5. Polypharmacy
Patients with obesity are likely to have complications or comorbidities [Citation3,Citation84], and patients may turn to their HCP to ask about the resulting polypharmacy. The most likely questions are associated with T2D. Liraglutide 3.0 mg should not be used in conjunction with other GLP-1 RAs, and should not be used as an insulin substitute. Due to the efficacy of liraglutide 3.0 mg in glucose lowering, blood glucose should be monitored before and during treatment with liraglutide 3.0 mg, as any existing antidiabetic drug prescription may need to be reduced when liraglutide 3.0 mg is initiated, to reduce the risk of hypoglycemia [Citation54].
Despite slowed gastric emptying, interaction studies did not show any effect of GLP-1 RAs on the absorption of concomitant oral medications including atorvastatin, digoxin and lisinopril, oral contraceptives, acetaminophen, and the antifungal griseofulvin [Citation54]. No dose adjustment of these particular oral drugs is required when also taking liraglutide; however, cautious monitoring must be used with other orally administered treatments [Citation54], including frequent monitoring of patients who take warfarin or other Coumadin® (Bristol-Myers Squibb, New York, USA) derivatives upon initiation of liraglutide 3.0 mg [Citation52].
Treatment with liraglutide 3.0 mg may reduce further polypharmacy through reducing obesity-related complications with successful weight loss. The reduction in these comorbidities may encourage patients to persist with liraglutide 3.0 mg therapy, long term. For example, data from the SCALE trials demonstrated that liraglutide 3.0 mg improved cardiometabolic factors [Citation56–60]. This may present an opportunity to minimize polypharmacy in line with approved indications and doses, as cardiometabolic parameters begin to improve with successful weight loss.
Finally, long-term treatment with liraglutide 3.0 mg is usually required, as obesity is a chronic disease. Patients should have weight-management goals and their HCPs should create a plan with them to achieve these goals. Patients should attend a follow-up appointment following initiation of liraglutide 3.0 mg when advised by their HCPs, to review progress and renew their prescription.
3. Use of other GLP-1 RA therapy in obesity
As of June 2021, a second GLP-1 RA (semaglutide) has been approved by the FDA for chronic weight management [Citation85]. Semaglutide has 94% sequence similarity to human GLP-1, with further modifications made compared with liraglutide, thus increasing its half-life to ~1 week [Citation46,Citation86,Citation87]. It was first developed for use in T2D as a once-weekly s.c. administration [Citation46], but has since been approved in a once-daily oral formulation for the same indication [Citation47]. In trials for diabetes, semaglutide s.c. proved very efficacious in weight loss – even more so than liraglutide (e.g. SUSTAIN 10, weight loss with semaglutide 1.0 mg of −5.8 kg versus with liraglutide 1.2 mg of −1.9 kg) [Citation88]. A daily dose of 0.05–0.4 mg semaglutide (with a dose-escalation period) induced clinically relevant weight loss across all treatment groups of 6%–14% over 52 weeks (versus 2% weight loss with placebo), and was well tolerated in patients with overweight or obesity and without diabetes (n = 957) [Citation89].
The safety and efficacy of once-weekly-administered semaglutide 2.4 mg for obesity have been assessed and published in four phase 3, 68-week trials (STEP clinical program) [Citation90–93]. The STEP 1 trial focused on the use of semaglutide 2.4 mg as an adjunct to lifestyle intervention [Citation90]. STEP 2 compared semaglutide 2.4 mg versus 1.0 mg versus placebo [Citation93]. STEP 3 explored the efficacy of semaglutide 2.4 mg versus placebo, both combined with IBT [Citation91]. STEP 4 compared continued treatment with semaglutide 2.4 mg versus switch to placebo [Citation92]. Only STEP 2 enrolled patients with T2D [Citation93].
The mean reduction in body weight from baseline to week 68 was 9.6%–16.0% in the semaglutide group versus 2.4%–5.7% with placebo, across the STEP 1–3 trials [Citation90,Citation91,Citation93]. Although direct cross-study comparisons of liraglutide and semaglutide should be interpreted with caution, compared with liraglutide (6%–8%), semaglutide appears to have greater weight-reducing potential. Also, more patients treated with semaglutide in the STEP trials achieved >5% and >10% weight loss (68.6%–86.6% and 45.6%–75.3%, respectively) compared with liraglutide 3.0 mg (46%–63% and 23%–33%, respectively). The results of an ongoing study of the effect of semaglutide on cardiovascular outcomes in patients with overweight or obesity (SELECT, NCT03574597) are expected in 2023 and should further illustrate the potential of semaglutide in CVD.
Tirzepatide, a dual glucose-dependent insulinotropic polypeptide/GLP-1 RA, has also shown to be promising for weight loss in patients with T2D [Citation94]. This treatment is currently being investigated in a phase 3 trial enrolling patients with obesity or overweight (SURMOUNT-1, NCT04184622).
4. Conclusion
Liraglutide 3.0 mg is a once-daily s.c. GLP-1 RA injection that can result in clinically meaningful weight loss. In addition to weight loss, liraglutide 3.0 mg has numerous positive effects with regard to improvement in cardiometabolic risk factors, including blood pressure, lipid profile, and glycemic control. The beneficial effects of liraglutide 3.0 mg on cardiometabolic risk factors may enable the use or dosage of concomitant drugs to be reduced, which may improve adherence and persistence.
5. Expert opinion
The management of obesity-related complications requires regular, face-to-face contact with HCPs, who are, therefore, well placed to address obesity. HCPs play a key role in advising patients on the initiation and maintenance of GLP-1 RAs and so will be able to aid patients with adherence to treatment and de-prescribing of metabolic syndrome-related medications as those parameters improve with successful weight loss.
However, patient concerns about polypharmacy and gastrointestinal side effects can be a barrier to the adoption of liraglutide 3.0 mg in routine clinical practice, although HCPs can emphasize to patients that treatment with liraglutide 3.0 mg may reduce further polypharmacy through reducing obesity-related complications with successful weight loss. Furthermore, GI side effects can be minimized through dose escalation, and by advising patients to make dietary changes. HCPs are advised to discuss potential side effects before drug initiation to manage expectations. It is worth emphasizing to patients that the most prevalent GI side effects, including nausea, vomiting, and diarrhea, are usually mild to moderate and transient. Education for HCPs regarding GI side effects remains a key area for improvement. Also, further research into reducing the frequency of these side effects with liraglutide 3.0 mg could be beneficial, particularly for the small proportion of patients who experience persistent side effects.
Further research into GLP-1 RAs will build on the current knowledge of these molecules and could increase uptake. The focus should be real-world data for liraglutide/semaglutide, as well as its impact on obesity-related comorbidities, particularly cardiovascular disease. The use of once weekly s.c. semaglutide 2.4 mg for chronic weight management has recently been approved by the US FDA, because of the promising data presented by the STEP clinical program. With the success of oral semaglutide in diabetes, oral semaglutide could be investigated and made available for weight management. Oral formulations of other GLP-1 RAs could also be made available in the future.
Other promising areas of research in the GLP-1 RA field that could be explored are agonists that target multiple receptors, and molecules with longer half-lives. Having been associated with weight reduction in patients with T2D, the dual receptor agonist tirzepatide has the potential to be used for weight loss too [Citation95]. Phase 2 studies into efpeglenatide, a once-monthly injectable GLP-1 RA, have shown promising results in terms of efficacy and safety profile [Citation96]. While the development of both tirzepatide and efpeglenatide is primarily targeted at glycemic control in patients with diabetes, significant weight loss has been observed with these therapies [Citation95,Citation96], and research could be carried out into the effect in patients with overweight and obesity. Longer dosing intervals than once weekly may have beneficial effect on patient adherence. Additionally, as response to GLP-1 RAs could vary between patients, combination of GLP-1 RAs and other medications that target different receptors may provide other effective treatment options [Citation97]. Although human studies have not yet been published, reduced energy intake and body-weight loss of a GLP-1 RA combined with amylin have been observed in diet-induced obese rats [Citation98].
In the near future, we expect that GLP-1 RAs will be more commonly prescribed for patients with obesity. It is possible that there could be a larger proportion of GLP-1 RA prescriptions for oral formulations, and there could be greater use of both dual receptor agonists and combination therapies.
Article highlights
In people with overweight or obesity, glucagon-like peptide-1 receptor agonists confer multiple metabolic benefits, including weight loss
This review focuses on the initiation and maintenance of liraglutide 3.0 mg, an FDA-approved glucagon-like peptide-1 receptor agonist for long-term weight management, and provides clinical trial and real-world data describing weight-loss efficacy, cardiometabolic risk factors, adverse events, and patient persistence in patients with overweight or obesity
Practical considerations are provided with the aim of equipping healthcare providers with the information to optimize patients’ use of this anti-obesity medication
Declaration of interest
D Patel is an advisor/consultant for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Insulet, Novo Nordisk, and Sanofi, and is on speakers’ bureaus for Amarin, AstraZeneca, Boehringer Ingelheim, Dexcom, Eli Lilly, Merck, Novo Nordisk, Xeris, and Zealand. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
A reviewer of this manuscript declares a history of consulting work with Novo Nordisk in 2017-2019. Peer reviewers on this manuscript have no other relevant financial or other relationships to disclose
Acknowledgments
Editorial support (in the form of writing assistance, collating author comments, assembling tables/figures, grammatical editing, and referencing) was provided by Nicola Winstone, Jin Heppell, and Helen Marshall of Ashfield MedComms, an Ashfield Health company.
Data availability statement
Data sharing is not applicable to this article, as no data were generated or analyzed.
Additional information
Funding
References
- Obesity Collaborators GBD, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13–27.
- Church TS, Kuk JL, Ross R, et al. Association of cardiorespiratory fitness, body mass index, and waist circumference to nonalcoholic fatty liver disease. Gastroenterology. 2006;130(7):2023–2030. .
- Guh DP, Zhang W, Bansback N, et al. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9(1):88. .
- Li C, Ford ES, Zhao G, et al. Prevalence of self-reported clinically diagnosed sleep apnea according to obesity status in men and women: National Health and Nutrition Examination Survey, 2005-2006. Prev Med. 2010;51(1):18–23. .
- Caterson ID, Hubbard V, Bray GA, et al. Prevention conference VII: obesity, a worldwide epidemic related to heart disease and stroke: group III: worldwide comorbidities of obesity. Circulation. 2004;110(18):e476–83. .
- Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424. .
- Alford S, Patel D, Perakakis N, et al. Obesity as a risk factor for Alzheimer’s disease: weighing the evidence. Obes Rev. 2018;19(2):269–280. .
- Garvey WT, Mechanick JI, Brett EM, et al. AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY. Endocr Pract. 2016;22(Suppl):31–203.
- Kolb R, Sutterwala FS, Zhang W. Obesity and cancer: inflammation bridges the two. Curr Opin Pharmacol. 2016;2977–2989.
- American Medical Association. 2012 Resolutions 2012. [cited 2021 Jun 30]. Available from: https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/hod/a12-resolutions_0.pdf
- Bray GA, Kim KK, Wilding JPH. Obesity: a chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes Rev. 2017;18(7):715–723.
- European Association for the Study of Obesity. 2015 Milan Declaration: a Call to Action on Obesity 2015. [cited 2021 Jun 30]. Available from: https://cdn.easo.org/wp-content/uploads/2018/12/16195534/EASO-Milan-Declaration-FINAL.pdf
- European Medicines Agency. Guideline on clinical evaluation of medicinal products used in weight management 2014. [cited 2021 Jun 30]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-evaluation-medicinal-products-used-weight-management-revision-1_en.pdf
- Obesity Canada. Obesity in Canada Accessed 14th November 2019. [cited 2021 Jun 30]. Available from: https://obesitycanada.ca/obesity-in-Canada/
- Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the National ACTION study. Obesity. 2018;26(1):61–69. .
- World Health Organization. 2020 [ cited 2021 Jun 30]. Available from: https://www.who.int/topics/obesity/en/
- Apovian CM, Garvey WT, Ryan DH. Challenging obesity: patient, provider, and expert perspectives on the roles of available and emerging nonsurgical therapies. Obesity. 2015;23(Suppl 2(0 2)):S1–S26.
- Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines and the obesity society. Circulation. 2014;129(25 Suppl 2):S102–38. .
- World Health Organization. Obesity: preventing and managing the global epidemic: report of a WHO consultation. WHO Technical Report Series 8941999.
- Endocrine Society. Endocrine Society Clinical Practice Guidelines 2020 [cited 2021 Jun 30]. Available from: https://www.endocrine.org/clinical-practice-guidelines
- Bays HEMW, Christensen S, Wells S, et al. Obesity algorithm eBook, presented by the obesity medicine association. www.obesityalgorithm.org. 2019.: Obesity Medicine Association; 2021. [cited 2021 Jun 30]. Available from: https://obesitymedicine.org/obesity-algorithm/.
- Varkevisser RDM, van Stralen MM, Kroeze W, et al. Determinants of weight loss maintenance: a systematic review. Obes Rev. 2019;20(2):171–211.
- Gasoyan H, Tajeu G, Halpern MT, et al. Reasons for underutilization of bariatric surgery: the role of insurance benefit design. Surg Obes Relat Dis. 2019;15(1):146–151.
- Love KM, Mehaffey JH, Safavian D, et al. Bariatric surgery insurance requirements independently predict surgery dropout. Surg Obes Relat Dis. 2017;13(5):871–876.
- Imbus JR, Voils CI, Funk LM. Bariatric surgery barriers: a review using Andersen’s Model of health services use. Surg Obes Relat Dis. 2018;14(3):404–412.
- González-Muniesa P, Mártinez-González M-A, Hu FB, et al. Obesity. Nat Rev Dis Primers. 2017;3(1):17034.
- Patel D. Pharmacotherapy for the management of obesity. Metabolism. 2015;64(11):1376–1385.
- Berthoud H-R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21(6):888–896.
- Cooke D, Bloom S. The obesity pipeline: current strategies in the development of anti-obesity drugs. Nat Rev Drug Discov. 2006;5(11):919–931.
- Simpson KA, Martin NM, Bloom SR. Hypothalamic regulation of appetite. Expert Rev Endocrinol Metab. 2008;3(5):577–592.
- Hall KD, Hammond RA, Rahmandad H. Dynamic interplay among homeostatic, hedonic, and cognitive feedback circuits regulating body weight. Am J Public Health. 2014;104(7):1169–1175.
- Mojsov S, Heinrich G, Wilson IB, et al. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem. 1986;261(25):11880–11889.
- Kreymann B, Williams G, Ghatei MA, et al. Glucagon-like peptide-1 7-36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304.
- Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403(2):261–280.
- Pyke C, Heller RS, Kirk RK, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–1290. .
- Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742.
- Nauck MA, Heimesaat MM, Behle K, et al. Effects of glucagon-like peptide 1 on counterregulatory hormone responses, cognitive functions, and insulin secretion during hyperinsulinemic, stepped hypoglycemic clamp experiments in healthy volunteers. J Clin Endocrinol Metab. 2002;87(3):1239–1246. .
- Nauck MA, Kleine N, �rskov C, et al. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–744. .
- Flint A, Raben A, Astrup A, et al. Glucagon-like peptide 1 promotes satiety and suppresses energy intake in humans. J Clin Invest. 1998;101(3):515–520. .
- Krieger JP. Intestinal glucagon-like peptide-1 effects on food intake: physiological relevance and emerging mechanisms. Peptides. 2020;131:170342.
- Hutch CR, Sandoval D. The role of GLP-1 in the metabolic success of bariatric surgery. Endocrinology. 2017;158(12):4139–4151.
- Knudsen LB, Lau J. The discovery and development of liraglutide and semaglutide. Front Endocrinol (Lausanne). 2019;10:155.
- AstraZeneca. Byetta® (exenatide) injection, Prescribing Information. [cited 2021 Jun 30]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021773s9s11s18s22s25lbl.pdf
- AstraZeneca. Bydureon (exenatide injection), Prescribing Information 2012 2017 Aug 4. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022200s000lbl.pdf
- Eli Lilly and Company. Trulicity (dulaglutide), Prescribing Information [cited 2021 Jun 30]. http://pi.lilly.com/ca/trulicity-ca-pm.pdf
- Novo Nordisk. Ozempic semaglutide injection - Prescibing Information. [cited 2021 Jun 30]. Available from:https://www.novo-pi.com/ozempic.pdf2019
- Novo Nordisk. Rybelsus - Prescribing Information [cited 2021 Jun 30]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf2019
- Novo Nordisk AS. Victoza (liraglutide), Prescribing Information. [cited 2021 Jun 30]. Available from: https://www.novo-pi.com/victoza.pdf.
- Sanofi-Aventis US. Adlyxin (lixisenatide), Prescribing Information[cited 2021 Jun 30]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208471Orig1s000lbl.pdf
- Madsbad S. Review of head-to-head comparisons of glucagon-like peptide-1 receptor agonists. Diabetes Obes Metab. 2016;18(4):317–332.
- Secher A, Jelsing J, Baquero AF, et al. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. 2014;124(10):4473–4488.
- Novo Nordisk. Saxenda Summary of Product Characteristics 2015 [ cited 2021 Jun 30]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/saxenda
- Nordisk N. Saxenda Product Information: australia [ cited 2021 Jun 30]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-01008-1&d=202001281016933
- *Novo Nordisk. SAXENDA (liraglutide) Prescribing Information 2020. [cited 2021 Jun 30]. Available from: https://www.novo-pi.com/saxenda.pdf.
- Astrup A, Rossner S, Van Gaal L, et al. Effects of liraglutide in the treatment of obesity: a randomised, double-blind, placebo-controlled study. Lancet. 2009;374(9701):1606–1616.
- **Blackman A, Foster GD, Zammit G, et al., Effect of liraglutide 3.0 mg in individuals with obesity and moderate or severe obstructive sleep apnea: the SCALE sleep apnea randomized clinical trial. Int J Obes (Lond). 2016.40(8):1310–1319.
- **Davies MJ, Bergenstal R, Bode B, et al., Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 314(7): 687–699. 2015.
- **le Roux CW, Astrup A, Fujioka K, et al., 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet. 389(10077): 1399–1409. 2017.
- **Pi-Sunyer X, Astrup A, Fujioka K, et al., A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 373(1): 11–22. 2015.
- **Wadden TA, Hollander P, Klein S, et al., Weight maintenance and additional weight loss with liraglutide after low-calorie-diet-induced weight loss: the SCALE Maintenance randomized study. Int J Obes (Lond). 37(11): 1443–1451. 2013.
- Wadden TA, Tronieri JS, Sugimoto D, et al. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for obesity in primary care: the SCALE IBT randomized controlled trial. Obesity (Silver Spring). 2020;28(3):529–536.
- Garvey WT, Birkenfeld AL, Dicker D, et al. Efficacy and safety of Liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal Insulin: the SCALE Insulin randomized controlled trial. Diabetes Care. 2020;43(5):1085–1093.
- Wadden TA, Walsh OA, Berkowitz RI, et al. Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity (Silver Spring). 2019;27(1):75–86.
- Rondanelli M, Perna S, Astrone P, et al. Twenty-four-week effects of liraglutide on body composition, adherence to appetite, and lipid profile in overweight and obese patients with type 2 diabetes mellitus. Patient Prefer Adherence. 2016;10407–10413.
- Floras JS. Sleep Apnea and cardiovascular disease: an enigmatic risk factor. Circ Res. 2018;122(12):1741–1764.
- **Gorgojo-Martinez JJ, Basagoiti-Carreno B, Sanz-Velasco A, et al., Effectiveness and tolerability of orlistat and liraglutide in patients with obesity in a real-world setting: the XENSOR Study. Int J Clin Pract. 2019;73(11):e13399.
- Wharton S, Liu A, Pakseresht A, et al. Real-World Clinical Effectiveness of Liraglutide 3.0 mg for weight management in Canada. Obesity (Silver Spring). 2019;27(6):917–924.
- Ganguly R, Tian Y, Kong SX, et al. Persistence of newer anti-obesity medications in a real-world setting. Diabetes Res Clin Pract. 2018;143348–143356.
- Wharton S, Haase CL, Kamran E, et al. Weight loss and persistence with liraglutide 3.0 mg by obesity class in the real-world effectiveness study in Canada. Obes Sci Pract. 2020;6(4):439–444.
- Nauck MA, Jensen TJ, Rosenkilde C, et al. Neoplasms reported with liraglutide or placebo in people with type 2 diabetes: results from the LEADER randomized trial. Diabetes Care. 2018;41(8):1663–1671.
- Patel DK, Stanford FC. Safety and tolerability of new-generation anti-obesity medications: a narrative review. Postgrad Med. 2018;130(2):173–182.
- Ryder R, Thong K, Blann A, et al. Liraglutide pancreatitis: the ABCD nationwide liraglutide audit. Br J Diabetes Vasc Dis. 2013;13(5-6):253-259.
- Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734–739.
- Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322.
- Food and Drug Administration. FDA approves weight management drug for patients aged 12 and older 2020 [cited 2021 Jun 30]. Available from: https://www.fda.gov/drugs/drug-safety-and-availability/fda-approves-weight-management-drug-patients-aged-12-and-older
- Kelly AS, Auerbach P, Barrientos-Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–2128.
- Unger JR, Parkin CG. Glucagon-like peptide-1 (GLP-1) receptor agonists: differentiating the new medications. Diabetes Ther. 2011;2(1):29–39.
- Ross SA. Breaking down patient and physician barriers to optimize glycemic control in type 2 diabetes. Am J Med. 2013;126(9 Suppl 1):S38–48.
- Novo Nordisk. SAXENDA (liraglutide [rDNA origin] injection) solution for subcutaneous use prescribing information. Plainsboro, NJ: Novo Nordisk Inc. 2014. [cited 2021 Jun 30]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/206321Orig1s000lbl.pdf
- Novo Nordisk. Novo Nordisk needles 2020 [2020 Mar 16th]. Available from: https://www.novoneedles.com
- Centers for Disease Control and Prevention. Advancing team-based care through collaborative practice agreements: a resource and implementation guide for adding pharmacists to the care team 2017. [cited 2021 Jun 30]. Available from: https://www.cdc.gov/dhdsp/pubs/docs/CPA-Team-Based-Care.pdf
- Centers for Disease Control and Prevention. Pharmacy: collaborative practice agreements to enable collaborative drug therapy management 2017. [cited 2021 Jun 30]. Available from: https://www.cdc.gov/dhdsp/pubs/docs/Best_Practice_Guide_CDTM_508.pdf
- Tronieri JS, Wadden TA, Walsh O, et al. Measures of adherence as predictors of early and total weight loss with intensive behavioral therapy for obesity combined with liraglutide 3.0mg. Behav Res Ther. 2020;131:103639.
- Sharma AM. M, M, M & M: a mnemonic for assessing obesity. Obes Rev. 2010;11(11):808–809.
- Food and Drug Administration. FDA approves new drug treatment for chronic weight management, first since 2014 2021. [ cited 2021 09 June]: https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014
- Lau J, Bloch P, Schaffer L, et al. Discovery of the Once-Weekly glucagon-like peptide-1 (GLP-1) Analogue Semaglutide. J Med Chem. 2015;58(18):7370–7380.
- Blundell J, Finlayson G, Axelsen M, et al. Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes Obes Metab. 2017;19(9):1242–1251.
- Capehorn MS, Catarig AM, Furberg JK, et al. Efficacy and safety of once-weekly semaglutide 1.0 mg vs once-daily liraglutide 1.2 mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes Metab. 2019;46(2):100–109.
- O’Neil PM, Birkenfeld AL, McGowan B, et al. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018;392(10148):637–649.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-Weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989.
- Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413.
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs Placebo on weight loss maintenance in adults with overweight or obesity: the step 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425.
- Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet. 2021;397(10278):971–984.
- Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193.
- Thomas MK, Nikooienejad A, Bray R, et al. Dual GIP and GLP-1 receptor agonist tirzepatide improves beta-cell function and insulin sensitivity in type 2 diabetes. J Clin Endocrinol Metab. 2021;106(2):388–396.
- Del Prato S, Kang J, Trautmann ME, et al. Efficacy and safety of once-monthly efpeglenatide in patients with type 2 diabetes: results of a phase 2 placebo-controlled, 16-week randomized dose-finding study. Diabetes Obes Metab. 2020;22(7):1176–1186.
- Lee YH, Lee HW, Hj C. GLP-1 based combination therapy for obesity and diabetes. J Obes Metab Syndr. 2017;26(3):155–160.
- Liberini CG, Koch-Laskowski K, Shaulson E, et al. Combined amylin/GLP-1 pharmacotherapy to promote and sustain long-lasting weight loss. Sci Rep. 2019;9(1):8447.
