Abstract
It is well known that Michaelis–Menten kinetics is suitable for the response function in chemical reaction, when the reaction rate does not increase indefinitely when an excess of resource is available. However, the existing models for insulin therapies assume that the response function of insulin clearance is proportional to the insulin concentration. In this paper, we propose a new model for insulin therapy for both type 1 and type 2 diabetes mellitus, in which the insulin degradation rate assumes Michaelis–Menten kinetics. Our analysis shows that it is possible to mimic pancreatic insulin secretion by exogenous insulin infusions, and our numerical simulations provide clinical strategies for insulin–administration practices.
1. Modelling the glucose–insulin feedback system
Diabetes mellitus is a disorder of the glucose–insulin endocrine metabolic regulatory system. It is caused by the fact that either the pancreas does not produce enough insulin to consume the glucose, or the insulin is not used efficiently by the cells to metabolize the glucose. The diabetic population accounts for approximately 7% of the whole population in US, and the health expense is huge (≈132 billion US dollars annually, ADA, http://www.diabetes.org). Many researchers have been attracted to the study of glucose–insulin regulatory system with an ultimate goal of providing more efficient, effective, and economic insulin therapies for diabetics (e.g., Citation1,Citation6–9,Citation12 Citation13 Citation15 Citation17 Citation18 Citation21 Citation29 Citation30 Citation37 Citation39 Citation40 Citation43 Citation48 Citation49).
In the last two decades, several mathematical models have been proposed and studied with the aim of understanding the system better, investigating possible pathways to diabetes mellitus, or providing more reasonable insulin administration practices (Citation4 Citation5 Citation10 Citation13 Citation14 Citation25 Citation27 Citation28 Citation33 Citation35 Citation36 Citation41 Citation42 Citation44 Citation48 Citation49 and references therein.) For example, recently, according to the mass conservation law, Li et al. Citation28 proposed a two-delay model for understanding the self-constrained regulatory mechanism of the system better. With the explicit delays, the model is more accurate for depicting glucose–insulin endocrine metabolic dynamics. The model is given as follows.
However, the existing insulin therapy models are inadequate Citation13 Citation14 Citation48 Citation49, either only type 1 diabetes insulin administration is considered Citation49, or some factor in the system is oversimplified. For example, the insulin degradation rate is assumed to be proportional to insulin concentration Citation28 Citation45 Citation48. It is well known that in a chemical reaction, the change rate typically increases with the increasing resource, but does not increase indefinitely when an excess of resource is available. In the glucose–insulin metabolic system, the number of insulin receptors of each cell changes vice versa with the circulating insulin concentration level. An increased insulin circulating level reduces the number of insulin receptors per cell, and the decreased circulating level of insulin triggers the number of receptors to increase. The number of receptors is increased during starvation and decreased in obesity and acromegaly. But, the receptor affinity is decreased by excess gluco-corticoids. The affinity of the receptor for the second insulin molecule is significantly lower than for the first bound molecule. This may explain the negative cooperative interactions observed during high insulin concentrations. That is, as the concentration of insulin increases and more receptors become occupied, the affinity of the receptors for insulin decreases. Conversely, at low insulin concentrations, positive cooperation has been recorded. In other words, the binding of insulin to its receptor at low insulin concentrations seems to enhance the binding further Citation46. Thus, it is more realistic to assume that the insulin degradation rate obeys Michaelis–Menten kinetics given by
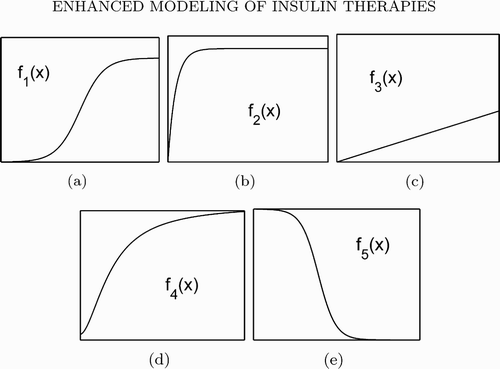
Throughout the paper, we assume the following conditions:
-
(H1) G in,
, (0, ∞)) are positive ω-periodic functions.
-
(H2) f 1(x), f 2(x), f 3(x),
are positive for x > 0.
, $f_2(0) {=} f_3(0)=0$, f 4(0)>0.
-
(H3) f′1(x), f′2(x), f′3(x), f′4(x) are positive on (0;∞).
-
(H4)
is positive on [0,∞), f′5(x) is negative on [0, ∞).
-
(H5) There exist positive numbers b 2, a 3, b 3 such that, for
,
and -
(H6)
.
Assumptions (H1)–(H6) are quite natural. Note that if f
2 takes the form in EquationEquation (5), then
. Thus, it is easy to see that conditions (H1)–(H5) cover the model functions in EquationEquations (5)–(8) used in Citation25
Citation27
Citation41
Citation44
Citation48 for numerical simulations. Assumption (H6) indicates that the maximum insulin clearance rate is larger than the addition of the maximum insulin infusion and production rates. Physiologically, the shapes of the functions are important instead of their forms Citation22 (). The shapes of the functions are detailed in earlier studies Citation22
Citation25
Citation28
Citation41.
Figure 1. Shapes of functions f 1 (a), f 2 (b), f 3 (c), f 4 (d) and f 5(e). Note: These figures are adapted from Citation28.
2. Mathematical analysis
In this section, the positivity and boundedness of solutions of EquationEquation (2) will be examined. We will also state results for the existence of a positive solution of EquationEquation (2)
and its stability. Detailed proofs of the existence and stability will be carried out in Appendices. Throughout this section, we assume conditions (H1–H6) are true.
Lemma 2.1
All solutions of
Equation
Equation (2)
exist for t>0, and they are positive and bounded from above.
Proof
The solution for EquationEquation (2) with the described initial conditions exist and is unique Citation24. Let (G(t), I(t)) be a solution of EquationEquation (2)
with initial conditions I(0)>0, G(0)>0, and
for
. If G(t) is not positive, then t>0, exists such that G(t)=0. Let
. Then, G(t
0)=0 and
. However,
Now we show the boundedness of (G(t), I(t)).
From EquationEquation (2), we have
Now considering the second equation of Equation(2), we have
Now if then I′(t)<0. In fact, we can easily prove, by Lemma 2.2,
Let (I(t), G(t)) be a solution of EquationEquation (2). We define
Lemma 2.2
Let f:R→R be a differentiable function. If
, then there are sequences
,
such that for all k,
,
and
.
Lemma 2.3
Equation
Equation (2)
is uniformly persistent, i.e. solutions of
Equation
Equation (2)
are eventually uniformly bounded from above and away from zero.
Proof
We only need to show that and
. By the fluctuation lemma, there exist a sequence
, such that
To have a positive periodic solution, the left side of the second equation of Equation(2) must change sign. If the rates of insulin injection and insulin production,
, are too large, then the rate of change of insulin, dI/dt, may be always positive and therefore EquationEquation (2)
does not have a periodic solution. In the following existence theorem, we assume that
is small enough so that inequality EquationEquation (16)
in Appendix 1 holds.
Theorem 2.4
If
is sufficiently small, then
Equation
Equation (2)
has a positive periodic solution (G*, I*).
Finally we state the stability result in the following theorem.
Theorem 2.5
The periodic solution (G*, I*) of
Equation
Equation (2)
is locally asymptotically stable if conditions
Equation(18)
and
Equation(19)
in Appendix 2 are satisfied.
Precise mathematical expressions of conditions Equation(18) and Equation(19)
are given in Appendix 2. Based on the expressions of Equation(18)
and Equation(19)
, and inequality EquationEquation (3)
, if the maximal insulin clearance rate d
1 is large, the delay τ1 is small, then, as a lower bound of
, σ0 can be large so that Ĉ>0. Similarly, if f′2 and f′3
f
4 are large, that is, the insulin utilizations are more effective, then [Dcirc]>0. These observations are in agreement with the study of Li and Kuang Citation25.
The proof of Theorem 2.4 is based on the Krasnoselskii fixed point theorem (Lemma A.1), and see Appendix 1 for details. The proof of Theorem 2.5 is based on a standard construction of a Lyapunov functional and will be carried out in Appendix 2.
3. Applications in clinical insulin therapies
In this section, we will discuss an application of model Equation(2) in clinical insulin therapies. The simulations demonstrate that the new model Equation(2)
is suitable for the study of the effectiveness of clinical insulin therapies. In particular, we will see that model Equation(2)
is more realistic. In a normal subject, the liver releases glucose into the blood. This helps the body to maintain cells functioning all 24 h. The pancreas responds by releasing a small but steady amount of insulin (basal) into the bloodstream day and night in a pulsatile manner. After meals, a large amount of insulin (bolus) is released enough to uptake the glucose produced when food is digested. The goal of intensive insulin therapy is to mimic the natural pattern of insulin release from the pancreas so that plasma glucose levels can be kept close to normal.
Currently, various insulin analogues are available for subcutaneous injection, for example, rapid-acting insulin analogues (Lispro and Aspart), short-acting insulin analogues (buffered regular insulin), intermediate-acting insulin analogues (Lente, NHP), and long-acting insulin analogues (Glargine and Ultralente). (adapted from Citation11) lists the time needed for the onset, peak, and duration of several types of insulin.
Table 1. Pharmacokinetics of available insulin products Citation11.
Our simulations are carried out with the same functions used in previous researches (Citation5
Citation25
Citation28
Citation41
Citation44). These functions, f
i
; i=1, 2, 3, 4, 5, take the following forms with experimentally determined parameters given in
Citation41
Citation44. Model Equation(2) utilizes the functions f
1 to f
5 in EquationEquations (5)–(8) and the parameter values in from Citation41
Citation44, which are based on experimental data. These experimental data were taken from normal subjects. With proper insulin treatments, patients can maintain plasma glucose within a normal range, although hypoglycaemia and hyperglycaemia can arise. Therefore, these functions and parameters can be used in the insulin therapy model Equation(2)
for diabetic patients.
Table 2. Parameters of the functions in EquationEquations (5)–(8).
The units of G and I in the functions (5)–(8) are in milligrams and respectively. They are converted to mg dl−1 and μU ml−1 when plotting the figures.
Now, we consider model Equation(2) with τ1=5 min,
, and d
2=2300.
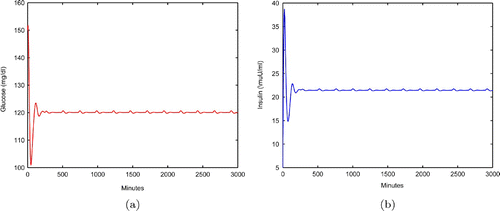
If there is no insulin injection, or , the profiles of glucose and insulin of EquationEquation (9)
is shown in . We see that the blood glucose level is always above 120, which is considered to be abnormal blood glucose level. Therefore, insulin injections are needed to bring the blood glucose level down to normal.
Figure 2. (a) Glucose profile of Equation(2) without insulin therapy. (b) Insulin profile of Equation(2)
without insulin therapy.
As an example, to demonstrate how model Equation(2) can be used in clinical insulin therapy strategies, we select the combination of Lispro and Glargine to mimic the bolus insulin and basal insulin infusion. For simplicity, we assume that G
in(t) and I
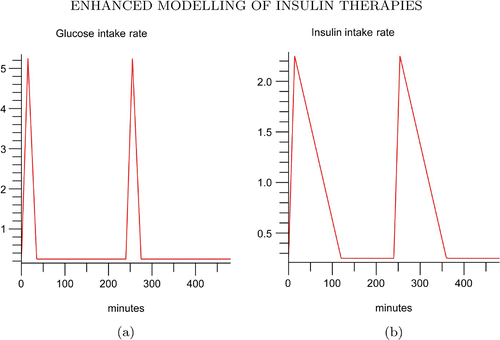
in(t) are periodic piecewise linear functions defined by the following two expressions over a period of ω=240 min. That is, we assume that both the meal digestion and the diffusion of subcutaneous insulin injection are linearly dependent on time. They can be extended to a periodic continuous function on [0, ∞). Namely, a subject consumes meal and is given insulin injection every 4 h. The maximum glucose intake was at 5 mg/min attained at 15 min mark. The whole duration of the glucose intake lasted for 35 min. EquationEquation (10)
is the glucose intake rate, G
in ().
We consider the combination of Glargine and Lispro insulins. The piecewise functions of in EquationEquation (11)
and
in EquationEquation (12)
mimic the insulin infusion rate if a patient takes Lispro or Glargine. The rapid-acting insulin mimics the bolus ultradian insulin secretion stimulated by the elevated glucose concentration level, while the long-acting insulin mimics the basal pulsatile insulin secretion. It is clear that the common period for G
in(t) and I
in(t) is ω=240 min.
With τ1=5 min and the simulation in (a) and (b) demonstrate the glucose and insulin concentration profiles of four injections a day of bolus insulin Lispro and one injection a day of basal insulin Glargine. The insulin injection rate is the combination of
and
. Therefore, I
in in EquationEquation (9)
takes the following expression.
Figure 4. Glucose and insulin profiles with the combination of Lispro and Glargine for Monod insulin-degradation model Equation(9) (a) Glucose profile of EquationEquation (9)
. (b) Insulin profile of EquationEquation (9)
.
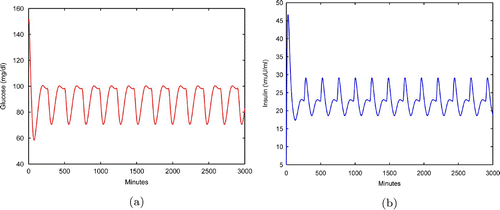
The glucose level shown in (c) for model Equation(9) is between 70 and 100 mg dl−1. It is seen that the profiles are close to the profiles of a normal subject. We should also point out that blood glucose levels could be close to 160 mg dl−1 after meals Citation32.
4. Discussions
To optimize the strategies of insulin adminstration, we choose the combination of insulin Glargine and Lispro as basal and bolus insulin, respectively, because clinical trials have shown that insulin Glargine plus insulin Lispro can improve glycaemic control of type 1 diabetes Citation2. In their study, the combination of insulin Glargine with insulin Lispro was compared with NPH insulin plus unmodified human insulin. It is reported that there was as much as 44% reduction of monthly rate of nocturnal hypoglycaemia with Glargine plus Lispro. These improvements in blood glucose control were obtained without any increase in episodes of hypoglycaemia. In fact, in another clinical trial by Murphy et al. Citation34, it has been documented that combination therapy with insulin Glargine plus insulin Lispro reduced the incidence of nocturnal hypoglycemia and was at least as effective as R/NPH insulin therapy in maintaining glycaemic control.
Currently, an insulin pump is the most advanced method of insulin administration for type 1 diabetes Citation26. With careful integration of the existing models for glucose absorption from the gut and/or subcutaneous insulin absorption and the regulation of glucose–insulin, an artificial pancreas can be created and put forward in clinical applications. The model proposed in this paper when integrated with the models proposed in earlier studies Citation25 Citation26, can form a solid foundation for an artificial pancreas.
Model Equation(2) does not take into account individual patient factors, such as age, treatment schedule, and exercise. Patient factors, including individual variations in insulin absorption, levels of exercise, local massage, and especially, local subcutaneous blood flow that can influence the effectiveness of an insulin regimen Citation16. The time taken to absorb one-half of an injected dose of insulin may vary by 25–50% among individual patients Citation19. Thus, it is important to implement individualized therapies, which are flexible to fit the needs of the patient. Model Equation(2)
can be adjusted to reflect the patient factors by incorporating a time delay in I
in.
In model Equation(2), we neglect the delayed effect of hepatic glucose production in f
5. In fact, the delayed effect on the dynamics of glucose and insulin for normal subjects under continuous and constant glucose infusion can be significant Citation25
Citation28. We will further investigate the delayed effect in a future research.
Experimental results by Rassam et al. Citation38 demonstrated that the injection of Lispro insulin 15–30 min before meal can optimally reduce postprandia hyperglycaemia. In our simulation, we assume that lispro insulin is injected 15 min before meal. Other regular insulins are recommended to be injected 30 or 45 min before meal. In practice, patients often inject insulin closer to mealtime, causing a higher postprandial serum glucose level and an increased potential for hypoglycaemia in the postabsorptive period. Thus, Lispro insulin results in more satisfactory postprandial glucose control.
In summary, we propose a new insulin therapy model using Michaelis–Menten kinetics. We are focusing on studying the dynamics of the model, and will continue the research and validate the model to fit actual data of the subjects.
Acknowledgements
This work is dedicated to Professor Ken Cooke. This work is partially supported by ASU MGIA-2006-08. The third author is also supported by DMS-0436341 and DMS/NIGMS-0342388. We are grateful to the anonymous referees and the editor whose careful reading, valuable comments, and suggestions greatly enhanced the original manuscript.
References
- Ahrén , B. and Pacini , G. 2004 . Importance of quantifying insulin secretion in relation to insulin sensitivity to accurately assess beta cell function in clinical studies . Eur. J. Endocrinol. , 150 : 97 – 104 .
- Ashwell , C. G. , Amiel , S. A. , Bilous , R. W. , Dashora , U. , Heller , S. R. , Hepburn , D. A. , Shutler , S. D. , Stephens , J. W. and Home , P. D. 2006 . Improved glycaemic control with insulin glargine plus insulin lispro: a multicentre, randomized, cross-over trial in people with Type 1 diabetes . Diabet. Med. , 23 : 285292
- Barbalat , I. 1959 . Systems d'equations differential d'oscillations nonlinearies . Rev. Roumaine Math. Pure. Appl. , 4 : 267 – 270 .
- Bennett , D. L. and Gourley , S. A. 2004 . Periodic oscillations in a model of the glucose-insulin interaction with delay and periodic forcing . Dyn. Syst. , 19 : 109 – 125 .
- Bennett , D. L. and Gourley , S. A. 2004 . Global stability in a model of the glucose-insulin interaction with time delay . Eur. J. Appl. Math. , 15 : 203 – 221 .
- Bergman , R. N. 2002 . Pathogenesis and prediction of diabetes mellitus: lessons from integrative physiology, in Irving L. Schwartz Lecture . Mount Sinai, J. Med. , 60 : 280 – 290 .
- Bergman , R. N. , Finegood , D. T. and Ader , M. 1985 . The assessment of insulin sensitivity in vivo . Endocr. Rev. , 6 ( 1 ) : 45 – 86 .
- Bergman , R. N. , Finegood , D. T. and Kahn , S. E. 2002 . The evolution of beta-cell dysfunction and insulin resistance in type 2 diabetes . Eur. J. Clin. Invest. , 32 ( Suppl. 3 ) : 35 – 45 .
- Bolie , V. W. 1961 . Coeffcients of normal blook glucose regulation . J. Appl. Physiol. , 16 : 783 – 788 .
- Dalla Man , C. , Caumo , A. and Cobelli , C. 2002 . The oral glucose minimal model: estimation of insulin sensitivity from a meal test . IEEE Trans. Biomed. Eng. , 49 : 419 – 429 .
- Daugherty , K. K. 2004 . Review of Insulin Therapy . J. Pharm. Pract. , 17 : 10 – 19 .
- De Gaetano , A. and Arino , O. 2000 . Mathematical modeling of the intravenous glucose tolerance test . J. Math. Biol. , 40 : 136 – 168 .
- Doran , C. V. , Chase , J. G. , Shaw , G. M. , Moorhead , K. T. and Hudson , N. H. 2005 . Derivative weighted active insulin control algorithms and intensive care unit trials . Control Eng. Pract. , 13 : 1129 – 1137 .
- Engelborghs , K. , Lemaire , V. , Belair , J. and Roose , D. 2001 . Numerical bifurcation analysis of delay differential equations arising from physiological modeling . J. Math. Biol. , 42 : 361 – 385 .
- Fernqvist , E. , Linde , B. , Ostman , J. and Gunnarsson , R. 1986 . Effects of physical exercise on insulin absorption in insulin-dependent diabetics. A comparison between human and porcine insulin . Clin. Physiol. , 6 : 487 – 497 .
- Goodyear , L. and Kahn , B. 1998 . Exercise, glucose transport, and insulin sensitivity . Ann. Rev. Med. , 49 : 235 – 261 .
- Gottesman , I. , Mandarino , L. and Gerich , J. 1983 . Estimation and kinetic analysis of insulin-independent glucose uptake in human subjects . Am. J. Physiol. Endocrinol. Metab. , 244 : E632 – E635 .
- Hildebrandt , P. 1991 . Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients . Acta Physiol. Scand. Suppl. , : 41 – 45 . 733 – 753 .
- Hirsch , I. B. 1999 . Type 1 Diabetes Mellitus and the use of flexible insulin regimens . Am. Fam. Physician , 60 : 2343 – 2352 . 2355 – 2356 .
- Hirsch , W. M. , Hanisch , H. and Gabriel , J. P. 1985 . Differential equation models of some parasitic infections:methods for the study of asymptotic behavior . Comm. Pure Appl. Math. , 38 : 733 – 753 .
- Keener , J. P. 2001 . Diffusion induced oscillatory insulin secretion . Bull. Math. Biol. , 63 : 625 – 641 .
- Keener , J. and Sneyd , J. 1998 . Mathematical Physiology , New York : Springer-Verlag .
- M. Krasnoselskii, Positive Solutions of Operator Equations, Noordhoff, Groningen, 1964
- Kuang , Y. 1993 . “ Delay differential equations with applications in population dynamics ” . In The Series of Mathematics in Science and Engineering , Vol. 191 , Boston : Academic Press .
- Li , J. and Kuang , Y. 2007 . Analysis of a model of the glucose-insulin regulatory system with two delays . SIAM J. Appl. Math. , 67 : 757 – 776 .
- J. Li and Y. Kuang, Pharmacokinetical models of subcutaneous injection of insulin analogues for type 1 diabetes, in review
- Li , J. , Kuang , Y. and Li , B. 2001 . Analysis of IVGTT glucose-insulin interaction models with time delay . Discrete Contin. Dyn. Syst. Ser. B , 1 : 103 – 124 .
- Li , J. , Kuang , Y. and Mason , C. 2006 . Modeling the glucose-insulin regulatory system and ultradian insulin secretory oscillations with two time delays . J. Theor. Biol. , 242 : 722 – 735 .
- Mahaffy , J. M. and Edelstein-Keshet , L. 2007 . Modeling cyclic waves of circulating T cells in autoimmune diabetes . SIAM J. Appl. Math. , 67 ( 4 ) : 951 – 937 .
- Makroglou , A. , Li , J. and Kuang , Y. 2006 . Mathematical models and software tools for the glucose-insulin regulatory system and diabetes: an overview . Appl. Numerical Math. , 56 : 559 – 573 .
- Mayfield , J. A. and White , R. D. 2004 . Insulin therapy for type 2 diabetes: Rescue, augmentation, and replacement of β -cell function . Am. Fam. Physician , 70 : 489 – 500 .
- Mooradian , A. D. and Thurman , J. E. 1999 . Drug therapy of postprandial hyperglycaemia . Drugs , 57 : 19 – 29 .
- Mukhopadhyay , A. , DeGaetano , A. and Arino , O. 2004 . Modeling the intra-venous glucose tolerance test: a global study for a single-distributed-delay model . Discrete Contin. Dyn. Syst. Ser. B , 4 : 407 – 417 .
- Murphy , N. P. , Keane , S. M. , Ong , K. K. , Ford-Adams , M. , Edge , J. A. , Acerini , C. L. and Dunger , D. B. 2003 . Randomized cross-over trial of insulin glargine plus lispro or NPH insulin plus regular human insulin in adolescents with Type 1 Diabetes on intensive insulin regimens, . Diabetes Care , 26 : 799 – 804 .
- Palumbo , P. , Panunzi , S. and De Gaetano , A. 2007 . Qualitative behavior of a family of delay-differential models of the glucose-insulin system . Discrete Contin. Dyn. Syst. Ser. B , 7 : 399 – 424 .
- Panunzi , S. , Palumbo , P. and De Gaetano , A. 2007 . A discrete single-delay model for the Intra-Venous Glucose Tolerance Test . Theor. Biol. Med. Model , 4
- Pöksen , N. , Hollingdal , M. , Juhl , C. , Butler , P. , Veldhuis , J. D. and Schmitz , O. 2002 . Pulsatile insulin secretion: detection, regulation, and role in diabetes . Diabetes , 51 : S245 – S254 .
- Rassam , A. G. , Zeise , T. M. , Burge , M. R. and Schade , D. S. 1999 . Optimal administration of lispro insulin in hyperglycemic type 1 diabetes, . Diabetes Care , 22 : 133 – 136 .
- Recasensa , M. , Aguileraa , E. , Mornigoa , R. , Casamitjanab , R. , Nicolettic , F. , Gomisa , R. and Conget , I. 2003 . Insulin lispro is as effective as regular insulin in optimising metabolic control and preserving – cell function at onset of type 1 diabetes mellitus . Diabetes Res. Clin. Pract. , 60 : 153 – 159 .
- Simon , C. and Brandenberger , G. 2002 . Ultradian oscillations of insulin secretion in humans . Diabetes , 51 : S258 – S261 .
- Sturis , J. , Polonsky , K. S. , Mosekilde , E. and Van Cauter , E. 1991 . Computer model for mechanisms underlying ultradian oscillations of insulin and glucose . Am. J. Physiol. , 260 : E801 – E809 .
- Sturis , J. , Scheen , A. , Leproult , R. , Polonsky , K. and Van Cauter , E. 1995 . 24-hour glucose profles during continuous or oscillatory insulin infusion . J. Clin. Invest. , 95 : 1464 – 1471 .
- Thompson , C. L. , Dunn , K. C. , Menon , M. C. , Kearns , L. E. and Braithwaite , S. S. 2005 . Hyperglycemia in the hospital . Diab. Spectr. , 18 ( 1 ) : 20 – 27 .
- Tolic , I. M. , Mosekilde , E. and Sturis , J. 2000 . Modeling the insulin-glucose feedback system: the signifcance of pulsatile insulin secretion . J. Theor. Biol. , 207 : 361 – 375 .
- Topp , B. , Promislow , K. , De Vries , G. , Miura , R. M. and Finegood , D. T. 2000 . A Model of β -cell mass, insulin, and glucose kinetics: pathways to diabetes . J. Theor. Biol. , 206 : 605 – 619 .
- Wanant , S. and Quon , A. J. 2000 . Insulin receptor binding kinetics: modeling and simulation studies . J. theor. Biol. , 205 : 355 – 364 .
- Wang , H. 2004 . Positive periodic solutions of functional differential equations . J. Diff. Equ. , 202 : 354 – 366 .
- Wang , H. , Li , J. and Kuang , Y. 2007 . Mathematical modeling and qualitative analysis of insulin therapies, . Math. Biosci. , 210 : 17 – 33 .
- Wilinska , M. E. , Chassin , L. J. , Schaller , H. C. , Schaupp , L. , Pieber , T. R. and Hovorka , R. 2005 . Insulin kinetics in type-1 diabetes: continuous and bolus delivery of rapid acting insulin . IEEE Trans. Biomed. Eng. , 52 : 3 – 12 .
Appendix 1. Proof of Theorem 2.4
In this section, we will give a proof of Theorem 2.4. A related result can be found in Citation47. Before we state the Krasnoselskii fixed point theorem, let us recall some concepts and conclusions of an operator in a cone in Citation23. Let X be a Banach space and K be a closed, non-empty subset of X. K is said to be a cone if Equation(1)
for all u, v∈K and all
and Equation(2)
u,− u ∈ K, imply u=0. Assume Ω is a bounded open subset in X with the boundary
, and let
be completely continuous, such that Tx ≠ x for
.
Lemma .1
Krasnoselskii's Fixed Point Theorem Citation23. Let X be a Banach space and let K∈X be a cone in X. Assume that Ω1, Ω2
are open subsets of X with
and let
-
and
or
-
and
First, since f 4 is bounded below and above, we can assume there exist two positive numbers m 4 and M 4, such that
We then define two open sets and
as
Note that and
Define the map by
Note that
In order to use the above fixed point theorem we need the following lemma.
Lemma .2
is compact and continuous.
Proof
We only consider the first component T 1(u, v). The proof for T 2(u, v) is the same. In view of the definition of T 1, for (u, v)∈K, we have
Lemma .3
If (u, v) is a fixed point of T in
, then (u, v) is a positive periodic solution of
Equation(2)
.
Proof
If and T
1(u, v)=u, then
Appendix 2: Proof of Theorem 2.5
In this section, we shall show that the periodic solution of EquationEquation (2)
is locally asymptotically stable. Our proof is based on the construction of Lyapunov functionals. Before we proceed to discuss stability, we state a lemma from Citation3 without proof, which will be employed to establish stability.
Lemma .4
Let h be a real number and f be a non-negative function defined on [h, ∞) such that f is integrable on [h, ∞) and is uniformly continuous on [h, ∞). Then
.
Now assume (G(t), I(t)) is a solution of EquationEquation (2) with initial condition I(0)>0, G(0)>0, and
for
. For simplicity we use the transformation
Note that both I and I* are bounded for t≥0. Therefore, there exist a positive number σ0 such that
Thus