Abstract
Patterns of contact in social behaviour and seasonality due to environmental influences often affect the spread and persistence of diseases. Models of epidemics with seasonality and patterns in the contact rate include time-periodic coefficients, making the systems nonautonomous. No general method exists for calculating the basic reproduction number, the threshold for disease extinction, in nonautonomous epidemic models. However, for some epidemic models with periodic coefficients and constant population size, the time-averaged basic reproduction number has been shown to be a threshold for disease extinction. We extend these results by showing that the time-averaged basic reproduction number is a threshold for disease extinction when the population demographics are periodic. The results are shown to hold in epidemic models with periodic demographics that include temporary immunity, isolation, and multiple strains.
1. Introduction
Periodicity in epidemics often occurs due to patterns of contact in social behaviour or due to seasonality from environmental factors Citation13 Citation23. Influenza is a disease that is well-known to have a recurrent seasonal pattern every year, a new viral strain is often introduced each year Citation23. Meningococcal meningitis in western Africa also varies seasonally, where there is a strong asso- ciation between annual epidemics and atmospheric circulation Citation29 Citation36. The human immune system follows a cyclic pattern in which the body may either become more susceptible or more resilient to certain diseases seasonally during the year Citation13 Citation23 Citation28. In addition to the disease parameters exhibiting periodicity, the underlying population demographics may also exhibit periodicity. In animal populations, the birth and death rates and carrying capacity may vary periodically due to environmental factors or to the reproductive cycle Citation8 Citation17 Citation18 Citation21 Citation31 Citation32 Citation38. Disease models with periodicity in births, deaths, and carrying capacity have been applied to the study of hantavirus in bank voles Citation31 Citation32 Citation38. Seasonality has been studied in many types of epidemic models, showing that periodic solutions exist, period doubling and chaos occur, or providing conditions for disease extinction Citation3 Citation6,Citation10–12,Citation14 Citation15 Citation17 Citation22 Citation23,Citation24–27,Citation30,Citation33–35.
In this investigation, we concentrate on providing conditions for disease extinction. The well-known disease extinction threshold in epidemic models is known as the basic reproduction number, the number of secondary infections caused by one infective individual in an entirely susceptible population Citation19. For autonomous differential equations or difference equations, the basic reproduction number can be calculated from the next generation matrix approach Citation5 Citation9 Citation37 or by linearization about a disease-free equilibrium (DFE). But for nonautonomous models, there is no standard procedure to calculate the basic reproduction number. We show, in some periodic nonautonomous epidemic models, expressed in terms of differential equations, that the basic reproduction number can be obtained from the corresponding autonomous model by using the time-average of the coefficients. Ma and Ma Citation24 and Greenhalgh and Moneim Citation14 Citation27 showed in some nonautonomous SIR-type epidemic models with periodic contact rate β (t) and constant population size that the basic reproduction number for the nonautonomous epidemic model is the same as that for the autonomous SIR epidemic model, where β (t) and other periodic coefficients are replaced by their averages, e.g.,
In the next section, we assume that, in the absence of disease, the population size satisfies a nonautonomous logistic equation with periodic birth rate, death rate, and carrying capacity. We show that solutions to this nonautonomous logistic equation are bounded and converge to a periodic solution. Then in Section 3, we study populations with periodic demographics, where disease is introduced, and the contact rate, recovery rate, and other rates may also be periodic. We assume that the disease does not result in additional mortality and that the mode of transmission can be either standard or mass action. In each of the models, it is shown that the basic reproduction number for the time-averaged autonomous epidemic model is also a basic reproduction number for the nonautonomous model. In particular, if
, then the population becomes disease-free, but if
, then the disease-free state is unstable. In Section 4, the effects of isolation are added to the epidemic model, and in Section 5, an epidemic model with multiple strains is studied. Some numerical examples are presented in Section 6 to illustrate the results. Then the results are summarized in the conclusion.
2. Periodic population dynamics
Let the total population size at time t be denoted as P(t). Let denote the intrinsic growth rate, the per capita birth rate minus the per capita density-independent death rate. Let k(t)P(t) denote the per capita density-dependent death rate. The parameters b(t), d(t), and k(t), defined for
, are continuous, nonnegative, bounded, and periodic with the same period p>0. The period p may not be the minimal period for some of the functions because we will allow some of them to be constant or to have different minimal periods. But p is the common period for all of the parameter functions b(t), d(t), and k(t) and the minimal period for at least one of the functions. Thus,
, and k(t+p)=k(t) for all t≥0. Let [bcirc], [dcirc], and [kcirc] denote their respective averages, e.g.,
| i. | [bcirc]>0; | ||||
| ii. |
| ||||
| iii. |
| ||||
We will show that solutions P(t) to Equation(1) are bounded above and below by positive constants and are periodic. The proof for boundedness of solutions to Equation(1)
follows closely a proof by Ireland et al. [Citation21, Lemma 1, pp. 43–44].
Theorem 2.1
Assume (i)–(iii) for model
Equation(1). Then there exists positive constants P
min
and P
max
such that
for all
.
Proof
Notice that . Let
(assumptions (ii) and (iii)). If P(t)>M, then P˙(t)<0. Hence, for all
.
To prove that P(t) is bounded below by a positive constant, choose δ>0 such that and
Let and
, where
and b
max>0. Then
for t∈[t
1, t
2]. If
,
for t∈[t
1, t
2]. In this case, it follows that
, which is a contradiction. Thus, it must be the case that
Making the change of variable in EquationEquation (1), u=1/P, we are led to the following linear differential equation:
Theorem 2.2
The solution P(t) of model
Equation(1)
converges uniformly to a unique periodic solution P*(t) with period p.
Proof
We prove that u(t) converges uniformly to the periodic solution u*(t) and u*(t) is unique. Hence, P(t) converges uniformly to the periodic solution P*(t). Let . Because u*(t) is periodic of period
for
. The periodic solution
3. SIRS epidemic model with seasonal births, deaths, and transitions
We consider an SIRS model with temporary immunity. The variable S denotes the number of susceptible individuals, variable I the number of infected and infectious individuals, and variable R the number of individuals that have recovered from the disease with temporary immunity. We assume that there are no disease-related deaths. Parameter γ (t) is the recovery rate and ρ(t) is the rate that recovered individuals lose their immunity. The periodic epidemic model is given by the following system of differential equations:
| a. |
| ||||
| b. |
| ||||
-
(iv)
, and
.
When , then model Equation(6)
simplifies to an SIR epidemic model with permanent immunity. When both parameters
, then model Equation(6)
simplifies to an SI epidemic model.
Applying the substitutions s=S/P, i=I/P, and r=R/P, we arrive at a system of proportions, where , and
| a. |
| ||||
| b. |
| ||||
Define the basic reproduction number as
| a. |
| ||||
| b. |
| ||||
For case (a) of standard incidence, the next theorem follows directly from Ma and Ma [Citation24, Theorem 7, p. 168] and Theorems 2.1 and 2.2. For case (b) of mass action incidence, these previous theorems can also be applied with a slight modification.
Theorem 3.1
Assume (i)– (iv) and
for t∈[0, p) for model
Equation(7)
. Let
be defined as in
Equation(8)
. If
, then the DFE (1, 0, 0) for model
Equation(7)
is locally asymptotically stable. If
, then the DFE (1, 0, 0) for model
Equation(7)
is unstable.
Proof
We verify the results for case (b), mass action incidence. Let . We analyse the DFE for the proportions (s, i, r)=(1, 0, 0). Linearization of i(t) in Equation(7)
leads to
with solution
Let . Consider the linearization of model Equation(7)
. For ε>0, there exists T>0 such that for
. Since ε can be chosen sufficiently small so that
, it follows that
as t→∞. But this means the disease-free state (1, 0, 0) for model Equation(7)
is unstable. ▪
It can be proved that global disease extinction occurs in case .
Theorem 3.2
Assume (i)–(iv) and
for t∈[0, p) for model
Equation(6)
. Let
be defined as in
Equation(8)
. If
, then disease extinction occurs in model
Equation(6)
,
, and
, where P*(t) is the periodic solution of model
Equation(1)
.
Proof
To show the global stability of the disease-free state, consider first the proportional model Equation(7). For ε>0, there exists T sufficiently large such that for
. For case (a), standard incidence,
. For case (b), mass action incidence, it follows for
. In both cases,
(see proof of Theorem 3.1), which means
,
, and
in model Equation(6)
as t→∞. ▪
4. SIXS model with seasonal isolation
Our results apply to an epidemic model with isolation, originally developed by Hethcote et al. Citation20, where we allow the parameters to be periodic functions. This model describes the long-term dynamical behaviour when there is isolation of infectious individuals. Quarantine or isolation (confinement of exposed or infectious individuals, respectively) have been practiced for human diseases such as leprosy, tuberculosis, measles, and scarlet fever and animal diseases such as foot and mouth disease, psittacosis, and rabies Citation20. The variable X represents the number of infected and infectious individuals I that are isolated. For example, isolation may mean bed rest at home during holidays or during school breaks which often occur seasonally. The periodic function ν(t) is the rate at which infected individuals are isolated; ε(t) is the rate at which infected individuals are removed from isolation because they are no longer infectious; they are put in the susceptible class. The periodic function γ (t) is the rate of recovery of infectious individuals; no immunity is assumed. Other parameters are the same as in the SIRS model in the previous section. The SIXS model has the following form:
-
(v)
, and
.
Rewriting system Equation(9), in terms of proportions, s=S/P, i=I/P, and x=X/P,
Theorem 4.1
Assume (i)– (iii), (v), and
for t∈[0, p) for model
Equation(10)
. Let
be defined as in
Equation(11)
. If
, then the DFE (1, 0, 0) for model
Equation(10)
is locally asymptotically stable. If
, then the DFE (1, 0, 0) for model
Equation(10)
is unstable.
Proof
The local stability can be determined by linearizing the system about the DFE,
If , then
and
as t→∞. The DFE (1, 0, 0) is unstable. ▪
Using arguments similar to the proof for the linearized system, we are able to prove that the infection dies out in model Equation(9) if
.
Theorem 4.2
Assume (i)–(iii), (v), and
for t∈[0, p) for model
Equation(9)
. Let
be defined as in
Equation(11)
. If
, then disease extinction occurs in model
Equation(9)
;
,
, and
, where P*(t) is the periodic solution of model
Equation(1)
.
Proof
Note that in EquationEquation (10),
. Applying the methods in the proofs of Theorem 3.2 and 4.1, it follows that
as t→∞ for
, which imply
, and
as t→∞. ▪
5. SIRS model with multiple strains and cross-immunity
We apply our results to SIRS models with multiple strains, models studied by Ackleh and Allen Citation1
Citation2. But we generalize the models by including periodic parameter functions. Each strain provides immunity from infection by other related strains, referred to as cross-immunity. Cross-immunity between strains can lead to competitive exclusion in which an aggressive strain can drive the other strains to extinction Citation1
Citation2. We assume that there are periodic contacts and the other coefficients may be periodic as well. Individuals may be infected with one of the n different strains, where the number of individuals infected and infectious with strain j is denoted as . Recovery from infection by strain j occurs at a rate γ
j
(t). We make the additional assumption that recovery may be only temporary; loss of immunity occurs at a rate ρ (t). We assume that there are no disease-related deaths, so that the total population size satisfies model Equation(1)
.
The model with multiple strains takes the following form:
| a. |
| ||||
| b. |
| ||||
-
(vi)
.
When there is just a single strain, n=1, the differential equations in EquationEquation (13) reduce to model Equation(6)
. When
, then model Equation(13)
simplifies to an epidemic model with permanent immunity. When the parameters
and
, then there is no immunity.
Rewriting system Equation(13) in terms of proportions,
, and r=R/P,
| a. |
| ||||
| b. |
| ||||
Define the strain reproduction number as
| a. |
| ||||
| b. |
| ||||
Then the basic reproduction number is defined as
Theorem 5.1
Assume (i)– (iii), (vi), and
for t∈[0, p) for model
Equation(14)
. Let
be defined as in
Equation(15)
. If
, then the DFE (1, 0, …, 0) for model
Equation(14)
is locally asymptotically stable. If
, then the DFE (1, 0, …, 0) for model
Equation(14)
is unstable.
Proof
Linearizing system Equation(14) about the DFE (1, 0, …, 0),
Theorem 5.2
Assume (i) – (iii), (vi), and
for t∈[0, p) for model
Equation(13)
. Let
be defined as in
Equation(15)
. If
, then disease extinction occurs in model
Equation(13)
,
, j=1, …, n,
, and
, where P*(t) is the periodic solution of model
Equation(1)
.
Proof
Note that i
j
in the proportional model Equation(13) satisfies
. Hence, it follows as in the proofs of Theorems 3.2 and 4.2 for
that there is extinction of I
j
. Disease extinction of all I
j
, j=1, …, n, requires all strain reproduction numbers to be less than one or
. ▪
6. Numerical examples
Several numerical examples illustrate our results for the SIRS model (Theorems 3.1 and 3.2) discussed in Section 3. Let
Figure 1. Solution P(t) to the periodic logistic differential Equationequation (1) with P(0)=1030 converges to a periodic solution P*(t) with period p=3 years and [Pcirc]=1516. Parameter functions b(t), d(t), and k(t) are defined in the text.
![Figure 1. Solution P(t) to the periodic logistic differential Equationequation (1) with P(0)=1030 converges to a periodic solution P*(t) with period p=3 years and [Pcirc]=1516. Parameter functions b(t), d(t), and k(t) are defined in the text.](/cms/asset/848f848f-d4f6-49d5-803c-bdc092f74a45/tjbd_a_330656_o_f0001g.gif)
For the SIRS epidemic model Equation(6), let the parameters for recovery and transmission be defined as
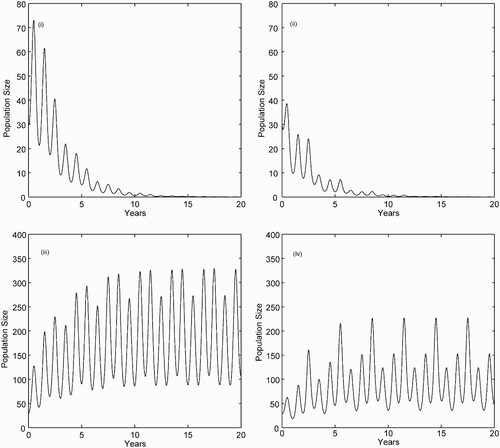
Figure 2. Number of infectious individuals I(t) for the SIRS model Equation(6). For solutions graphed in (i) and (iii), we assume standard incidence, whereas in (ii) and (iv), we assume mass action incidence. The parameters b(t), d(t), k(t), γ (t), ρ(t), and β(t) are defined in the text, S(0)=1000, I(0)=30, and R(0)=0. (i) Standard incidence, βˆ=3.6 and ˆℛ0=0.90. (ii) Mass action incidence, βˆ=0.0024 and ˆℛ0=0.91. (iii) Standard incidence, βˆ=4.4 and ˆℛ0=1.10. (iv) Mass action incidence, βˆ=0.003 and ˆℛ0=1.14.
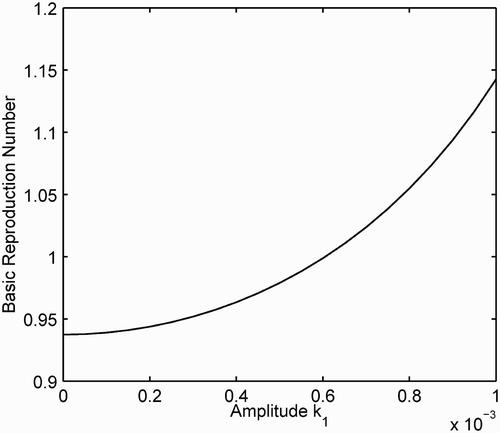
Calculation of for standard incidence is straightforward by applying EquationEquation (8)
because
, [bcirc]=2, and
. To calculate
for the mass action case requires the estimation of
The population size has a direct impact on the basic reproduction number in the case of mass action incidence (as can be seen in formula Equation(17)). Even when the average values of the parameter functions are constant, the amplitude of the density-dependent mortality function k(t) can change the dynamics of P*(t), which in turn affects
. Let
, where
. Let all other parameter functions be as defined previously with
. Then
and
().
7. Conclusion
The effects of seasonality in epidemic models reflect the population and disease dynamics that often occur in nature and provide greater biological realism of the mechanisms that may be behind the emergence of disease outbreaks in human and wildlife systems. For epidemic models with periodic coefficients, it is important to be able to estimate the basic reproduction number. We have shown that the basic reproduction number for periodic nonautonomous epidemic models can be computed from the corresponding time-averaged autonomous epidemic models in some cases. Periodicity can occur in contact rates, birth rates, death rates, carrying capacity, recovery, and isolation. These results extend previous results, where it was assumed that the population size remained constant Citation14 Citation24 Citation27. We have shown that the population size can also be periodic. The results hold for the standard and mass action incidence and can be applied to periodic nonautonomous epidemic models with temporary immunity, isolation, and multiple strains. An important restriction in our results is that there are no disease-related deaths. Additional mortality due to disease changes the dynamics of the total population size, P(t), and could result in total population extinction. Our results have applications to a zoonotic disease carried by wild rodents, hantavirus. Certain species of rodents serve as reservoirs for hantavirus infection, but no additional mortality results from the infection Citation4 Citation7. Bank voles, reservoirs for the hantavirus known as Puumala virus, have been shown to exhibit population cycles of either 3–4 years or 1 year in different parts of Europe Citation31 Citation32 Citation38.
Acknowledgements
Financial support was provided by the National Science Foundation, DMS-0718302 (LJSA) and the Fogarty International Center, no. #R01TW006986-02 under the NIH NSF Ecology of Infectious Diseases initiative (LJSA, CLW). We thank E.J. Allen and M. Langlais for helpful comments and for providing references for this work. In addition, we thank two anonymous reviewers for their helpful suggestions.
References
- Ackleh , A. S. and Allen , L. J.S. 2003 . Competitive exclusion and coexistence for pathogens in an epidemic model with variable population size . J. Math. Biol. , 47 : 153 – 168 .
- Ackleh , A. S. and Allen , L. J.S. 2005 . Competitive exclusion in SIS and SIR epidemic models with total cross immunity and density-dependent host mortality . Discrete Cont. Dyn. Sys. Series B , 5 : 175 – 188 .
- Alexander , M. E. and Moghadas , S. M. 2004 . Periodicity in an epidemic model with a generalized non-linear incidence . Math. Biosci. , 189 : 75 – 96 .
- Allen , L. J.S. , McCormack , R. K. and Jonsson , C. B. 2006 . Mathematical models for hantavirus infection in rodents . Bull. Math. Biol. , 68 : 511 – 524 .
- Allen , L. J.S. and van den Driessche , P. 2008 . The basic reproduction number in some discrete-time epidemic models . J. Differ. Equ. Appl. , (in press)
- Aron , J. L. and Schwartz , I. 1984 . Seasonality and period-doubling bifurcations in an epidemic model . J. Theor. Biol. , 110 : 665 – 679 .
- Chu , Y. K. , Owen , R. D. , Gonzalez , L. M. and Jonsson , C. B. 2003 . The complex ecology of hantavirus in Paraguay . Amer. J. Trop. Med. Hyg. , 69 : 263 – 268 .
- Cushing , J. M. 1998 . An Introduction to Structured Population Dynamics , Philadelphia : SIAM .
- Diekmann , O. , Heesterbeek , J. A.P. and Metz , J. A.J. 1990 . On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations . J. Math. Biol. , 28 : 365 – 382 .
- Dietz , K. Proceedings of aWorkshop on Mathematical Modeling in Medicine . Mainz, Berlin, Germany. The incidence of infectious diseases under the influence of seasonal fluctuations , Vol. 11 , pp. 1 – 15 . Lecture Notes in Biomathematics
- Dowell , F. 2001 . Seasonal variations in host susceptibility and cycles of certain infectious diseases . Emerg. Infect. Dis. , 7 : 369 – 374 .
- Dushoff , J. , Plotkin , J. B. , Levin , S. M. and Earn , D. J.D. 2004 . Dynamical resonance can account for seasonality of influenza epidemics . Proc. Natl Acad. Sci. , 101 : 16915 – 16916 .
- Grassly , N. C. and Fraser , C. 2006 . Seasonal infectious disease epidemiology . Proc. Roy. Soc. Lond. B , 273 : 2541 – 2550 .
- Greenhalgh , D. and Moneim , I. 2003 . SIRS epidemic model and simulations using different types of seasonal contact rate . Syst. Anal. Model. Simul. , 43 : 573 – 600 .
- Grossmann , Z. , Gumowski , I. and Dietz , K. 1977 . “ The incidence of infectious diseases under the influence of seasonal fluctuations: analytic approach ” . In Nonlinear Systems and Applications to Life Sciences , 525 – 546 . New York : Academic Press .
- Hartman , P. 1964 . Ordinary Differential Equations , New York : John Wiley and Sons, Inc .
- He , D. and Earn , D. J.D. 2007 . Epidemiological effects of seasonal oscillations in birth rates . Theor. Popul. Biol. , 72 : 274 – 291 .
- Henson , S. M. and Cushing , J. M. 1997 . The effect of periodic habitat fluctuations on a nonlinear insect population model . J. Math. Biol. , 36 : 201 – 226 .
- Hethcote , H. W. 2000 . The mathematics of infectious disease . SIAM Rev. , 42 : 599 – 653 .
- Hethcote , H. W. , Ma , Z. and Liao , S. 2002 . Effects of quarantine in six endemic models for infectious diseases . Math. Biosci. , 180 : 141 – 160 .
- Ireland , J. M. , Mestel , B. D. and Norman , R. A. 2007 . The effect of seasonal host birth rates on disease persistence . Math. Biosci. , 206 : 31 – 45 .
- Lloyd , A. L. and May , R. M. 1996 . Spatial heterogeneity in epidemic models . J. Theor. Biol. , 179 : 1 – 11 .
- Lofgren , E. 2007 . Influenza seasonality: underlying causes and modeling theories . J. Virol. , 81 : 5429 – 5436 .
- Ma , J. and Ma , Z. 2006 . Epidemic threshold conditions for seasonally forced SEIR models . Math. Biosci. Eng. , 3 : 161 – 172 .
- Moneim , I. A. 2007 . Seasonally varying epidemics with and without latent period: a comparative simulation study . Math. Med. Biol. , 24 : 1 – 15 .
- Moneim , I. A. 2007 . The effect of using different types of periodic contact rate on the behavior of infectious diseases: a simulation study . Comp. Biol. Med. , 37 : 1582 – 1590 .
- Moneim , I. A. and Greenhalgh , D. 2005 . Use of a periodic vaccination strategy to control the spread of epidemics with seasonally varying contact rate . Math. Biosci. Eng. , 13 : 131 – 150 .
- Nelson , R. J. , Demas , G. E. , Klein , S. L. and Kriegsfeld , L. J. 2002 . Seasonal Patterns of Stress, Immune Function and Disease , Cambridge : Cambridge University Press .
- Pascual , M. and Dobson , A. 2004 . Seasonal patterns of infectious diseases . PLoS Med. , 2 : 18 – 20 .
- Rohani , P. , Earn , D. J. , Finkenstadt , B. and Grenfell , B. T. 1998 . Population dynamic interference among childhood diseases . Proc. Roy. Soc. Lond. B , 265 : 2033 – 2041 .
- Sauvage , F. , Langlais , M. and Pontier , D. 2007 . Predicting the emergence of human hantavirus disease using a combination of viral dynamics and rodent demographic patterns . Epidemiol. Infect. , 135 : 46 – 56 .
- Sauvage , F. , Langlais , M. , Yuccoz , N. G. and Pontier , D. 2003 . Modeling hantavirus in fluctuating populations of bank voles: the role of indirect transmission on virus persistence . J. Anim. Ecol. , 72 : 1 – 13 .
- Schwartz , I. B. 1985 . Multiple recurrent outbreaks and predictability in seasonally forced nonlinear epidemic models . J. Math. Biol. , 21 : 347 – 361 .
- Schwartz , I. B. 1992 . Small amplitude, long period outbreaks in seasonally driven epidemics . J. Math. Biol. , 30 : 473 – 491 .
- Schwartz , I. B. and Smith , H. L. 1983 . Infinite subharmonic bifurcations in an SEIR model . J. Math. Biol. , 18 : 233 – 253 .
- Sultan , B. , Labadi , K. , Guegan , J. F. and Janicot , S. 2005 . Climate drives the meningitis epidemics onset in West Africa . PLoS Med. , 2 : 43 – 49 .
- van den Driessche , P. and Watmough , J. 2002 . Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission . Math. Biosci. , 180 : 29 – 48 .
- Wolf , C. , Langlais , M. , Sauvage , F. and Pontier , D. 2006 . A multi-patch epidemic model with periodic demography, direct and indirect transmission and variable maturation rate . Math. Popul. Stud. , 13 : 153 – 177 .